Back to Journals » Research and Reports in Tropical Medicine » Volume 15
Exploring the Role of Community Involvement in Reducing the Burden of Schistosomiasis and Other Neglected Tropical Diseases in Malawi: Where are We in the Fight Against Neglected Tropical Diseases?
Authors Lubanga AF , Bwanali AN, Munthali LE, Mphepo M, Chumbi GD, Kangoma M, Matola Y, Kaonga B, Moyo CS
Received 7 November 2023
Accepted for publication 24 February 2024
Published 28 February 2024 Volume 2024:15 Pages 51—58
DOI https://doi.org/10.2147/RRTM.S448425
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Mario Rodriguez-Perez
Adriano Focus Lubanga,1,2 Akim Nelson Bwanali,1,3 Leonard Eston Munthali,2 Mzati Mphepo,3 Gertrude Diana Chumbi,2 Melina Kangoma,2 Yankho Matola,2 Byenala Kaonga,2 Chitemwa Sithando Moyo2
1Education and Research, Clinical Research Education and Management Services Ltd (CREAMS), Lilongwe, Malawi; 2Department of Clinical Services, Kamuzu Central Hospital, Lilongwe, Malawi; 3Department of Clinical Services, Queen Elizabeth Central Hospital, Blantyre, Malawi
Correspondence: Adriano Focus Lubanga, Education and Research, Clinical Research Education and Management Services Ltd (CREAMS), Anderson House, Area 43, P.O Box 31045, Lilongwe, Malawi, Tel +265992744497, Email [email protected]; [email protected]
Abstract: Schistosomiasis has been endemic in Malawi since 1947. Despite the longevity of endemicity of the disease, it still maintains a high burden in Malawi. This could be attributed to insufficient coverage of preventive and therapeutic mass drug administration (MDA) which mainly targets school-aged children, leaving out adults who also bear a high burden of the disease. Additionally, despite well documented impact of community involvement in boosting up the effectiveness of health programmes, there is minimal community involvement in schistosomiasis control and prevention programmes. Therefore, this perspective seeks to discuss the historical background of schistosomiasis in Malawi, gaps in community engagement and participation and suggest ways of enhancing the role of the community in prevention and control programmes. Amongst other challenges, the control programmes are centralised, leading to minimal input at the district and community level as well as low awareness of schistosomiasis control and prevention methods at the community level. It is of utmost significance therefore to provide comprehensive schistosomiasis health education to the communities and devise a thorough outline of the specific roles and responsibilities of all stakeholders including community members in the fight against schistosomiasis and other neglected tropical diseases.
Keywords: schistosomiasis, community prevention, water, sanitation, hygiene, neglected tropical diseases
Background
Schistosomiasis is a neglected tropical disease (NTD) caused by parasitic trematode worms of the genus Schistosoma.1 Globally, the disease is estimated to affect over 240 million people every year, causing over 280, 000 deaths per year.2–4 Schistosomiasis has the third highest burden of diseases attributable to NTDs accounting for over 3.3 million disability-adjusted life years as of 2019.5 In the last decade, schistosomiasis has been only second to malaria in terms of worldwide morbidity.6,7 The burden is especially heavier in poor and marginalized communities with limited access to safe drinking water and adequate sanitation.8–11
The sub-Saharan Africa (SSA) has the greatest prevalence of schistosomiasis bearing over 90% of the global burden.6,9 Malawi, a low-income country within the SSA, is equally massively afflicted with the burden of schistosomiasis. The disease is one of the most common and prevalent NTDs in Malawi, with at least 50% of the population at risk of infection.12 There are two notable Schistosoma species prevalent in Malawi, namely, Schistosoma haematobium (S. haematobium) that causes urinary schistosomiasis and Schistosoma mansoni (S. mansoni) that causes intestinal schistosomiasis.12,13 S. haematobium is the most prevalent species in Malawi, found mainly in the lakeshore and Southern Region districts of the country.14 If left untreated, schistosomiasis gives rise to tragic consequences that include infertility, anaemia and poor child development.15–17 These reduce productivity and promote poverty, thereby impeding elimination of the disease and potential realization of Sustainable Development Goals (SDGs) in SSA.18,19
