Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Exploring the Potential Molecular Mechanism of Sijunzi Decoction in the Treatment of Non-Segmental Vitiligo Based on Network Pharmacology and Molecular Docking
Authors Du Z , Wang H , Gao Y , Zheng S , Kou X , Sun G , Song J, Dong J, Wang G
Received 5 January 2023
Accepted for publication 22 March 2023
Published 1 April 2023 Volume 2023:16 Pages 821—836
DOI https://doi.org/10.2147/CCID.S403732
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Ziwei Du,1 Hepeng Wang,1 Yang Gao,1 Shumao Zheng,1 Xiaoli Kou,1 Guoqiang Sun,1 Jinxian Song,2 Jingfei Dong,3 Genhui Wang4
1Department of Dermatology, Hebei Academy of Traditional Chinese Medicine, Shijiazhuang, Hebei, 050031, People’s Republic of China; 2Department of Dermatology, Quyang County People’s Hospital, Baoding, People’s Republic of China; 3Department of Clinical Laboratory, Hebei Provincial Hospital of Traditional Chinese Medicine, Shijiazhuang, People’s Republic of China; 4Department of Dermatology, Hebei Provincial Hospital of Traditional Chinese Medicine, Shijiazhuang, People’s Republic of China
Correspondence: Yang Gao, Department of Dermatology, Hebei Academy of Traditional Chinese Medicine, No. 209 Jianhua South Street, Shijiazhuang, Hebei, 050031, People’s Republic of China, Tel +86-15833969687, Email [email protected]
Background: Non-segmental vitiligo is a common decolorized skin disease. The purpose of this study was to reveal the active components of Sijunzi decoction (SJZD) and the target genes for the treatment of non-segmental vitiligo.
Methods: Based on TCMSP and GEO databases, effective components and targets of SJZD in the treatment of non-segmental vitiligo were revealed by network pharmacology. GO and KEGG were used to analyze the biological functions of SJZD targets. The Cytoscape-cytoHubba plugin was used to identify hub target genes. SsGSEA method was used to analyze the infiltration level of immune cells in non-segmental vitiligo. Molecular docking was performed to predict the interaction between active compounds and hub target genes. Finally, real-time PCR detection was also performed.
Results: It was found that 104 active compounds may be effective ingredients in the treatment of non-segmental vitiligo. These 104 compounds acted on 42 differentially expressed target genes. KEGG analysis showed that target genes were significantly enriched in immune-related pathways such as MAPK and TNF signaling pathways. A total of 6 hub target genes (AKT1, CASP3, PPARG, SIRT1, TNF and TP53) were identified using the Cytoscape-cytoHubba plugin. Molecular docking showed that active compounds quercetin, kaempferol, formononetin and naringenin had good binding to hub target genes. We also found that Type 2 T helper cells, CD56bright natural killer cell and CD56dim natural killer cell infiltration levels were abnormal in non-segmental vitiligo and correlated with AKT1.
Conclusion: The results of this study indicate that quercetin, kaempferol, formononetin and naringenin in SJZD may play an important role in the treatment of non-segmental vitiligo by acting on AKT1, CASP3, PPARG, SIRT1, TNF and TP53 to regulate immune cell infiltration and multiple signaling pathways.
Keywords: Sijunzi decoction, non-segmental vitiligo, network pharmacology, molecular docking, immune, gene
Introduction
Vitiligo is a common depigmentation skin disease that causes white spots and patches on the body due to a lack of pigment cells in the epidermis.1 Two forms of the disease are well recognised: segmental and non-segmental vitiligo (the commonest form).2 Non-segmental vitiligo is associated with innate immune activation, inflammasome activation, oxidative stress, and loss of melanocyte adhesion.3 In addition to the physical effects of vitiligo, it is more likely to cause psychological trauma. Drug therapy, phototherapy and surgery are commonly used in the treatment of vitiligo.4 Due to non-segmental vitiligo is prone to relapse,5 the current treatment is still not ideal. Therefore, it is necessary to constantly explore new therapeutic methods.
Sijunzi decoction (SJZD) is a traditional Chinese medicine. SJZD is composed of four kinds of Chinese herbal medicines: ginseng, poria, atractylodes and licorice.6 Crude polysaccharide (SJZDP), polysaccharide fraction (S-3) and homogeneous polysaccharide (S-3-AG) separated from SJZD have immune-enhancing effects, and may have different immune regulation effects on intestinal immunity, specific immunity and non-specific immunity due to their different monosaccharide compositions.7 Mechanism of action study has shown that SJZD can enhance the immune function of mice by regulating the janus kinase (JAK)-signal transducer and activator of transcription (STAT) signaling pathway.8 SJZD may also help reduce intestinal injury after burns and prevent intestinal bacterial translocation.9 The pectin polysaccharide in SJZD can also promote the antioxidant defense of SW480 cells.10 So far, no studies have found SJZD in the treatment of skin diseases, including vitiligo. Exploring the potential therapeutic effect of SJZD on non-segmental vitiligo is not only conducive to deepening the understanding of the medicinal value of SJZD, but also pioneering new therapeutic drugs for the treatment of vitiligo.
