Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 15
Exploring the Association Between Emphysema Phenotypes and Low Bone Mineral Density in Smokers with and without COPD
Authors González J , Rivera-Ortega P, Rodríguez-Fraile M , Restituto P, Colina I, Calleja MD, Alcaide AB , Campo A , Bertó J, Seijo L, Pérez-Warnisher MT, Zulueta JJ , Varo N, de-Torres JP
Received 12 April 2020
Accepted for publication 2 July 2020
Published 27 July 2020 Volume 2020:15 Pages 1823—1829
DOI https://doi.org/10.2147/COPD.S257918
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Richard Russell
Jessica González, 1 Pilar Rivera-Ortega, 2 Macarena Rodríguez-Fraile, 3 Patricia Restituto, 4 Inmaculada Colina, 5 María de los Desamparados Calleja, 6 Ana B Alcaide, 2 Aránzazu Campo, 2 Juan Bertó, 2 Luis Seijo, 7 Maria Teresa Pérez-Warnisher, 7 Javier J Zulueta, 2 Nerea Varo, 4 Juan P de-Torres 8
1Pulmonary Department, Hospital Universitari Arnau de Vilanova, Lleida, Spain; 2Pulmonary Department, Clinica Universidad de Navarra, Pamplona, Spain; 3Nuclear Medicine Department, Clínica Universidad de Navarra, Pamplona, Spain; 4Biochemistry Department, Clínica Universitaria de Navarra, Pamplona, Spain; 5Department of Internal Medicine, Clínica Universidad de Navarra, Pamplona, Spain; 6Department of Endocrinology & Nutrition, Clínica Universidad de Navarra, Pamplona, Spain; 7Pulmonary Department, Clínica Universidad de Navarra, Madrid, Spain; 8Respirology Division, Queen’s University, Kingston, ON, Canada
Correspondence: Jessica González
Pulmonary department, Hospital Universitari Arnau de Vilanova, Lleida, Spain
Email [email protected]
Rationale: Emphysema and osteoporosis are tobacco-related diseases. Many studies have shown that emphysema is a strong and independent predictor of low bone mineral density (BMD) in smokers; however, none of them explored its association with different emphysema subtypes.
Objective: To explore the association between the different emphysema subtypes and the presence of low bone mineral density in a population of active or former smokers with and without chronic obstructive pulmonary disease (COPD).
Methods: One hundred and fifty-three active and former smokers from a pulmonary clinic completed clinical questionnaires, pulmonary function tests, a low-dose chest computed tomography (LDCT) and a dual-energy absorptiometry (DXA) scans. Subjects were classified as having normal BMD or low BMD (osteopenia or osteoporosis). Emphysema was classified visually for its subtype and severity. Logistic regression analysis explored the relationship between the different emphysema subtypes and the presence of low BMD adjusting for other important factors.
Results: Seventy-five percent of the patients had low BMD (78 had osteopenia and 37 had osteoporosis). Emphysema was more frequent (66.1 vs 26.3%, p=< 0.001) and severe in those with low BMD. Multivariable analysis adjusting for other significant cofactors (age, sex, FEV 1, and severity of emphysema) showed that BMI (OR=0.91, 95% CI: 0.76– 0.92) and centrilobular emphysema (OR=26.19, 95% CI: 1.71 to 399.44) were associated with low BMD.
Conclusion: Low BMD is highly prevalent in current and former smokers. BMI and centrilobular emphysema are strong and independent predictors of its presence, which suggests that they should be considered when evaluating smokers at risk for low BMD.
Keywords: emphysema, COPD, low bone mineral density, osteopenia, osteoporosis; smokers
Introduction
Tobacco smoking has long been identified as a risk factor for osteoporosis. Many studies have shown that smokers have decreased bone mineral density (BMD) with increased risk fracture compared to nonsmokers, particularly at the hip.1 Additionally, a recent study demonstrates that current smokers show a more rapid BMD decline over time compared to former smokers.2
It is well known that chronic obstructive pulmonary disease (COPD) is one of the most common diseases related to cigarette smoke, and several and large epidemiologic studies have demonstrated an increased prevalence of osteopenia and osteoporosis in these patients.3 The prevalence of low BMD in the absence of steroid use in patients with mild airflow limitation4,5 suggests a pathogenic link between the lung and the skeleton different from traditional osteoporosis risk factors.