The World Health Organization (WHO) aims to achieve control and elimination of schistosomiasis as a public health problem and move towards interruption of its transmission by the year 2030.20 In line with this, in February 2022, the WHO launched new guidelines with 6 evidence-based recommendations to aid the accomplishment of the control and elimination goals.1 The main strategy employed is large-scale MDA of praziquantel for annual preventive chemotherapy in all age-groups from 2-years old not just school children.21–24 Malawi carries MDA that currently targets school-aged children (aged 5–14 years) annually with high coverage rates.25 However, MDA has not been shown to lead to elimination of infection.26,27 Fittingly, the WHO advocates for the use of an integrated approach including Water, Sanitation and Hygiene (WASH) strategies, snail control and behavioural change interventions to achieve elimination of schistosomiasis.1,23 This is more appropriate because in addition to living in an endemic country, lack of hygiene and recreational habits for school-aged children such as swimming in infested water bodies increases the population’s vulnerability to infection.1,28 Furthermore, exposure to infested waters for adults during income-generating activities like fishing sustains the transmission cycle of the infection.12 Provision of safe water and adequate hygiene has been proven an expedient tool for decreasing the incidence of schistosomiasis and other water-borne diseases.29,30 However, in most African countries, including Malawi, access to WASH services largely remains a challenge.
Despite the evident need of supplemental WASH interventions, delivery of appropriate and sustainable interventions, remains a challenge in rural and marginalized settings which experience weak and unstable health systems.31,32 Studies done across SSA reveal limited knowledge on transmission and control of schistosomiasis in the region, inevitably leading to poor health-seeking behaviours and adoption of interventions.8,33 Few studies that have investigated community practices on schistosomiasis control and prevention have reported that poor WASH practices were driven by a combination constrained access to WASH services, misconceptions towards schistosomiasis and discrepancy between personal preferences and available interventions.30,33,34 In communities, where access to WASH interventions remains a huge challenge, exploring the involvement of the community in planning and execution of control measures for NTDs remains crucial toward disease control. Community involvement is recognised as a crucial pillar for the success of disease control and elimination.35,36 However, studies in SSA show that most health programmes run with little community involvement which contributes to their lack of success.37,38 This perspective, therefore, seeks to discuss the historical background of schistosomiasis in Malawi, gaps in community engagement and participation and suggest ways of enhancing the role of the community in prevention and control programmes.
Historical Background and Epidemiological Distribution of Schistosomiasis in Malawi
Schistosomiasis has been endemic in Malawi for several decades.39 The wide distribution of the disease in the plains and plateaus of Malawi has been reported for over 70 years.14,40 While urinary schistosomiasis is highly prevalent in the lakeshore and southern region districts, intestinal schistosomiasis predominates on the central plains and in the Northern Region’s districts.41,42 The first schistosomiasis mapping exercise, carried out in Malawi in 2003, demonstrated both a widespread occurrence of infection and a marked variability in infection prevalence.43 The disease is ranked among the top 20 causes of outpatient visits to health facilities with 40–50% of the population being at risk of the infection.44 The average reported national prevalence of the diseases reaches as high as 50%.41 Figure 1 shows maximum point prevalence of schistosome infection and location of S. mansoni and S. haematobium surveys in Malawi. Snails have largely been implicated in the transmission of the disease. In Malawi, two snail species are known to act as intermediate hosts for urinary schistosomiasis, namely the well-recognised host Bulinus globosus and the recently implicated Bulinus nyassanus, while Biomphalaria pfeifferi acts as intermediate host for intestinal schistosomiasis.14
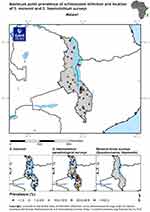 |
Figure 1 Shows maximum point prevalence of schistosome infection and location of S. mansoni and S. haematobium surveys in Malawi. |
Efforts to Combat Schistosomiasis in Malawi
Various strategies are known and have been utilised for schistosomiasis control and prevention in high burden areas. In 2020, the WHO launched a set of recommended guidelines charting routes to the control or elimination of schistosomiasis.1 The WHO strategy to control and eliminate human schistosomiasis includes preventive chemotherapy for at-risk groups, access to improved drinking water, and improved sanitation, hygiene education, environmental management and snail control.23 This new road map for 2021–2030, targets elimination of schistosomiasis as a public health problem by 2030 and the interruption of schistosomiasis transmission in humans in selected countries by 2030.45
Since 2009, Malawi has had ongoing national scale treatment program for schistosomiasis control.12,25 Due to the high endemicity of the disease in all districts in the country, the national treatment programs covered all the districts. By 2018, through MDA, all districts endemic for S. mansoni and S. haematobium had received four or more rounds of annual treatment with praziquantel and albendazole for soil-transmitted helminths (STH). These MDA campaigns were coupled with impact surveys to determine the prevalence and intensity of the disease.12 The survey went further to categorise high prevalent areas within particular districts.