Network pharmacology integrates systemic biology and pharmacology to promote drug development and reveal potential treatment mechanism. In addition, network pharmacology is particularly concerned about complex “drug-gene-target-disease” interactive network.11 Molecular docking technology is used to place small-molecule ligands in the binding region of macromolecular receptors through computer simulation and predict the binding energy (binding affinity) and binding mode (conformation) of the two by calculating physical and chemical parameters.12 Then, find the conformation with the lowest binding energy for ligand and receptor. The smaller the binding energy, the more stable the binding between ligand and receptor. Binding energy less than −5.0 kJ/mol (Note: −5.0 kJ/mol = −1.19423 kcal/mol) is the basis for screening candidate targets of active ingredients.13,14 Molecular docking has become an important tool to help understand how compounds interact with their molecular targets and for drug discovery and development.15 In this study, we explored the multi-target mechanism of SJZD in the treatment of non-segmental vitiligo based on network pharmacology and molecular docking. In addition, immune correlation analysis and receiver operating characteristic (ROC) analysis were performed for hub target genes.
Materials and Methods
Screening of SJZD Active Compounds and Target Genes
SJZD includes four medicinal materials, namely ginseng, poria, atractylodes and licorice. The compounds contained in the four medicinal materials were screened out using the TCMSP database (http://lsp.nwu.edu.cn/tcmsp.php). Then, active compounds with therapeutic efficacy are selected by absorption, distribution, metabolism and excretion (ADME). Screening indicators were set as oral bioavailability (OB) ≥30% and drug-likeness (DL) ≥0.18.16–18 The target genes of active compounds are screened out in TCMSP, and the selected target genes must be annotated by DrugBank database (https://www.drugbank.ca/) or validated. Meanwhile, target genes were normalized using UniProt database (https://www.uniprot.org/).
Screening of Non-Segmental Vitiligo Datasets and Identification of Differentially Expressed Genes (DEGs)
In this study, GSE65127 datasets were selected from Gene Expression Omnibus (GEO) database (https://www.ncbi.nlm.nih.gov/geo/). The GSE65127 dataset contains skin tissue sample data from 10 non-segmental vitiligo patients and 10 healthy controls. The gene expression matrices in the GSE65127 was downloaded and annotated by GPL570 platform annotation file. Convert gene probes to gene symbols. Multiple probes corresponding to the same gene were averaged. The limma package was used for differential expression analysis. The screening criteria for DEGs were set P <0.05 and |log2 fold change | (|log2FC|) >0.1. Subsequently, drug target genes were intersected with DEGs to obtain differentially expressed target genes related to non-segmental vitiligo.
Functional Enrichment Analysis of Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG)
GO includes three ontologies, namely molecular function (MF), cellular component (CC) and biological process (BP).19 KEGG is a database for systematic analysis of gene function.20 To understand the possible functions involved in drug target genes and non-segmental vitiligo related differentially expressed target genes, GO and KEGG functional enrichment analyses were performed based on the David database. False discovery rate (FDR) <0.05 was considered statistically significant.
Construction of Drug-Compound-Gene-Disease Network
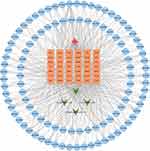
Active compounds acting on differentially expressed target genes were selected. Subsequently, complex networks are constructed based on interactions between medicinal materials, compounds, differentially expressed target genes, and non-segmental vitiligo. Cytoscape v3.7.2 was used to visualize the drug-compound-gene-disease network.
Identification of Multicentric Hub Genes
A protein-protein interaction (PPI) network of differentially expressed target genes was constructed based on STRING database and imported into Cytoscape for visualization. The cytoHubba plugin in Cytoscape software includes 11 topological analysis methods, Degree, Edge Percolated Component (EPC), Maximum Neighborhood Component (MNC), Density of Maximum Neighborhood Component (DMNC), Maximal Clique Centrality (MCC), Bottleneck, EcCentricity, Closeness, Radiality, Betweenness and Stress.21 Seven methods (Betweenness, Closeness, Degree, EPC, MCC, MNC and Stress) in cytoHubba plug-in were used to screen the PPI network. The top 10 node genes scored by each method were selected, and then multicentric hub genes were screened by the UpSet package. Subsequently, receiver operating characteristic (ROC) analysis of hub gene was performed using pROC package to determine the diagnostic accuracy. The area under the curve (AUC) was used to assess accuracy. AUC >0.7 indicated that the gene had better diagnostic accuracy.22
Infiltration of Immune Cells
Gene sets marking each immune cell type were obtained from Charoentong’s study.23 The ssGSEA algorithm was used to quantify the relative abundance of each immune cell infiltration in the immune microenvironment (IME). The Wilcoxon test was used to compare the difference in immune cell infiltration between non-segmental vitiligo group and normal control group. Subsequently, the correlation between multicentric hub genes and immune cell infiltration was also analyzed.
Molecular Docking
To investigate the interaction relationship between compounds and hub genes, we performed molecular docking studies to explore the binding activity of the two. 3D structure files of hub gene proteins and compounds were downloaded from RCSB PDB (http://www.rcsb.org/pdb/home/home.do) and TCMSP databases, respectively. Protein receptors were first treated with water molecule removal in PyMol,24 followed by hydrogenation and other pretreatments in AutoDockTools.25 The compounds were also preprocessed in AutoDockTools. Then, molecular docking calculations were performed and the results were visualized using PyMol.
Real-Time PCR Detection
Non-segmental vitiligo is also more common in children.26 Children with vitiligo were often accompanied by anxiety, inferiority and depression. Therefore, the parents of these children are eager to seek treatment to alleviate their children’s pain. Blood samples from 21 children (7 control blood samples, 7 non-segmental vitiligo blood samples and 7 non-segmental vitiligo blood samples treated with SJZD) treated in our hospital were collected for real-time PCR. Total RNA of blood samples was extracted by RNAliquid overspeed whole blood (liquid samples) total RNA extraction kit (Beijing HT-biotech Co., Ltd., RN2602, China). Then, reverse transcription and real-time PCR detection were performed using the FastKing cDNA First Strand Synthesis Kit (TIANGEN, KR116, China) and SuperReal PreMix Plus (SYBR Green) (TIANGEN, FP205, China), respectively. Subsequently, a real-time PCR detection program was run using a Model Gene-9660 quantitative PCR instrument. The 2−ΔΔCt method was used for relative quantitative analysis of expression data.27 GAPDH and ACTB were used as reference genes.