Smokers with and without COPD also have the destruction of lung parenchyma called emphysema. In fact, two recent studies suggest that emphysema could be an independent marker of low BMD in smokers; one performed in patients with COPD6 and the other also including patients without COPD.7 The similarities between emphysema and osteoporosis, with potential mechanisms linking the two processes (loss of extracellular matrix and the association with inflammatory mediators, such as tumor necrosis factor-α)8,9 support this hypothesis. Interestingly, both studies showed in the multivariate analysis that emphysema remained a significant predictor of low BMD, whereas airflow obstruction severity did not. These findings were later replicated in other studies.10,11
However, none of these studies have explored the association of different emphysema subtypes (centrilobular, panlobular and paraseptal) and the presence of low BMD. Centrilobular emphysema (CLE) is associated with a higher smoking history,12,13 a unique systemic chronic inflammation12 and a protease-antiprotease misbalance,14 that is also associated with osteoporosis.15,16 Therefore, we hypothesized that it is likely that CLE emphysema is associated with low BMD. The main objective of our study is to describe the association of different emphysema subtypes with the presence of low BMD.
Methods
Participants
One hundred and fifty-three consecutive active and former smokers from the pulmonary department of the Clínica Universidad de Navarra were invited to participate between August 2014 and March 2016. Study subjects were men 50 years of age or older and postmenopausal women, with a smoking history of ≥10 pack-years. Those who had a previous diagnosis of osteoporosis and/or were using preventive treatment for osteoporosis were excluded. None of the subjects were under oral corticosteroid treatment. All subjects signed an informed consent prior to enrollment and the protocol was approved by the Institution’s ethics committee (Comité de Ética Clínica Universidad de Navarra, number151/2014). The study was conducted in accordance with the Declaration of Helsinki.
Patients were evaluated during their initial visit and each subject underwent a medical history and physical examination, including a questionnaire administered by the same investigator that registered their age, race, body mass index (BMI), menopause information, fracture history (including family history), tobacco and alcohol intake history, medication use in the past and present, and number of respiratory exacerbations in those with a previous diagnosis of COPD. These exacerbations were defined following the global initiative for chronic obstructive lung disease (GOLD) guidelines17 as an acute worsening of respiratory symptoms that result in the use of additional therapy.18 Patients underwent pulmonary function tests (PFTs), 6-minute walking distance (6MWD), bone densitometry (BMD measurement) and a low-dose chest-computed tomography (LDCT).
Figure 1 shows the Flowchart of the included individuals. One hundred and sixty-four patients were initially evaluated but 11 patients were excluded because they met the exclusion criteria. One hundred and fifty-three patients were finally included in the analysis (69 females and 84 males), 104 patients with COPD and 49 without.
 |
Figure 1 Flowchart showing the inclusion of the participant. |
Pulmonary Function Tests (PFTs)
Airway function (spirometry, lung volumes and diffusing capacity) was measured in all participants using a flow spirometer (Vmax22; SensorMedics, Yorba Linda, CA) according to guidelines of the American Thoracic Society.19 Results were expressed as a percentage of the predicted value according to the European Community Lung Health Survey.20 All post-bronchodilation measurements were determined 15 minutes after the inhalation of 400 µg of salbutamol. The presence and severity of airflow obstruction was determined using criteria of the Global Initiative for Chronic Obstructive Lung Disease (GOLD; post bronchodilation forced expiratory volume in 1 s/forced vital capacity (FEV1/FVC) ratio <70%).17 The 6MWD was performed following the current American Thoracic Society guidelines.21
Bone Density by Dual X-Ray Absorptiometry
A dual X-ray absorptiometry (DEXA) technique with a Lunar iDXA scan (General Electric Co) was used to measure the bone mineral density of the lumbar spine (L1 to L4), femoral neck, total hip and in some cases non-dominant forearm (33%). Diagnosis of osteoporosis was based on the lowest T-score of these locations and defined according to the world health organization (WHO) criteria (osteoporosis: T-score≤-2.5; osteopenia or low bone mass: T-score <-1.0 and >-2.5; and normal bone mass: T-score ≥ −1.0).22
All DXA scans were interpreted by a physician certified by ISCD and following standard quality control procedures, including daily phantom scanning. Coefficient of variation was 1% for L1-L4 BMD and 1.1% for total hip BMD.