In recent years, more efforts have been taken to control NTDs in Malawi. In 2011, Malawi developed its first National Strategy Plan to cover all NTDs to last for a span of 5 years (2011–2016).14 This also included creation of an NTD secretariat committee to monitor progress made. There is also an established National Schistosomiasis and Soil-Transmitted Helminths Control Programme run by the Ministry of Health.12 This program was integrated into the School Health and Nutrition Programme, an initiative led by the Ministry of Education and the Ministry of Health. This allowed the efficient mass administration of drugs among school going children, who share a high burden of the disease, particularly in lake-shore areas. Similarly, in 2015, Malawi launched the NTD Master plan for 2015–2020, with the vision of transforming Malawi into a nation free from NTDs by 2020.46 Among others, the master plan advocated for an integrated approach among the most prevalent endemic NTDs in Malawi, to accelerate the implementation of NTDs prevention and control activities in a coordinated manner in the country.
The country also enjoyed massive donor support through Schistosomiasis Control Initiative Foundation which led to high availability of key drugs (praziquantel and albendazole) for MDA campaigns.25 Coupled with task shifting to community-based health surveillance assistants (HSAs) and community-based volunteers, MDA enjoyed massive gains in coverage rates, reaching as high as 87% in some areas.25 Furthermore, deliberate efforts have been made to integrate schistosomiasis control programmes into the district implementation plans (DIP), to ensure more ownership and control of the programmes.
Gaps and Notable Challenges in the Fight Against Schistosomiasis in Malawi
Even though the history of schistosomiasis control in Malawi dates back to as early as 1960’s, the control programmes have largely concentrated on few modes of controlling the disease.12,13 The National Schistosomiasis Control Programme has largely been centralized, with the schistosomiasis control and prevention running as a vertical program, largely focusing on regular MDA in high burden areas through schools and villages.25 Even though in recent years efforts have been made to decentralize and integrate schistosomiasis control programs with other key NTDs, little is still documented in terms of efforts that have been taken toward implementations of integrated programmes.
The programmes have also largely focused on school-aged children, owing to the known high burden of the disease among this particular age group.25 However, recent studies have demonstrated a high prevalence of the disease among adults as well, especially in high burden areas.13,44 Largely focusing on school-going children simply means adults, and children who are not in school are largely left out of the programmes.
A recent study conducted to assess the implementation and effectiveness of MDA for the prevention of schistosomiasis and STH revealed low knowledge among the general population on the transmission cycle and prevention of schistosomiasis.25 This low knowledge points towards gaps in community sensitization and awareness of the disease which could consequently deter community engagement in control programmes. With schistosomiasis being endemic in the country, with cases being reported as early as 1948, it would be expected that by now, community awareness of the disease would be considerably high coupled with active involvement in control and prevention efforts.40
Even though the MDAs have achieved high coverage in the targeted districts, the prevalence of the disease still remains high in most districts.25,43 It is unlikely that Malawi will achieve the goal of reducing the burden of schistosomiasis and STH to levels of no public health importance in Malawi by 2025. The persistence of the disease also reflects limited efforts on other measures of controlling it as recommended in the WHO guidelines. The guidelines advocate for improved access to improved drinking water, sanitation, hygiene education, environmental management and snail control.23 However, it is evidently clear that Malawi’s effort is largely centred on chemotherapy neglecting the other equally important measures of controlling the disease.