Statistical Analysis
The limma package was used to screen DEGs in vitiligo based on P <0.05 and |log2FC| >0.1. GO and KEGG functional enrichment analyses were also performed based on the David database. FDR <0.05 was considered statistically significant. In addition, Wilcoxon test was used to compare the difference in immune cell infiltration between non-segmental vitiligo group and normal control group. In real-time PCR, t-test was used to evaluate the statistical significance.
Results
Mining and Screening of Active Compounds and Target Genes of SJZD
Totally, 135 compounds were found in SJZD. Among the 135 compounds, 22 were from ginseng, 15 were from poria, 7 were from atractylodes, and 92 were from licorice. The compound kaempferol is found in both licorice and ginseng. Totally, 252 target genes were obtained after annotation and standardization in DrugBank and UniProt databases. Top 10 active compounds with the number of target genes are shown in Table 1, among which 2-[(3R)-8,8-dimethyl-3,4-dihydro-2H-pyrano[6,5-f]chromen-3-yl]-5-methoxyphenol and stigmasterol have the same number of target genes. In the CC term of GO analysis, the target genes were mainly distributed in cytosol, plasma membrane, cytoplasm and nucleus. In the MF term of GO analysis, the target genes were mainly involved in protein binding and identical protein binding. In the BP term of GO analysis, the target genes were mainly involved in positive regulation of transcription from RNA polymerase II promoter, signal transduction and positive regulation of transcription, DNA-templated (Figure 1A). KEGG enrichment analysis revealed that target genes were enriched in a variety of immune signaling pathways (Figure 1B). These results indicate that SJZD contains multiple compounds that synergistically regulate multiple pathways by acting on multiple target genes.
 |
Table 1 Top 10 Active Compounds with the Number of Target Genes |
Identification of Differentially Expressed Target Genes Related to Non-Segmental Vitiligo
In the GSE65127 dataset, 3187 DEGs were identified in non-segmental vitiligo patients according to P <0.05 and |log2FC| >0.1. Among them, 1663 DEGs were up-regulated and 1,524 DEGs were down-regulated. The volcano and heat map of DEGs are shown in Figure 2A and B. Subsequently, drug target genes were intersected with DEGs, and 42 differentially expressed target genes related to non-segmental vitiligo were obtained (Figure 2C). In the CC term of GO analysis, the differentially expressed target genes were mainly distributed in cytoplasm, nucleoplasm and nucleus. In the MF term of GO analysis, the differentially expressed target genes were mainly involved in protein binding and identical protein binding. In the BP term of GO analysis, the differentially expressed target genes were mainly involved in positive regulation of apoptotic process and positive regulation of transcription from RNA polymerase II promoter (Figure 2D). KEGG analysis showed that differentially expressed target genes were significantly enriched in immune-related pathways such as MAPK and TNF signaling pathways (Figure 2E). These results indicate that multiple compounds of SJZD may play a role in the treatment of non-segmental vitiligo by acting on multiple target genes and synergistically regulating multiple pathways.
Construction of Drug-Compound-Gene-Disease Network
There were 104 compounds acting on 42 differentially expressed target genes. Top 10 active compounds with the number of differentially expressed target genes are shown in Table 2. Subsequently, a drug-compound-gene-disease network was constructed based on non-segmental vitiligo, 4 medicinal materials, 42 differentially expressed target genes and 104 active compounds. Cytoscape v3.7.2 was used to visualize the network (Figure 3).
 |
Table 2 Top 10 Active Compounds with the Number of Differentially Expressed Target Genes |
Identification of Hub Target Genes
A PPI network of 42 differentially expressed target genes was constructed (Figure 4A). Seven groups of central genes were screened by Betweenness, Closeness, Degree, EPC, MCC, MNC and Stress in the cytoHubba plug-in. PPI networks of the top 10 node genes in the Betweenness, Closeness, Degree, EPC, MCC, MNC and Stress algorithms were constructed, respectively (Figure 4B–H). Finally, six hub differentially expressed target genes (multicentric genes, hub target genes) were screened by UpSet package (Figure 4I). These six hub target genes are AKT1, CASP3, PPARG, SIRT1, TNF and TP53. In order to understand the diagnostic value of AKT1, CASP3, PPARG, SIRT1, TNF and TP53, ROC analysis was performed (Figure 5). The results showed that the AUC values of AKT1, CASP3, PPARG, SIRT1, TNF and TP53 were all greater than 0.7, which suggested that AKT1, CASP3, PPARG, SIRT1, TNF and TP53 had high diagnostic accuracy and might be potential diagnostic markers for non-segmental vitiligo.
 |
Figure 5 ROC analysis of AKT1 (A), CASP3 (B), PPARG (C), SIRT1 (D), TNF (E) and TP53 (F). Abbreviations: ROC, receiver operating characteristic; AUC, area under the curve. |
Immune Cell Infiltration in the IME of Non-Segmental Vitiligo
To evaluate infiltration status of 23 immune cells in non-segmental vitiligo IME by ssGSEA method. Compared with the control group, the infiltration degree of Type 2 T helper cells in the non-segmental vitiligo group was higher, while the infiltration degree of CD56bright natural killer cell and CD56dim natural killer cell was lower (Figure 6A). In addition, the infiltration degree of other immune cells had no significant difference between the two groups. Subsequently, the correlation between immune cell infiltration and AKT1, CASP3, PPARG, SIRT1, TNF and TP53 was analyzed. The results showed that AKT1 was negatively correlated with CD56bright natural killer cell and CD56dim natural killer cell and positively correlated with Type 2 T helper cell (Figure 6B). The negative correlation between AKT1 and CD56dim natural killer cell was the highest, which was −0.81.
