Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Exploring the Potential of Hesperidin in Preventing Hypertrophic Scars: Insights from a Rabbit Ear Model
Authors Yang P, Zhong J , Zhao X, Ali K , Wu L, Bu Z
Received 2 July 2023
Accepted for publication 13 October 2023
Published 19 October 2023 Volume 2023:16 Pages 2957—2963
DOI https://doi.org/10.2147/CCID.S428587
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Anne-Claire Fougerousse
Ping Yang,1,* JianBo Zhong,1,* XingYun Zhao,1 Kamran Ali,1,2 Liming Wu,1 ZhangYu Bu1
1Department of Dermatology, Affiliated Hangzhou First People’s Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang, People’s Republic of China; 2Department of Oncology, The Fourth Affiliated Hospital, International Institutes of Medicine, Zhejiang University School of Medicine, Yiwu, Zhejiang, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Liming Wu, Department of Dermatology, Affiliated Hangzhou First People’s Hospital, Zhejiang University School of Medicine, No. 261, Huansha Road, Hangzhou, People’s Republic of China, Tel/Fax +86 13750837205, Email [email protected]
Background: Hypertrophic scars, commonly occurring after trauma or surgery in critical areas like the head and joints, pose significant challenges to both physical and mental health due to their impact on skin function and aesthetics. While the complex pathogenesis involves fibroblast activation and collagen deposition, effective treatments are lacking, underscoring the importance of exploring pathogenesis and targeted therapies. Hesperidin, a bioactive compound in citrus fruits with diverse health benefits, including anti-fibrotic and anti-angiogenic effects, is the focus of this study with the aim of investigating its impact on hypertrophic scar formation, given its potential to promote blood flow and improve skin microcirculation.
Objective: This study aimed to observe the inhibitory effect of topical hesperidin on hypertrophic scars in rabbits.
Methods: A total of 8 healthy adult New Zealand white rabbits were used to establish a rabbit hypertrophic scarring animal model. Five wounds were created on each rabbit’s two ears, with three wounds on the left ear (groups A, B, and C) and two wounds on the right ear (groups D and E). After six days of wound drying postoperatively, the wounds were locally treated with medication as followed: group A, 0.1% hesperidin; group B, 1% hesperidin; group C, 1% triamcinolone acetonide; group D, Vaseline; and group E, blank control without any medication. After 28 days, the scar tissue samples were collected for histological examination.
Results: The results showed that the scar texture of groups B and C was softer and lighter in color, and the number of fibroblasts, capillaries, and inflammatory cells in the tissue was significantly less than those in the other three groups. The hypertrophic scar indices of groups B and C were significantly smaller than those of groups A, D, and E, and the difference was statistically significant (P < 0.05). There were no significant difference between groups B and C.
Conclusion: Topical application of hesperidin demonstrated promising potential for reducing hypertrophic scar formation in rabbits.
Plain Language Summary: Hypertrophic scars are a common problem that can occur after trauma or surgery, causing functional and aesthetic issues in areas such as the head, face, neck, and joints. The formation of these scars involves complex processes including fibroblast activation and collagen deposition. Effective treatments for hypertrophic scars are currently lacking, and understanding their pathogenesis and developing targeted therapies are important.
Hesperidin (HDN), a compound found in plants like bitter orange and citrus peel, has various biological activities and has been found to have anti-fibrotic and anti-angiogenic effects in some studies. Traditional Chinese medicine believes that scars are caused by Qi stagnation and blood stasis, and that hesperidin has been shown to improve skin microcirculation, aligning with the principles of blood activation and stasis removal.
This study aimed to observe the effects of hesperidin on hypertrophic scar formation using animal models. Eight rabbits were divided into different groups and treated with different formulations of hesperidin or other control substances. Scar tissue samples were collected and examined after 28 days.