A recent study also demonstrated possible intermixing between human and animal schistosomes.47 The potential interaction of human and animal species with possible hybridization calls for a one health approach toward disease mitigation.48 However, little is known about one health approach toward controlling NTDs in Malawi.
The Role of the Community in Reducing the Burden of Schistosomiasis and Other NTDs in Malawi and SSA
Eliminating high endemic diseases as public health problems requires joint efforts from health program implementers and the community.31 In most developing countries, disease prevalence and incidence are heightened because of the interplay of economic, social, and biological factors.49 While most health programs focus on dealing with the biological component of the disease, the social and economic aspects also remain crucial toward mitigating the impact of most NTDs. Controlling any endemic diseases at the community level requires well-implemented and relevant sensitization and education of community members and implementation of strategies that integrate and involve the community affected by the disease.34,35 Community engagement activities have largely been utilized in controlling infectious diseases particularly in low- and middle-income countries.35,50 This includes a range of approaches to involve communities in the improvement of their health and wellbeing.
The high burden of schistosomiasis and other NTDs reflects the neglect of the community in planning and execution of disease control programs. An effective elimination strategy for schistosomiasis needs to be comprehensive and directly feature the affected community members in planning and implementation of control Measures. In Malawi, most strategies to control schistosomiasis have largely been “top-down” in nature. These are developed based on evidence acquired from research and are thus imposed on the communities. This reduces community ownership of the programs and hence sustainability of the programmes.36 A study conducted in Tanzania revealed that using already existing village governance structures for WASH for schistosomiasis control contributed toward reduction in prevalence of schistosomiasis and diarrhoeal diseases and led to an increased awareness of WASH interventions for sustaining gains in NTD control.51 The study measured public health benefits associated with the intervention and linked these to specific activities which included managing committee functioning, promoting health awareness, self-organisation and data handling, income generation and interaction between village bodies. Even though it was an exploratory study, the insights generated reveal the important of community directed interventions toward control of NTDs.
The NTD report of a situation analysis conducted between February and April 2017, in Nigeria under the NTD COUNTDOWN project also highlighted that involving the community as primary stakeholders in NTD control programs implementation can help attain and sustain wider treatment coverage.52–54 The Nigerian mass administration of medicines is designed to engage community input through advisory committees or coalitions that are involved in decision-making. These groups nominate and reward their own community-based distributors, who assist in tailoring interventions towards specific target groups. Community-based distributors have been key in NTD control and have been utilised everywhere, including Malawi. However, most of the times, these community-based distributors are not equipped with comprehensive knowledge to carry disease awareness campaigns during the distribution of the drugs.
Similarly, a study conducted in Kenya investigating the capacity of local communities to address the burden of NTDs revealed firstly, the need for inter-sectoral collaboration between governments and affected populations for inclusive and sustainable NTD solutions.31 Secondly, the study recommended the need for a “bottom-up” approach that enhances capacity building, sensitization, and behaviour change for improved uptake of NTD interventions.
Indeed, most NTDs have available interventions that work. The most significant challenge remains how to deliver interventions to affected populations in areas experiencing weak health systems.31 For countries such as Malawi, where various health disparities exist when it comes to access to universal health coverage, the community needs to be actively involved in designing most health interventions. The need to engage the community in planning disease control and mitigation measures is becoming more evident now than ever. In the control on Schistosomiasis, the community may be involved at multiple levels, including development of WASH interventions, snail control, and conduction of awareness campaigns. An increase in the awareness of community members regarding schistosomiasis and STH is important for them to understand these diseases and to play a central role in their prevention and control.
Recommendations for the Future
Community engagement and participation are expedient tools in reducing the burden of NTDs. Multiple studies have reported that in addition to improving the effectiveness and ownership of health programmes, community engagement enhances sustainability of programmes.35,36,50 It is, therefore, essential to build up community participation. This could be done through ways suggested in the following paragraphs.