Molecular Docking
The active compounds of medicinal materials were screened according to the hub target genes, and it was found that ginsenoside RH2, kaempferol, naringenin, quercetin and formononetin all interacted with at least two hub target genes. Moreover, kaempferol, naringenin, quercetin and formononetin were top 10 active compounds with the number of differentially expressed target genes. Therefore, kaempferol, naringenin, quercetin and formononetin were selected to perform molecular docking with the six hub target genes. The results of the lowest binding energy when the hub target genes dock with the active compounds are shown in Table 3, and the corresponding molecular docking effect is shown in Figure 7. The binding energy of kaempferol to TNF was the lowest at −6.09 kcal/mol and formed six hydrogen bonds with GLN-102, PRO-100, ARG-103 residues.
 |
Table 3 Minimum Binding Energy in Molecular Docking of Core Genes and Active Compounds |
Real-Time PCR Detection of CASP3, AKT1, SIRT1 and TNF
Based on the results of GEO integration analysis, CASP3, AKT1, SIRT1 and TNF were randomly selected for verification by real-time PCR (Figure 8). All primers used for real-time PCR detection are shown in Table 4. Compared with normal controls, the expression levels of CASP3, AKT1, SIRT1 and TNF in non-segmental vitiligo group showed an upward trend. The expression trend of these genes was consistent with the GEO integration analysis results. Compared with the non-segmental vitiligo group, the expression levels of CASP3, AKT1, SIRT1 and TNF in the SJZD treatment group showed a downward trend. However, the lack of significance of real-time PCR results may be caused by the small sample size and heterogeneity among patients. Therefore, a large number of samples need to be collected for further research.
 |
Table 4 Sequence of Primers Used for Real-Time PCR Detection |
 |
Figure 8 Real-time PCR detection of CASP3 (A), AKT1 (B), SIRT1 (C) and TNF (D). |
Discussion
In this study, 135 active compounds were obtained in SJZD. Among them, 104 active compounds may be effective ingredients in the treatment of non-segmental vitiligo. The most promising active compounds were quercetin, kaempferol, formononetin and naringenin. Quercetin, kaempferol, formononetin and naringenin were all flavonoids. Quercetin can inhibit oxidative stress and has powerful anti-inflammatory effects.28,29 Adding quercetin to the diet may also affect the innate immunity of crayfish and protect crayfish from white spot syndrome virus infection.30 A study showed that quercetin could weaken the effect of hydrogen peroxide on endoplasmic reticulum morphology and tyrosinase output in melanocytes.31 Kaempferol decreased the expression of major proinflammatory cytokines in psoriatic lesions and down-regulated the signal of proinflammatory nuclear factor kappa B (NF-κB) in the skin.32 Moreover, kaempferol can also enhance the formation of melanin.33 Formononetin can reduce lung inflammation and significantly reduce oxidative stress by inhibiting the activation of NF-κB, c-Jun N-terminal kinase (JNK) and nuclear factor erythroid 2-related factor 2 (Nrf2) signaling pathways in allergic asthma.34 Formononetin treatment down-regulates proinflammatory cytokines and inhibits the activation of NOD-like receptor protein 3 (NLRP3) inflammasome in mice with autoimmune hepatitis.35 Naringenin is an immunomodulator with anti-inflammatory properties.36 Naringenin has antioxidant activity, which can relieve oxidative stress and inhibit the production of inflammatory mediators.37 Furthermore, naringenin also induced melanogenesis in B16-F10 melanoma cells through Wnt-β-catenin signaling pathway.38 Moreover, in the present study, quercetin, kaempferol, formononetin and naringenin were not only the compounds with the top 10 number of differentially expressed target genes but also molecular docking with hub differentially expressed target genes showed binding energy less than −5.0 kJ/mol. Binding energy less than −5.0 kJ/mol is the basis for screening candidate targets of active ingredients.13,14 Therefore, we hypothesized that the active ingredients of SJZD in the treatment of non-segmental vitiligo are mainly flavonoids, which may play a role by anti-oxidative stress, inhibiting inflammatory cytokines and promoting melanin formation.