Low-Dose Chest CT (LDCT)
Patients were scanned using a sixty-four slice multidetector CT scanner (Somatom Sensation 64, Somatom Definition, Siemens Healthcare, Erlangen, Germany) at a low-dose setting (120 kV tube voltage, 40 mAs tube current, 64x0.6 mm slice collimation, 0.5 s gantry rotation time, 1.4 pitch, 1 mm slice thickness, 1 mm reconstruction interval). Examinations were acquired with patients in the supine position, in cranio-caudal direction and at end-inspiration. Resulting images were reconstructed with a high convolution reconstruction algorithm (B60) and lung window.23
Assessment of Emphysema on LDCT
A pulmonologist (JG) visually assessed the emphysema presence, type and severity, using validated criteria established by the Fleischner Society.24 Following these guidelines, pulmonary emphysema was classified into three subtypes: centrilobular (CLE), panlobular (PLE) and paraseptal (PSE). To determine the emphysema severity, a five-level semiquantitative scale based on criteria used in the National Emphysema Treatment Trial was used.25 However, no division of the lung into different zones was undertaken. Therefore, severity was assessed using this scoring system throughout the whole lung.
Statistical Analysis
Statistical analysis was performed using STATA. Normal distribution of samples was assessed by the Shapiro–Wilks tests. Quantitative data are represented as mean ± SD or median (interquartile range), depending on the data distribution; relative frequencies were used for qualitative data. Differences between study groups were evaluated by the Student’s t test for normally distributed variables, the Mann–Whitney U-test for non-normally distributed variables, and χ2 statistics for categorical variables. Uni- and multivariable logistic regression analyses were performed to study the potential independent association between low BMD and the studied parameters. The multivariable analysis included age, sex, BMI, FEV1, severity and subtype of emphysema.
Results
According to the results of the DXA scan, 38 patients (24.8%) had normal BMD and 115 (75.2%) low BMD, including 78 (50.9%) subjects with osteopenia and 37 (24.2%) with osteoporosis. Subjects with low BMD tended to be women (50.9% vs 26.3%; p=0.008) with lower BMI (26.4 vs 29.7 Kg/m2; p=<0.001) (Table 1). No significant differences were found in the smoking history or in the use of inhaled steroids. No differences were found in the proportion of COPD patients in both groups. However, emphysema was more frequently found in the group with low BMD (66.1% vs 26.3%; p=<0.001). There were also differences between the emphysema subtypes (p=<0.001) with CLE, alone (31.3% vs 7.9%) or in combination with PSE (30.4% vs 13.2%), being most frequent in the low BMD group. Furthermore, emphysema was more severe in this group of patients. Regarding the lung function, FEV1 and DLCO were lower in the group with low BMD that walked fewer meters in the 6MWD.
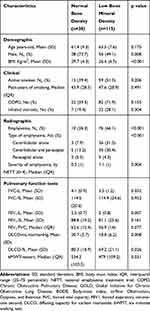 |
Table 1 Baseline Clinical, Radiographic and Densitometric Characteristics of Patients with and without Low Bone Mineral Density (Osteopenia and Osteoporosis) |
In the univariate analysis, the presence and severity of emphysema was associated with the presence of low BMD (OR=5.45; CI: 2.40–12.37; p=<0.001; OR=1.83; CI: 1.19–2.81 p=0.006, respectively) (Table 2). Interestingly, CLE subtype showed a greater risk of having a low BMD (OR=8.61; CI: 2.40–30.79; p=0.001), that decreased when its presence is combined with PSE (OR=5.02; CI: 1.74–14.43; p=0.003). However, PSE alone does not seem to have an effect on the presence of low BMD (OR=1.79; CI: 0.32–9.92; p=0.503). Other predictive factors for low BMD were male sex (OR=0.34; CI: 0.15–0.77; p=0.010), BMI (OR=0.84; CI: 0.76–0.92; p=<0.001), FEV1 (OR=0.43; CI: 0.24–0.80; p=0.008) DLCO (OR=0.90; CI: 0.83–0.97; p=0.012) and the 6MWD (OR=0.99; CI: 0.98–0.99; p=0.034). A spirometric diagnosis of COPD did not show any relationship with the presence of low BMD (OR=1.74; CI: 0.80–3.78; p=0.157).
 |
Table 2 Predictors of Low Bone Mineral Density (Osteopenia/Osteoporosis) in Current and Former Smokers, Univariate Analysis |
Multivariable logistic regression analysis was constructed based on a model of risk factors that included those that either reached a statistically significant difference in the univariable analysis or are well-known factors associated with low BMD. The model included age, sex, BMI, FEV1, severity and type of emphysema. Only BMI and the presence of CLE were significantly associated with low BMD (Table 3). The relationship between the different emphysema subtypes and the risk of low BMD, was only with those that had CLE alone or in combination with PSE while those patients with PSE alone were not associated with the presence of low BMD.