For successful communication engagement programmes, communities should have sufficient knowledge on schistosomiasis and other NTDs. They need to understand the burden, transmission cycle, their role in sustaining or cutting the transmission cycle of schistosomiasis as well as other prevention and control measures. This could be done through comprehensive schistosomiasis education programmes through mass media, health facility-based education or house-to-house visits. Furthermore, it is important to explain the health programmes in play and the specific roles and responsibilities of the community members.
Additionally, it is necessary to foster a “bottom-up” community engagement approach. This calls for communities to be involved at all stages of implementing schistosomiasis control and prevention programmes. Communities should have an active role in the designing, implementation, monitoring and evaluation stages of health programmes.36 This ensures programmes that are context-specific and culturally acceptable.35 Such an open approach brings in a sense of ownership and collective action amongst community members, and improves commitment toward achieving intended outcomes. In turn, sustainable and effective programmes ensue.
Another important gateway to the community is to utilize the existing community leadership structures. Traditional leaders, religious leaders and school teachers could be trained on schistosomiasis prevention methods and act as agents of change to take an active role in ensuring schistosomiasis control measures are being observed in their communities.
Furthermore, schistosomiasis control programmes should be decentralized and coordinated at a district level. As highlighted in the preceding sections, the centralized programmes lack effective coordination and coverage. Transitioning to a decentralized approach could enable District Health Management Teams (DHMT) to work effectively with all stakeholders within the district and target the high burden areas within the district. This could result in improved coordination and successful implementation and completion of schistosomiasis control and prevention programmes.
Finally, studies have suggested that it is critical to involve women in community WASH projects.35 Culturally defined roles of women in the communities tend to hinder their active involvement in health programmes. However, exclusion of women in WASH projects has been attributed to high failure rate and poor management of the projects.55 This has been explained by the active role of women in water management at the household level. Women collect, store and take care of water for domestic purposes. Therefore, they deserve an active role in community WASH programmes which could ensure effective implementation of the WASH projects.
Conclusion
Despite the long history of schistosomiasis in Malawi, there is still a high burden of the disease. Effective interventions, especially MDA and WASH, are well known. However, such community-based interventions fail to achieve optimal coverage and implementation due to a remarkable lack of community engagement. This is due to poor awareness of the disease in communities and lack of effective coordination of the health programmes. Therefore, it is imperative to conduct comprehensive community education campaigns as well as define specific responsibilities of all relevant stakeholders in order to ensure effective implementation of schistosomiasis control and prevention programmes. This could put Malawi on track toward achieving the goal of interrupting transmission of schistosomiasis by the year 2030.
Data Sharing Statement
All materials used for preparation of this manuscript are available upon request from the corresponding author.
Ethics Approval and Consent to Participate
Ethical Approval was not required for the preparation of this manuscript.
Funding
The authors declare that they did not receive any funding for this work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. WHO guideline on control and elimination of human schistosomiasis. Available from: https://www.who.int/publications-detail-redirect/9789240041608.
2. Faust CL, Osakunor DNM, Downs JA, et al. Schistosomiasis control: leave no age group behind. Trends Parasitol. 2020;36:582–591. doi:10.1016/j.pt.2020.04.012
3. Savioli L, Albonico M, Colley DG, et al. Building a global schistosomiasis alliance: an opportunity to join forces to fight inequality and rural poverty. Infect Dis Poverty. 2017;6:65. doi:10.1186/s40249-017-0280-8