In our analysis, AKT serine/threonine kinase 1 (AKT1), caspase 3 (CASP3), peroxisome proliferator activated receptor gamma (PPARG), sirtuin 1 (SIRT1), tumor necrosis factor (TNF) and tumor protein p53 (TP53) were identified as hub genes, indicating that they are important targets of SJZD and have important biological significance. AKT phosphorylation is abnormal in vitiligo, and AKT activation is associated with keratinocyte differentiation.39 Moreover, AKT1 is a potential target of Huangqi SJZD.40,41 CASP3 is a type of cysteine aspartic protease, and its expression was significantly higher in the diseased skin of vitiligo mice than in the non-diseased skin of the same experimental mice.42 SJZD may play a role in the treatment of ulcerative colitis by regulating the expression of IL-6 and CASP3 and participating in cell apoptosis, inflammation and other pathways.43 The expression and function of PPAR signaling pathway (PPARA, PPARD and PPARG) play important roles in melanocyte proliferation, differentiation and melanogenesis.44,45 Moreover, isorhamnetin and kaempferide, the compounds of Vernonia anthelmintica (L.), may promote melanogenesis by targeting PPAR signaling pathway and other signaling pathways to treat vitiligo.45 PPARG is an important target of Huangqi SJZD in Alzheimer’s disease.46 Sirtuins play a role in inflammatory skin diseases, hyperproliferative skin diseases, autoimmune diseases, skin fungal infections and other skin diseases.47 SIRT1 also plays an important role in vitiligo, but the expression trend is contrary to this article, and further research is needed.48 Compared with normal skin tissues, the expression of TNF (also known as TNF-α) in vitiligo patients’ skin tissues was significantly higher. Moreover, TNF also affects melanocyte survival and melanin synthesis in vitiligo.49 Serum TNF is a risk factor for generalized vitiligo in Iraqi patients. Serum TNF level is high in patients with active vitiligo.50 A study found that SJZD can alleviate TNF-induced damage to intestinal epithelial cell barrier function.51 In addition, Jiawei SJZD may play an anti-tumor role in liver cancer by regulating TNF and other molecules to regulate non-specific immune function.52 TP53 may have some potential effects on skin damage in vitiligo.53 Therefore, to explore the targeting effect of SJZD on AKT1, CASP3, PPARG, SIRT1, TNF and TP53 is beneficial to the treatment of non-segmental vitiligo. In addition, we also found that the AUC values of AKT1, CASP3, PPARG, SIRT1, TNF and TP53 were all greater than 0.7, which suggested that AKT1, CASP3, PPARG, SIRT1, TNF and TP53 had high diagnostic accuracy and might be potential diagnostic markers for non-segmental vitiligo.
GO and KEGG enrichment analysis to identify the important functional terms and pathways that may be involved in the treatment of non-segmental vitiligo by SJZD to reveal the potential molecular regulatory mechanism of SJZD. MAPK signaling pathway and TNF signaling pathway are important pathways involved in differentially expressed target genes in KEGG analysis. MAPK signaling pathway inhibition mediates inflammatory reprogramming and sensitizes tumors to targeted activation of the innate immune sensor RIG-I.54 MAPK signaling pathway also plays an important role in protecting melanocytes from oxidative stress, protecting keratinocytes from damage and mediating melanogenesis in vitiligo.48,55,56 Quercetin and kaempferol can mediate the production of inflammatory cytokines and chemokines through MAPK signaling pathway.57–59 TNF signaling plays an important role in a variety of physiological and pathological processes, including regulating immune responses and inducing inflammation.60 TNF is a key factor of TNF signaling pathway, which is highly expressed in vitiligo patients and also affects melanocyte survival and melanin synthesis.49,50 Studies have shown that quercetin, kaempferol and naringenin can reduce the production of proinflammatory factor TNF-α, inhibit inflammation and play a role in immune regulation.61–63 In addition, differentially expressed target genes were also enriched in hepatitis B, human cytomegalovirus infection, kaposi sarcoma-associated herpesvirus infection, Epstein-Barr virus infection and other inflammatory and immune-related pathways. Therefore, we hypothesized that SJZD may regulate multiple signaling pathways by targeting different targets and then regulate the disease progression of non-segmental vitiligo.
By ssGSEA method analysis, we found that compared with the control group, the infiltration degree of Type 2 T helper cells in the non-segmental vitiligo group was higher, while the infiltration degree of CD56bright natural killer cell and CD56dim natural killer cell was lower. In this study, we also found that AKT1 was negatively correlated with CD56bright natural killer cells and CD56dim natural killer cells, and positively correlated with Type 2 T helper cells. Moreover, binding energy of AKT1 with quercetin, kaempferol and naringenin is less than −5.0 kJ/mol, which is an important target of quercetin, kaempferol and naringenin. Therefore, we hypothesized that the active ingredient in SJZD targeted regulation of AKT affects the levels of Type 2 T helper cells, CD56bright natural killer cells and CD56dim natural killer cells in the IME of patients with non-segmental vitiligo, and then regulates the immune regulatory system of non-segmental vitiligo.
However, we have to admit that this study also has some limitations. Firstly, the data and information used in this study are from public databases, and the results lack a lot of in vitro and in vivo validation. Secondly, the sample size of real-time PCR validation is too small and the results lack significance, so a large number of samples need to be collected for further research. Thirdly, the molecular mechanism of SJZD acting on vitiligo is still unclear, so a large number of experimental studies on the identified compounds, target genes and signaling pathways are needed. In summary, we analyzed the potential role of SJZD in non-segmental vitiligo through network pharmacology, and identified important active compounds, target genes and signaling pathways. These results indicate that quercetin, kaempferol, formononetin and naringenin in SJZD may play an important role in the treatment of non-segmental vitiligo by acting on AKT1, CASP3, PPARG, SIRT1, TNF and TP53 to regulate immune cell infiltration and multiple signaling pathways.
Data Sharing Statement
The data analyzed in this study were obtained from TCMSP and GEO databases. The persistent accessible web links for TCMSP and GEO are http://lsp.nwu.edu.cn/tcmsp.php and https://www.ncbi.nlm.nih.gov/geo/, respectively. Accession numbers of the non-segmental vitiligo dataset used in the current study are GSE65127 in GEO. All data generated or analyzed during this study are included in this published article.
Ethics Approval and Consent to Participate
The present study was approved by the Ethics Committee of Hebei Academy of Traditional Chinese Medicine (20220129). This study complied with the Declaration of Helsinki. Written informed consent was obtained from all participants’ parents.