The results showed that hesperidin-treated scars had a softer texture and lighter color than the control scars. Histological analysis revealed reduced scar thickness, more organized collagen fibers; and fewer fibroblasts, capillaries, and inflammatory cells in the hesperidin-treated groups. The 1% hesperidin group showed comparable effects to the corticosteroid triamcinolone acetonide group.
These findings suggest that hesperidin has anti-inflammatory, anti-fibroblast proliferation, and anti-angiogenic properties and effectively inhibits hypertrophic scar formation in rabbits. Further research is needed to determine its efficacy and safety in human, and to establish appropriate dosages for therapeutic use. Overall, hesperidin holds promise as a potential treatment for preventing and treating skin scars.
Keywords: hesperidin, hypertrophic scar, rabbit ear
Introduction
Hypertrophic scarring (HS) is a common problem after trauma and surgery, affecting the function and aesthetics of the skin, especially in the head, face, neck, and joint areas, which seriously affects the physical and mental health of patients.1 The pathogenesis of hypertrophic scars is complex, and many studies have suggested that the activation of fibroblasts and deposition of collagen play a key role in scar formation.2 The biological activity of fibroblasts is regulated by the microenvironment in the surrounding matrix, and capillaries play a crucial role in establishing this microenvironment.3 However, effective treatments for hypertrophic scars are still lacking. Therefore, it is particularly important to explore their possible pathogenesis and targeted therapies.
Hesperidin (HDN) is widely found in natural plants such as bitter orange, tangerine peel, and citrus peel, and is the main active ingredient of the fruit of the Rutaceae citrus genus.4 It has multiple biological activities, such as anti-tumor, anti-inflammatory, antibacterial, antiviral, gastrointestinal function enhancement, and antioxidant effects.5,6 In recent years, some studies in the field of tumors have found that it has anti-fibrotic and anti-angiogenic effects.5,6 Traditional Chinese medicine believes that scars are mainly caused by Qi stagnation and blood stasis, so they are often treated with blood-activating and stasis-removing methods.1 Studies have shown that local application of 1% and 0.1% hesperidin can increase local skin blood flow and improve skin microcirculation, consistent with the theory of blood activation and stasis removal.7 However, there is currently no research on the use of hesperidin in hypertrophic scars at home or abroad. Therefore, this study aimed to observe the effects of hesperidin on hypertrophic scar formation in animal experiments.
Materials and Methods
Experimental Animals
Eight healthy adult rabbits (males and females not specified) aged between 8–12 months and weighing 2.0–2.5 kg were provided by the Animal Experiment Center of Zhejiang Chinese Medical University and were housed by them.
Reagents Preparation
Orange peel glycoside concentrate powder was provided by Xi’an Sheng Qing Biological Technology Co., Ltd. 1% HDN cream: 99 g Vaseline was heated and melted, and then 1 g HDN powder was added and stirred evenly. The cream was obtained after cooling. 0.1% HDN cream: 99.9 g of Vaseline was heated and melted, and then 0.1 g HDN powder was added and stirred evenly. 1% triamcinolone acetonide cream: 99 g Vaseline was heated and melted, and then 1 g of triamcinolone acetonide injection was slowly added while stirring.
Animal Model Establishment
Eight healthy adult New Zealand white rabbits (of unspecified sex) were included in the study. Anesthesia of the marginal vein of the ear was achieved using 3% pentobarbital sodium (30 mg/kg). The surgical procedure was performed with strict adherence to the aseptic techniques. A wound measuring 1 cm×1 cm was created along the longitudinal axis of the rabbit ear abdomen, ensuring a distance of more than 1 cm between each wound to avoid visible blood vessels. The full-thickness skin and cartilage membrane of the rabbit’s ear were excised and the wound edges were cauterized using a hot iron. Postoperatively, the wounds were left exposed to allow natural healing. Previous studies have indicated that wound epithelialization typically takes 19–21 days. Once epithelialization occurred, a firm mass was palpated, protruding above the skin surface, displaying a pale red color, and its proliferative extent did not surpass the original wound boundary. The mass reached its peak height at approximately 28–32 days.