4. Schistosomiasis (Bilharzia). Available from: https://www.who.int/health-topics/schistosomiasis.
5. Uzoegbo SC, Jackson LJ, Bloch SCM. A systematic review and quality appraisal of the economic evaluations of schistosomiasis interventions. PLoS Negl Trop Dis. 2022;16:e0010822. doi:10.1371/journal.pntd.0010822
6. Mitra A, Mawson A. Neglected tropical diseases: epidemiology and global burden. TropicalMed. 2017;2:36. doi:10.3390/tropicalmed2030036
7. Mnkugwe RH, Minzi OS, Kinung’hi SM, et al. Prevalence and correlates of intestinal schistosomiasis infection among school-aged children in North-Western Tanzania. PLoS One. 2020;15(2):e0228770. doi:10.1371/journal.pone.0228770
8. Koffi AJD, Doumbia M, Fokou G, et al. Community knowledge, attitudes and practices related to schistosomiasis and associated healthcare-seeking behaviours in northern Côte d’Ivoire and southern Mauritania. Infect Dis Poverty. 2018;7(1):70. doi:10.1186/s40249-018-0453-0
9. McManus DP. Defeating schistosomiasis. N Engl J Med. 2019;381(26):2567–2568. doi:10.1056/NEJMe1913771
10. McManus DP, Dunne DW, Sacko M, et al. Schistosomiasis. Nat Rev Dis Primers. 2018;4(1):1–19. doi:10.1038/s41572-018-0013-8
11. Feasey N, Wansbrough-Jones M, Mabey DCW, et al. Neglected tropical diseases. Br Med Bul. 2010;93(1):179–200. doi:10.1093/bmb/ldp046
12. Kayuni SA, Alharbi MH, Makaula P, et al. Male genital schistosomiasis along the shoreline of Lake Malawi: baseline prevalence and associated knowledge, attitudes and practices among local fishermen in Mangochi District, Malawi. Front Public Health. 2021;9:590695. doi:10.3389/fpubh.2021.590695
13. Kayuni S, Lampiao F, Makaula P, et al. A systematic review with epidemiological update of male genital schistosomiasis (MGS): a call for integrated case management across the health system in sub-Saharan Africa. Parasite Epidemiol Control. 2019;4:e00077. doi:10.1016/j.parepi.2018.e00077
14. Makaula P, Sadalaki JR, Muula AS, et al. Schistosomiasis in Malawi: a systematic review. Parasit Vectors. 2014;7(1):570. doi:10.1186/s13071-014-0570-y
15. Neglected tropical diseases | global health | CDC; 2023. Available from: https://www.cdc.gov/globalhealth/ntd/index.html.
16. Ochola EA, Karanja DMS, Elliott SJ, Dikomitis L. The impact of Neglected Tropical Diseases (NTDs) on health and wellbeing in sub-Saharan Africa (SSA): a case study of Kenya. PLoS Negl Trop Dis. 2021;15(2):e0009131. doi:10.1371/journal.pntd.0009131
17. Adenowo AF, Oyinloye BE, Ogunyinka BI, et al. Impact of human schistosomiasis in sub-Saharan Africa. Braz J Infect Dis. 2015;19(2):196–205. doi:10.1016/j.bjid.2014.11.004
18. Morton S, Pencheon D, Squires N. Sustainable Development Goals (SDGs), and their implementation: a national global framework for health, development and equity needs a systems approach at every level. Br Med Bul. 2017;124(1):81–90. doi:10.1093/bmb/ldx031
19. Engels D, Zhou X-N. Neglected tropical diseases: an effective global response to local poverty-related disease priorities. Infect Dis Poverty. 2020;9(1):10. doi:10.1186/s40249-020-0630-9
20. Makau-barasa LK, Onduma N, Yotebieng K, et al. African institutions will lead on the road to end neglected tropical diseases. Front Trop Dis. 2023;2023:1–5.
21. Casulli A, Brindley PJ. New global targets for NTDs in the WHO roadmap 2021–2030. PLoS Negl Trop Dis. 2021;15(5):e0009373. doi:10.1371/journal.pntd.0009373
22. New WHO guidelines for schistosomiasis | pediatric praziquantel consortium. Available from: https://www.pediatricpraziquantelconsortium.org/what-we-do/new-who-guidelines-schistosomiasis.
23. The 2022 WHO schistosomiasis guideline – new ways for HUGS to control schistosomiasis. LSTM. Available from: https://www.lstmed.ac.uk/news-events/blogs/the-2022-who-schistosomiasis-guideline-%E2%80%93-new-ways-for-hugs-to-control.