Funding
2018 Scientific Research Program of Hebei Administration of Traditional Chinese Medicine (2018063).
Disclosure
The authors declare that they have no conflicts of interest.
References
1. Ahmed Jan N, Masood S. Vitiligo. In: StatPearls. Treasure Island (FL): StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC; 2022.
2. Ezzedine K, Eleftheriadou V, Whitton M, van Geel N. Vitiligo. Lancet. 2015;386(9988):74–84. doi:10.1016/S0140-6736(14)60763-7
3. Speeckaert R, van Geel N. Vitiligo: an update on pathophysiology and treatment options. Am J Clin Dermatol. 2017;18(6):733–744. doi:10.1007/s40257-017-0298-5
4. Karagaiah P, Valle Y, Sigova J, et al. Emerging drugs for the treatment of vitiligo. Expert Opin Emerg Drugs. 2020;25(1):7–24. doi:10.1080/14728214.2020.1712358
5. Taneja N, Sreenivas V, Sahni K, Gupta V, Ramam M. Disease stability in segmental and non-segmental vitiligo. Indian Dermatol Online J. 2022;13(1):60–63. doi:10.4103/idoj.IDOJ_154_21
6. Wang T, Feng Y, Wang H. The mechanisms of sijunzi decoction in the treatment of chronic gastritis revealed by network pharmacology. Evid Based Complement Alter Med. 2020;2020:8850259. doi:10.1155/2020/8850259
7. Gao B, Peng Y, Peng C, Zhang Y, Li X. A comparison of characterization and its actions on immunocompetent cells of polysaccharides from sijunzi decoction. Evid Based Complement Alter Med. 2019;2019:9860381. doi:10.1155/2019/9860381
8. Xiong B, Qian H. Effects of Sijunzi decoction and Yupingfeng powder on expression of janus kinase-signal transducer and activator of transcription signal pathway in the brain of spleen-deficiency model rats. J Trad Chin Med. 2013;33(1):78–84. doi:10.1016/S0254-6272(13)60105-3
9. Guo L, Dong ND, Xiong AB, Liu ZY, Liu CR, He XC. 四君子汤加味防治烫伤后大鼠肠道损伤和细菌移位实验研究 [An experimental study on the prevention and treatment of postburn intestinal injury and bacterial translocation by Sijunzi decoction in scalded rats]. Zhonghua shao shang za zhi. 2003;19(2):89–93. Chinese.
10. Huang C, Zhu Z, Cao X, et al. A pectic polysaccharide from sijunzi decoction promotes the antioxidant defenses of SW480 cells. Molecules. 2017;22(8):1341. doi:10.3390/molecules22081341
11. Fang J, Wang C, Zheng J, Liu Y, Sailor G. Network pharmacology study of Yishen capsules in the treatment of diabetic nephropathy. PLoS One. 2022;17(9):e0273498. doi:10.1371/journal.pone.0273498
12. Kitchen DB, Decornez H, Furr JR, Bajorath J. Docking and scoring in virtual screening for drug discovery: methods and applications. Nat Rev Drug Discov. 2004;3(11):935–949. doi:10.1038/nrd1549
13. Liu S, Wang R, Lou Y, Liu J. Uncovering the mechanism of the effects of pien-tze-huang on liver cancer using network pharmacology and molecular docking. Evid Based Complement Alter Med. 2020;2020:4863015. doi:10.1155/2020/4863015
14. Hsin KY, Matsuoka Y, Asai Y, et al. systemsDock: a web server for network pharmacology-based prediction and analysis. Nucleic Acids Res. 2016;44(W1):W507–W513. doi:10.1093/nar/gkw335
15. Pinzi L, Rastelli G. Molecular docking: shifting paradigms in drug discovery. Int J Mol Sci. 2019;20(18):4331. doi:10.3390/ijms20184331
16. Xu X, Zhang W, Huang C, et al. A novel chemometric method for the prediction of human oral bioavailability. Int J Mol Sci. 2012;13(6):6964–6982. doi:10.3390/ijms13066964
17. Ru J, Li P, Wang J, et al. TCMSP: a database of systems pharmacology for drug discovery from herbal medicines. J Cheminform. 2014;6:13. doi:10.1186/1758-2946-6-13
18. Dong Y, Zhao Q, Wang Y. Network pharmacology-based investigation of potential targets of astragalus membranaceous-angelica sinensis compound acting on diabetic nephropathy. Sci Rep. 2021;11(1):19496. doi:10.1038/s41598-021-98925-6
19. Zhao Y, Fu G, Wang J, Guo M, Yu G. Gene function prediction based on gene ontology hierarchy preserving hashing. Genomics. 2019;111(3):334–342. doi:10.1016/j.ygeno.2018.02.008
20. Kanehisa M, Goto S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28(1):27–30. doi:10.1093/nar/28.1.27
21. Chin CH, Chen SH, Wu HH, Ho CW, Ko MT, Lin CY. cytoHubba: identifying hub objects and sub-networks from complex interactome. BMC Syst Biol. 2014;8(Suppl4):S11. doi:10.1186/1752-0509-8-S4-S11
22. Šimundić AM. Measures of diagnostic accuracy: basic definitions. Ejifcc. 2009;19(4):203–211.
23. Charoentong P, Finotello F, Angelova M, et al. Pan-cancer immunogenomic analyses reveal genotype-immunophenotype relationships and predictors of response to checkpoint blockade. Cell Rep. 2017;18(1):248–262. doi:10.1016/j.celrep.2016.12.019
24. Baugh EH, Lyskov S, Weitzner BD, Gray JJ, Uversky VN. Real-time PyMOL visualization for Rosetta and PyRosetta. PLoS One. 2011;6(8):e21931. doi:10.1371/journal.pone.0021931
25. El-Hachem N, Haibe-Kains B, Khalil A, Kobeissy FH, Nemer G. AutoDock and AutoDockTools for protein-ligand docking: beta-site amyloid precursor protein cleaving enzyme 1(BACE1) as a case study. Methods Mol Biol. 2017;1598:391–403.