Experimental Grouping and Drug Administration
A total of five wounds were created on each ear of each rabbit, with three wounds on the left ear (designated as groups A, B, and C) and two wounds on the right ear (designated as groups D and E). Local administration of drugs commenced six days post-surgery once the wounds had dried. The groups were categorized based on their respective drug treatments as follows: Group A received 0.1% HDN, Group B received 1% HDN, Group C received 1% triamcinolone acetonide, Group D was treated with Vaseline, and Group E served as the untreated control (blank control) without medication. Scar tissue samples from all five groups were collected and examined for relevant indicators 28 days after the surgical procedure.
Specimen Collection
Anesthesia of the marginal vein in the ear was achieved using 3% pentobarbital sodium (30 mg/kg), and tissue samples were obtained from the scar edge situated at the interface between normal rabbit ear skin and scar tissue, including sections of normal skin. The specimens were vertically sectioned to the skin surface to ensure that the entire layer of rabbit ear tissue was collected without disruption. Samples designated for hematoxylin and eosin (HE) staining were fixed in 4% neutral formalin and subsequently embedded in paraffin.
Observation Indicators and Methods
Scar proliferation in each group was visually assessed using with naked eye. The pathological characteristics of the scars were examined under a light microscope following hematoxylin and eosin (HE) staining of scar tissue samples obtained from each group. These observations included an assessment of the dermis layer thickness, predominant constituents of the dermis, morphology and structure of collagen fibers, and the presence of collagen nodules. The hypertrophic index (HI) was determined as follows. Using a micrometer, the relative increase in thickness was measured under a microscope. The scar height (SH) was compared to the height of normal skin (dermis height, DH), and the HI was calculated using the formula HI = SH/DH.
Statistical Methods
The experimental data were statistically analyzed using SPSS 23.0. Descriptive statistics were used to express quantitative data as the mean ± standard deviation (mean ± SD). Inter-group comparisons were performed using t-tests, and a p-value less than 0.05 was considered statistically significant.
Results
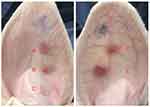
Observation of Scar Formation in Rabbit Ears by Naked Eye
The scars in groups B and C exhibited a softer texture and lighter color than those in groups D and E, which had a harder texture and redder color. The scars in group A displayed a texture and color that fell between those observed in groups B and C and D and E (Figure 1).
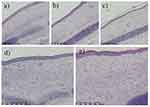
Observation of Tissue Slices Stained with Hematoxylin and Eosin Under a Light Microscope
In group A, the dermis exhibited evident thickening with a more disorganized arrangement of collagen fibers. Notably, there was a substantial increase in fibroblast and capillary proliferation, along with a noticeable infiltration of inflammatory cells in the deeper layers of the dermis. In groups B and C, the dermis within the scars displayed slight thickening compared to the surrounding normal tissue. Collagen fibers and fibroblasts exhibited a more ordered arrangement. Moreover, there was a significant reduction in the presence of fibroblasts, capillaries, and inflammatory cells within the scar tissue compared with the other three groups. Conversely, in groups D and E, the dermis within the scars exhibited a considerable increase in thickness compared with the surrounding normal tissue. Collagen fibers displayed a dense and disordered arrangement, forming a spiral pattern. Additionally, pronounced fibroblast and capillary proliferation was observed, accompanied by a large number of inflammatory cells within the scar tissue. Figure 2 provides a visual representation of the observed differences in the dermal characteristics among the various experimental groups.
Scar Hyperplasia Index
The B group exhibited a statistically significant difference compared to both the D group and E group (p-value < 0.05). Similarly, the C group demonstrated a statistically significant difference compared to both the D group and E groups (p-value < 0.05).