24. Kura K, Hardwick RJ, Truscott JE, et al. The impact of mass drug administration on Schistosoma haematobium infection: what is required to achieve morbidity control and elimination? Parasites Vectors. 2020;13(1):554. doi:10.1186/s13071-020-04409-3
25. Makaula P, Kayuni SA, Mamba KC, et al. An assessment of implementation and effectiveness of mass drug administration for prevention and control of schistosomiasis and soil-transmitted helminths in selected southern Malawi districts. BMC Health Serv Res. 2022;22(1):517. doi:10.1186/s12913-022-07925-3
26. King CH, Kittur N, Binder S, et al. Impact of Different mass drug administration strategies for gaining and sustaining control of schistosoma mansoni and schistosoma haematobium infection in Africa. Am J Trop Med Hyg. 2020;103(1_Suppl):14–23. doi:10.4269/ajtmh.19-0829
27. Wang W, Liang Y. Mass Drug Administration (MDA) for schistosomiasis. J Infect Dis. 2015;211(5):848–849. doi:10.1093/infdis/jiu506
28. Bartlett AW, Sousa-Figueiredo JC, van Goor RC, et al. Burden and factors associated with schistosomiasis and soil-transmitted helminth infections among school-age children in Huambo, Uige and Zaire provinces, Angola. Infect Dis Poverty. 2022;11(1):73. doi:10.1186/s40249-022-00975-z
29. Social capital, collective action and access to water in rural Kenya - PubMed. Available from: https://pubmed.ncbi.nlm.nih.gov/25181474/.
30. Kalumbi LR, Thaulo C, MacPherson EE, et al. Perspectives and practices on water, sanitation, and hygiene from a fishing community along lake Malombe, Southern Malawi. Int J Environ Res Public Health. 2020;17(18):6703. doi:10.3390/ijerph17186703
31. Ochola EA, Karanja DMS, Elliott SJ. Local tips, global impact: community-driven measures as avenues of promoting inclusion in the control of neglected tropical diseases: a case study in Kenya. Infect Dis Poverty. 2022;11:88. doi:10.1186/s40249-022-01011-w
32. Optimising the performance of frontline implementers engaged in the NTD programme in Nigeria: lessons for strengthening community health systems for universal health coverage | human Resources for Health | full Text. Available from: https://human-resources-health.biomedcentral.com/articles/10.1186/s12960-019-0419-8.
33. Sacolo H, Chimbari M, Kalinda C. Knowledge, attitudes and practices on schistosomiasis in sub-Saharan Africa: a systematic review. BMC Infect Dis. 2018;18:46. doi:10.1186/s12879-017-2923-6
34. Musuva RM, Awiti A, Omedo M, et al. Community knowledge, attitudes and practices on schistosomiasis in Western Kenya-The SCORE project. Am J Trop Med Hyg. 2014;90:646–652. doi:10.4269/ajtmh.13-0488
35. Tsekleves E, Fonseca Braga M, Abonge C, et al. Community engagement in water, sanitation and hygiene in sub-Saharan Africa: does it WASH? J Water Sanitat Hygien Develop. 2022;12:143–156. doi:10.2166/washdev.2022.136
36. Vanderslott S, Van Ryneveld M, Marchant M, et al. How can community engagement in health research be strengthened for infectious disease outbreaks in Sub-Saharan Africa? A scoping review of the literature. BMC Public Health. 2021;21(1):633. doi:10.1186/s12889-021-10348-0
37. Campbell C, Nair Y, Maimane S, et al. Hearing community voices: grassroots perceptions of an intervention to support health volunteers in South Africa. SAHARA J. 2008;5(4):162–177. doi:10.1080/17290376.2008.9724916
38. Ankomah SE, Fusheini A, Derrett S. Barriers and facilitators of Patient-public engagement for health system improvement in Sub-Saharan Africa: a systematic scoping review. Health Policy Open. 2021;2:100055. doi:10.1016/j.hpopen.2021.100055
39. Cetron MS, Chitsulo L, Sullivan JJ, et al. Schistosomiasis in Lake Malawi. Lancet. 1996;348(9037):1274–1278. doi:10.1016/S0140-6736(96)01511-5