26. Kartal D, Borlu M, Çınar SL, Kesikoğlu A, Utaş S. Thyroid abnormalities in paediatric patients with vitiligo: retrospective study. Postepy dermatologii i alergologii. 2016;33(3):232–234. doi:10.5114/ada.2016.60617
27. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi:10.1006/meth.2001.1262
28. Sul OJ, Ra SW. Quercetin prevents LPS-induced oxidative stress and inflammation by modulating NOX2/ROS/NF-kB in lung epithelial cells. Molecules. 2021;26(22):6949. doi:10.3390/molecules26226949
29. Chen T, Zhang X, Zhu G, et al. Quercetin inhibits TNF-α induced HUVECs apoptosis and inflammation via downregulating NF-kB and AP-1 signaling pathway in vitro. Medicine. 2020;99(38):e22241. doi:10.1097/MD.0000000000022241
30. Zhang Y, Xiao C, Zhu F. Effects of dietary quercetin on the innate immune response and resistance to white spot syndrome virus in Procambarusclarkii. Fish Shellfish Immunol. 2021;118:205–212. doi:10.1016/j.fsi.2021.09.012
31. Guan C, Xu W, Hong W, et al. Quercetin attenuates the effects of H2O2 on endoplasmic reticulum morphology and tyrosinase export from the endoplasmic reticulum in melanocytes. Mol Med Rep. 2015;11(6):4285–4290. doi:10.3892/mmr.2015.3242
32. Liu C, Liu H, Lu C, et al. Kaempferol attenuates imiquimod-induced psoriatic skin inflammation in a mouse model. Clin Exp Immunol. 2019;198(3):403–415. doi:10.1111/cei.13363
33. Heriniaina RM, Dong J, Kalavagunta PK, Wu HL, Yan DS, Shang J. Effects of six compounds with different chemical structures on melanogenesis. Chin J Nat Med. 2018;16(10):766–773. doi:10.1016/S1875-5364(18)30116-X
34. Yi L, Cui J, Wang W, et al. Formononetin attenuates airway inflammation and oxidative stress in murine allergic asthma. Front Pharmacol. 2020;11:533841. doi:10.3389/fphar.2020.533841
35. Liu G, Zhao W, Bai J, Cui J, Liang H, Lu B. Formononetin protects against concanavalin-A-induced autoimmune hepatitis in mice through its anti-apoptotic and anti-inflammatory properties. Biochem Cell Biol. 2021;99(2):231–240. doi:10.1139/bcb-2020-0197
36. Zeng W, Jin L, Zhang F, Zhang C, Liang W. Naringenin as a potential immunomodulator in therapeutics. Pharmacol Res. 2018;135:122–126. doi:10.1016/j.phrs.2018.08.002
37. Rodríguez-García C, Sánchez-Quesada C, Dietary JJG. Flavonoids as cancer chemopreventive agents: an updated review of human studies. Antioxidants. 2019;8(5):137. doi:10.3390/antiox8050137
38. Huang YC, Yang CH, Chiou YL. Citrus flavanone naringenin enhances melanogenesis through the activation of Wnt/β-catenin signalling in mouse melanoma cells. Phytomedicine. 2011;18(14):1244–1249. doi:10.1016/j.phymed.2011.06.028
39. Kim NH, Lee AY. Reduced aquaporin3 expression and survival of keratinocytes in the depigmented epidermis of vitiligo. J Invest Dermatol. 2010;130(9):2231–2239. doi:10.1038/jid.2010.99
40. Cui Y, Wang H, Wang D, et al. Network pharmacology analysis on the mechanism of Huangqi Sijunzi decoction in treating cancer-related fatigue. J Healthc Eng. 2021;2021:9780677. doi:10.1155/2021/9780677
41. Cui Y, Mi J, Feng Y, et al. 黄芪四君子汤治疗乳腺癌癌因性疲乏的疗效及机制:基于94例临床随机对照试验和网络药理学 [Huangqi Sijunzi decoction for treating cancer-related fatigue in breast cancer patients: a randomized trial and network pharmacology study]. Nan fang yi ke da xue xue bao. 2022;42(5):649–657. Chinese. doi:10.12122/j.issn.1673-4254.2022.05.04
42. Al Robaee AA, Alzolibani AA, Rasheed Z. Autoimmune response against tyrosinase induces depigmentation in C57BL/6 black mice. Autoimmunity. 2020;53(8):459–466. doi:10.1080/08916934.2020.1836489
43. Meng-Long Z, Xiao-Yan H, Ya-Lu C, et al. 基于网络药理学探讨四君子汤治疗溃疡性结肠炎的作用机制及实验验证 [Mechanism and experimental verification of Sijunzi Decoction in treatment of ulcerative colitis based on network pharmacology]. Zhongguo Zhong yao za zhi. 2020;45(22):5362–5372. Chinese. doi:10.19540/j.cnki.cjcmm.20200810.405
44. Kang HY, Chung E, Lee M, Cho Y, Kang WH. Expression and function of peroxisome proliferator-activated receptors in human melanocytes. Br J Dermatol. 2004;150(3):462–468. doi:10.1111/j.1365-2133.2004.05844.x
45. Wang JY, Chen H, Wang YY, et al. Network pharmacological mechanisms of Vernonia anthelmintica (L.) in the treatment of vitiligo: isorhamnetin induction of melanogenesis via up-regulation of melanin-biosynthetic genes. BMC Syst Biol. 2017;11(1):103. doi:10.1186/s12918-017-0486-1
46. Zhang W, Lv M, Shi Y, Mu Y, Yao Z, Yang Z. Network pharmacology-based study of the underlying mechanisms of Huangqi Sijunzi decoction for Alzheimer’s disease. Evid Based Complement Alter Med. 2021;2021(6480381):1–3.