The scar hyperplasia indices in groups B and D was significantly lower than those in both the D group and E group (Table 1).
 |
Table 1 Scar Hyperplasia Index of Each Group at 28 Days Post-Surgery |
Discussion
Hypertrophic scars are a prevalent issue that often arise after severe trauma and surgical procedures, causing both psychological and physiological distress in patients. Currently, scar treatment poses challenges, and despite the availability of diverse methods, outcomes remain unsatisfactory. Hence, it is crucial to explore more effective treatment approaches.1,8 In recent years, an increasing number of studies have identified drugs derived from traditional Chinese medicine. These drugs have demonstrated definite therapeutic effects and fewer side effects, thereby offering a broader range of scar treatment options.1,9
During wound repair, transforming growth factor-β (TGF-β) is widely recognized as the most important cytokine.10 Multiple studies have demonstrated that the development of pathological scars is linked to excessive activation of TGF-β and its downstream signaling pathways, which in turn promote fibroblast proliferation.11,12 Hesperidin has been demonstrated in several previous studies to inhibit cancer cell proliferation and induce cell apoptosis.13–17 Additionally, it inhibits angiogenesis.18 However, the precise mechanisms underlying this action remains unclear. Recent investigations have revealed its regulatory and anti-fibrotic effects on immunity, with TGF-β1 playing a pivotal role in these processes.19 Hesperidin can inhibit the synthesis or secretion of extracellular matrix components induced by TGF-β1 in human embryonic lung fibroblasts.19 Several studies have indicated that hesperidin exhibits a protective effect against chemically induced liver cancer, potentially through inhibition of the TGF-β1/Smad3 signaling pathway.20 Furthermore, recent studies have shown that hesperidin effectively inhibits liver fibrosis. Its anti-liver fibrosis effect is believed to be associated with the regulation of the TGF-β1/Smad signaling pathway, leading to the inhibition of hepatic stellate cell activation and proliferation.21,22 Studies have suggested that the TGF-β1/Smad signaling pathway plays a critical role in mediating the anti-tumor, anti-angiogenic, and anti-fibrotic effects of hesperidin.23 An in vitro cell study demonstrated that hesperidin exerts an inhibitory effect on the TGF-β signaling pathway. This indicates that hesperidin can reduce the probability of binding of TβRII to TGF-β1, thereby reducing receptor dimerization and activation. Consequently, downstream signal transduction mediated by Smad3 phosphorylation is inhibited, leading to a cascade of biological effects.24 In conclusion, the TGF-β/Smad signaling pathway has been identified as a critical factor in scar hypertrophy, and hesperidin has been shown to effectively inhibit this pathway. Consequently, it is plausible to speculate that hesperidin has the potential to prevent scar formation.
In this study, we conducted a preventative treatment by topically applying varying concentrations of hesperidin to rabbit ear scars during the formation process. Our findings revealed that the scar color and hardness in the 1% HDN (hesperidin) group were similar to those in the 1% hydrocortisone group and significantly lighter compared to the negative control group. In addition, the 0.1% HDN group demonstrated intermediate results between the two groups. The scar proliferation index in the 1% HDN group was comparable to that in the 1% hydrocortisone group and significantly lower than that in control group. However, no significant difference was observed between the 0.1% HDN and the control group. Histological staining further demonstrated that the dermis of scars in the 1% HDN and 1% hydrocortisone groups exhibited reduced thickness compared with the surrounding normal tissue. Additionally, these groups exhibited a more organized arrangement of collagen fibers and fibroblasts, as well as fewer fibroblasts, capillaries, and inflammatory cells compared to the other three groups.
Our results suggested that hesperidin possesses anti-inflammatory, anti-fibroblast proliferation, and anti-angiogenic properties, thereby inhibiting rabbit ear scar formation. Moreover, the therapeutic effect of hesperidin appears to be positively correlated with its concentration within a certain range, with the inhibitory effect of 1% hesperidin on scars comparable to that of glucocorticoids. Notably, hesperidin demonstrated definite pharmacological effects in inhibiting scar formation in a rabbit ear model, while exhibiting minimal adverse reactions. Therefore, investigating its pharmacological effects and specific mechanisms for preventing scar formation is important and may contribute to the development of effective new drugs for the prevention and treatment of skin scars in humans.