40. Ransford ON. Schistosomiasis in the Kota Kota district of Nyasaland. Trans R Soc Trop Med Hyg. 1948;41(5):617–628. doi:10.1016/S0035-9203(48)90438-6
41. Mtethiwa AHN, Nkwengulila G, Bakuza J, et al. Extent of morbidity associated with schistosomiasis infection in Malawi: a review paper. Infect Dis Poverty. 2015;4(1):25. doi:10.1186/s40249-015-0053-1
42. Kapito-Tembo AP, Mwapasa V, Meshnick SR, et al. Prevalence distribution and risk factors for Schistosoma hematobium infection among school children in Blantyre, Malawi. PLoS Negl Trop Dis. 2009;3(1):e361. doi:10.1371/journal.pntd.0000361
43. Kayuni S, Peeling R, Makaula P. Prevalence and distribution of schistosoma haematobium infection among school children living in southwestern shores of Lake Malawi. Malawi Med J. 2017;29(1):16–23. doi:10.4314/mmj.v29i1.4
44. Nyangulu W, Sadimba C, Nyirenda J, et al. The prevalence of schistosoma mansoni infection among adults with chronic non-communicable diseases in Malawi. Trop Med Int Health. 2022;50(1):56. doi:10.1186/s41182-022-00450-3
45. Rollinson D, Sankar G, Stephens M, et al. Increasing efficiencies from integrating control and elimination programmes for soil-transmitted helminths and schistosomiasis. Int Health. 2022;14(1):111–112. doi:10.1093/inthealth/ihab029
46. Makaula P, Kayuni SA, Mamba KC, et al. Mass drug administration campaigns: comparing two approaches for schistosomiasis and soil-transmitted helminths prevention and control in selected Southern Malawi districts. BMC Health Serv Res. 2024;24(1):11. doi:10.1186/s12913-023-10489-5
47. Díaz AV, Walker M, Webster JP. Reaching the World Health Organization elimination targets for schistosomiasis: the importance of a one health perspective. Philos Trans R Soc B. 2023;378(1887):20220274. doi:10.1098/rstb.2022.0274
48. Mackenzie JS, Jeggo M. The one health approach—why is it so important? Trop Med Infect Dis. 2019;4(2):88. doi:10.3390/tropicalmed4020088
49. Léger E, Borlase A, Fall CB, et al. Prevalence and distribution of schistosomiasis in human, livestock, and snail populations in northern Senegal: a one health epidemiological study of a multi-host system. Lancet Planet Health. 2020;4(8):e330–e342. doi:10.1016/S2542-5196(20)30129-7
50. Akondeng C, Njamnshi WY, Mandi HE, et al. Community engagement in research in sub-Saharan Africa: approaches, barriers, facilitators, ethical considerations and the role of gender – a systematic review protocol. BMJ Open. 2022;12(5):e057922. doi:10.1136/bmjopen-2021-057922
51. Madon S, Malecela MN, Mashoto K, et al. The role of community participation for sustainable integrated neglected tropical diseases and water, sanitation and hygiene intervention programs: a pilot project in Tanzania. Soc Sci Med. 2018;202:28–37. doi:10.1016/j.socscimed.2018.02.016
52. Das JK, Salam RA, Arshad A, et al. Community based interventions for the prevention and control of non-helmintic NTD. Infect Dis Poverty. 2014;3(1):24. doi:10.1186/2049-9957-3-24
53. Marchal B, Van Dormael M, Pirard M, et al. Neglected tropical disease (NTD) control in health systems: the interface between programmes and general health services. Acta Trop. 2011;Suppl 120:S177–S185. doi:10.1016/j.actatropica.2011.02.017
54. Meredith SEO, Cross C, Amazigo UV. Empowering communities in combating river blindness and the role of NGOs: case studies from Cameroon, Mali, Nigeria, and Uganda. Health Res Policy Syst. 2012;10:16. doi:10.1186/1478-4505-10-16
55. Towards a pro-community-based water resource management system in Northwest Cameroon: practical evidence and lessons of best practices. SpringerLink. Available from: https://link.springer.com/article/10.1007/s10708-019-10085-3.
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