47. Serravallo M, Jagdeo J, Glick SA, Siegel DM, Brody NI. Sirtuins in dermatology: applications for future research and therapeutics. Arch Dermatol Res. 2013;305(4):269–282. doi:10.1007/s00403-013-1320-2
48. Becatti M, Fiorillo C, Barygina V, et al. SIRT1 regulates MAPK pathways in vitiligo skin: insight into the molecular pathways of cell survival. J Cell Mol Med. 2014;18(3):514–529. doi:10.1111/jcmm.12206
49. Singh M, Mansuri MS, Kadam A, et al. Tumor necrosis factor-alpha affects melanocyte survival and melanin synthesis via multiple pathways in vitiligo. Cytokine. 2021;140:155432. doi:10.1016/j.cyto.2021.155432
50. Ahmed R, Sharif D, Jaf M, Amin DM. Effect of TNF-α −308G/A (rs1800629) promoter polymorphism on the serum level of TNF-α among Iraqi patients with generalized vitiligo. Clin Cosmet Investig Dermatol. 2020;13:825–835. doi:10.2147/CCID.S272970
51. Lu Y, Li L, Zhang JW, Zhong XQ, Wei JA, Han L. Total polysaccharides of the Sijunzi decoction attenuate tumor necrosis factor-α-induced damage to the barrier function of a Caco-2 cell monolayer via the nuclear factor-κB-myosin light chain kinase-myosin light chain pathway. World J Gastroenterol. 2018;24(26):2867–2877. doi:10.3748/wjg.v24.i26.2867
52. Chen L, Jin T, Ning C, Wang S, Wang L, Lin J. 加味四君子汤对H22肝癌小鼠的抑瘤作用和免疫功能的影响 [Anti-tumor and immune-modulating effect of Jiawei Sijunzi decoction in mice bearing hepatoma H22 tumor]. Nan fang yi ke da xue xue bao. 2019;39(2):241–248. Chinese. doi:10.12122/j.issn.1673-4254.2019.02.18
53. Dey-Rao R, Sinha AA. Vitiligo blood transcriptomics provides new insights into disease mechanisms and identifies potential novel therapeutic targets. BMC Genom. 2017;18(1):109. doi:10.1186/s12864-017-3510-3
54. Brägelmann J, Lorenz C, Borchmann S, Nishii K, Wegner J. MAPK-pathway inhibition mediates inflammatory reprogramming and sensitizes tumors to targeted activation of innate immunity sensor RIG-I. Nat Commun. 2021;12(1):5505. doi:10.1038/s41467-021-25728-8
55. Li XS, Tang XY, Su W, Li X. Vitexin protects melanocytes from oxidative stress via activating MAPK-Nrf2/ARE pathway. Immunopharmacol Immunotoxicol. 2020;42(6):594–603. doi:10.1080/08923973.2020.1835952
56. Mamat N, Lu XY, Kabas M, Aisa HA. Potential anti-vitiligo properties of cynarine extracted from Vernonia anthelmintica (L.) willd. Int J Mol Med. 2018;42(5):2665–2675.
57. Cheng SC, Huang WC, Pang J-H, Wu Y-H, Cheng C-Y. Quercetin Inhibits the production of IL-1β-induced inflammatory cytokines and chemokines in ARPE-19 cells via the MAPK and NF-κB signaling pathways. Int J Mol Sci. 2019;20(12):2957. doi:10.3390/ijms20122957
58. Meng LQ, Yang FY, Wang MS, et al. Quercetin protects against chronic prostatitis in rat model through NF-κB and MAPK signaling pathways. Prostate. 2018;78(11):790–800. doi:10.1002/pros.23536
59. Suchal K, Malik S, Gamad N, et al. Kaempferol attenuates myocardial ischemic injury via inhibition of MAPK signaling pathway in experimental model of myocardial ischemia-reperfusion injury. Oxid Med Cell Longev. 2016;2016:7580731. doi:10.1155/2016/7580731
60. Varfolomeev E, Vucic D. Intracellular regulation of TNF activity in health and disease. Cytokine. 2018;101:26–32. doi:10.1016/j.cyto.2016.08.035
61. Bhaskar S, Helen A. Quercetin modulates toll-like receptor-mediated protein kinase signaling pathways in oxLDL-challenged human PBMCs and regulates TLR-activated atherosclerotic inflammation in hypercholesterolemic rats. Mol Cell Biochem. 2016;423(1–2):53–65. doi:10.1007/s11010-016-2824-9
62. Park SE, Sapkota K, Kim S, Kim H, Kim SJ. Kaempferol acts through mitogen-activated protein kinases and protein kinase B/AKT to elicit protection in a model of neuroinflammation in BV2 microglial cells. Br J Pharmacol. 2011;164(3):1008–1025. doi:10.1111/j.1476-5381.2011.01389.x
63. Niu X, Wu C, Li M, et al. Naringenin is an inhibitor of T cell effector functions. J Nutr Biochem. 2018;58:71–79. doi:10.1016/j.jnutbio.2018.04.008
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.