Conclusion
Topical application of hesperidin has shown to effectively reduced hypertrophic scar formation in rabbits. However, further studies are necessary to assess its efficacy and safety in human, and to determine the appropriate therapeutic dosages.
Data Sharing Statement
The data are accessible within the manuscript, and specific details can be provided upon request.
Ethics Approval
The study was approved by the Zhejiang Chinese Medical University Animal Ethics Committee and followed the ethics and welfare guidelines for animals of Zhejiang Chinese Medical University, Institutional Animal Care and Use Committee (IACUC), (Approval No. IACUC-20191125-03).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
Zhejiang Province Chinese Medicine Science and Technology Plan Project, Excellent Young Talent Fund, 2020 ZQ03.
Disclosure
Ping Yang and JianBo Zhong are co-first authors for this study. The authors declare no conflicts of interest in this work.
References
1. Zhang T, Wang X-F, Wang Z-C, et al. Current potential therapeutic strategies targeting the TGF-β/Smad signaling pathway to attenuate keloid and hypertrophic scar formation. Biomed Pharmacother. 2020;129:110287. doi:10.1016/j.biopha.2020.110287
2. Ud-Din S, Bayat A. New insights on keloids, hypertrophic scars, and striae. Dermatol Clin. 2014;32(2):193–209. doi:10.1016/j.det.2013.11.002
3. Zhu H, Tamot B, Quinton M, Walton J, Hacker RR, Li J. Influence of tissue origins and external microenvironment on porcine foetal fibroblast growth, proliferative life span and genome stability. Cell Prolif. 2004;37(3):255–266. doi:10.1111/j.1365-2184.2004.00310.x
4. Valls RM, Pedret A, Calderón-Pérez L, et al. Effects of hesperidin in orange juice on blood and pulse pressures in mildly hypertensive individuals: a randomized controlled trial (Citrus study). Eur J Nutr. 2021;60(3):1277–1288. doi:10.1007/s00394-020-02279-0
5. Ortiz AD, Fideles SO, Reis CH, et al. Therapeutic effects of citrus flavonoids neohesperidin, hesperidin and its aglycone, hesperetin on bone health. Biomolecules. 2022;12(5):626. doi:10.3390/biom12050626
6. Aggarwal V, Tuli HS, Thakral F, et al. Molecular mechanisms of action of hesperidin in cancer: recent trends and advancements. Exp Biol Med. 2020;245(5):486–497. doi:10.1177/1535370220903671
7. Man M-Q, Yang B, Elias PM. Benefits of hesperidin for cutaneous functions. Evid Based Complement Alternat Med. 2019;2019:2676307. doi:10.1155/2019/2676307
8. Menchaca AD, Style CC, Olutoye OO. A review of hypertrophic scar and keloid treatment and prevention in the pediatric population: where are we now? Adv Wound Care. 2022;11(5):255–279. doi:10.1089/wound.2021.0028
9. Chen D, Li Q, Zhang H, et al. Traditional Chinese medicine for hypertrophic scars-A review of the therapeutic methods and potential effects. Front Pharmacol. 2022;13:1025602. doi:10.3389/fphar.2022.1025602
10. Lichtman MK, Otero-Vinas M, Falanga V. Transforming growth factor beta (TGF-β) isoforms in wound healing and fibrosis. Wound Repair Regen off Publ Wound Heal Soc and Eur Tissue Repair Soc. 2016;24(2):215–222.
11. Wu C, Jiang J, Boye A, Jiang Y, Yang Y. Compound Astragalus and Salvia miltiorrhiza extract suppresses rabbits’ hypertrophic scar by modulating the TGF-β/Smad signal. Dermatology. 2014;229(4):363–368. doi:10.1159/000365784
12. Zhai X, Ding J, Tang Z. Resveratrol inhibits proliferation and induces apoptosis of pathological scar fibroblasts through the mechanism involving TGF-β1/smads signaling pathway. Cell Biochem Biophys. 2015;71(3):1267–1272. doi:10.1007/s12013-014-0317-6
13. Choi EJ. Hesperetin induced G1-phase cell cycle arrest in human breast cancer MCF-7 cells: involvement of CDK4 and p21. Nutr Cancer. 2007;59(1):115–119. doi:10.1080/01635580701419030
14. Nalini N, Aranganathan S, Kabalimurthy J. Chemopreventive efficacy of hesperetin (citrus flavonone) against 1,2-dimethylhydrazine-induced rat colon carcinogenesis. Toxicol Mech Methods. 2012;22(5):397–408. doi:10.3109/15376516.2012.673092
15. Patil JR, Chidambara Murthy KN, Jayaprakasha GK, Chetti MB, Patil BS. bioactive compounds from Mexican lime (Citrus aurantifolia) juice induce apoptosis in human pancreatic cells. J Agric Food Chem. 2009;57(22):10933–10942. doi:10.1021/jf901718u
16. Sivagami G, Vinothkumar R, Bernini R, et al. Role of hesperetin (a natural flavonoid) and its analogue on apoptosis in HT-29 human colon adenocarcinoma cell line--A comparative study. Food Chem Toxicol an Int J Publ Br Ind Biol Res Assoc. 2012;50(3–4):660–671. doi:10.1016/j.fct.2011.11.038
17. Zarebczan B, Pinchot SN, Kunnimalaiyaan M, Chen H. Hesperetin, a potential therapy for carcinoid cancer. Am J Surg. 2011;201(3):
18. Choi EJ, Kim GD, Chee K-M, Kim G-H. Effects of hesperetin on vessel structure formation in mouse embryonic stem (mES) cells. Nutrition. 2006;22(9):947–951. doi:10.1016/j.nut.2006.05.004
19. Zhou Z, Kandhare AD, Kandhare AA, Bodhankar SL. Hesperidin ameliorates bleomycin-induced experimental pulmonary fibrosis via inhibition of TGF-beta1/Smad3/AMPK and IkappaBalpha/NF-kappaB pathways. EXCLI J. 2019;18:723–745. doi:10.17179/excli2019-1094
20. Mahmoud AM, Mohammed HM, Khadrawy SM, Galaly SR. Hesperidin protects against chemically induced hepatocarcinogenesis via modulation of Nrf2/ARE/HO-1, PPARγ and TGF-β1/Smad3 signaling, and amelioration of oxidative stress and inflammation. Chem Biol Interact. 2017;277:146–158. doi:10.1016/j.cbi.2017.09.015
21. Wu F, Jiang L, He X, Zhu P, Li J. Effect of hesperidin on TGF-beta1/Smad signaling pathway in HSC. China J Chinese Mater Medica. 2015;40(13):2639–2643.
22. Nasehi Z, Kheiripour N, Taheri MA, Ardjmand A, Jozi F, Shahaboddin ME. Efficiency of hesperidin against liver fibrosis induced by bile duct ligation in rats. Biomed Res Int. 2023;2023:5444301. doi:10.1155/2023/5444301
23. Kim H-J, Jin B-R, H-J A. Hesperidin ameliorates benign prostatic hyperplasia by attenuating cell proliferation, inflammatory response, and epithelial-mesenchymal transition via the TGF-β1/Smad signaling pathway. Biomed Pharmacother. 2023;160:114389. doi:10.1016/j.biopha.2023.114389
24. Yang Y, Wolfram J, Shen H, Fang X, Ferrari M. Hesperetin: an inhibitor of the transforming growth factor-β (TGF-β) signaling pathway. Eur J Med Chem. 2012;58:390–395. doi:10.1016/j.ejmech.2012.10.028
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.