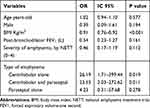 |
Table 3 Predictors of Low Bone Mineral Density (Osteopenia/Osteoporosis) in Current and Former Smokers, Multivariate Analysis |
Discussion
The most important and novel finding of the present study in a population of active or former smokers is that the presence of a specific emphysema phenotype (CLE) and a low BMI were the best predictors of the presence of a low BMD in the bone densitometry. But most importantly, this association was independent of the most common risk factors for low BMD and of the presence and degree of airflow obstruction.
Evidence shows that BMI is one of the modifiable factors affecting BMD,26 being protective in older subjects if high27–30 and considered a high-risk factor for osteoporosis if low.31,32 Moreover, many studies corroborate that low BMI is associated with low BMD and fractures.33,34 Even more, a study showed that voluntary weight loss in overweight elderly women increases bone loss suggesting their tight relationship.35 Interestingly, the fundamental basis of emphysema is loss of lung tissue and recent data from the literature shows that emphysema is often associated with less tissue in other body compartments such as BMI, muscle wasting measured as a lower fat-free mass index (FFMI)36,37 as well as low BMD.6,7,10 Likewise, a recent study shows that at baseline, patients with more severe emphysema had lower BMI, lower FFMI and a higher prevalence of self-reported osteoporosis.38 The results of our study are in accordance with this groundswell of literature and support the hypothesis of tissue wasting associated with those smokers that developed emphysema.
The independent association of radiographic emphysema and low BMD observed in several studies and in ours suggest a common pathogenic link between lung parenchymal destruction and bone loss. There is a growing body of literature promoting a central pathophysiologic role of chronic inflammation in emphysema to explain this link. Accelerated bone loss and osteoporosis are explained by the imbalance between bone formation by osteoblasts and bone resorption by osteoclasts, which are managed by a complex network of inflammatory cells and cytokines.39,40 For example, interleukin (IL)-1, tumor necrosis factor (TNF)-α, IL-6 and IL-17, all stimulators of osteoclastic bone resorption, have been implicated in emphysema in both human and animal models.8,9,41–45 Moreover, it has been shown a correlation between plasma TNF-α levels with plasma collagen type I cross-linked C-telopeptide (CTX) levels, a marker of bone resorption in patients with severe obstructive lung disease,46 indicating an increased bone turnover in these patients. This inflammatory theory could result in protease-antiprotease imbalance leading to tissue destruction in both lungs and bone, being these certain inflammatory proteins markers of bone turnover or bone mineral density. In this regard, Bolton et al16 reported higher levels of metalloproteinase 9 (MMP-9) in patients with COPD and osteoporosis compared with a control group of COPD without osteoporosis. Furthermore, the relationship between MMP-9 and bone density was independent of lung function. Interestingly, these inflammatory proteins such as TNF-α and MMP-9 have been also implicated in emphysema.9,43,47 This is just a proposed explanation for the potential link between emphysema and low BMD, because unfortunately none of these inflammatory makers were measured in the present study. Further, well-designed studies should explore this pathogenically link to explain our findings.
The finding that CLE is the phenotype specifically associated with low BMD independently of the most important risk factor is novel and important. This emphysema subtype is the one usually associated with tobacco smoking,12,13 with a higher chronic systemic inflammation response and a specific lung repair mechanism unique to the exposure and probably due to a different genetic predisposition, although this has not been shown yet. Patients with CLE have higher white blood cells counts12 and a unique protease-antiprotease balance, characterized by a higher expression of matrix metalloproteinase 9 (MMP9) and transforming growth beta 1 (TGB1),14 which could explain the potential link with osteoporosis.15,16 In contrast, patients with paraseptal emphysema (PSE) usually have less respiratory symptoms and do not affect the lung function decline typically seen in patients with CLE.12 Furthermore, PSE occurs even in the absence of tobacco exposure and may depend on age and genetic susceptibility, but again this needs to be shown.
The main strength of the present study is that all major risk factors for low BMD in smokers were considered, including lung function and COPD diagnosis, presence of radiological emphysema and its types, usually not explored in other groups. Another strength is that an equal number of men and women were included in our sample. The present study also had several limitations. Firstly, it is a relatively small sample size with a low number of patients with osteoporosis, but they were very well characterized and studied. Secondly, the findings of the present work should be replicated in another cohort with more cases of PLE and PSE alone to externally validate its most important messages.
In summary, the present study shows that in active or former adult smokers, the specific subtype of CLE associates with low BMD and low BMI. These findings if reproduced in other cohorts could have important clinical implications for the management of these patients.
Disclosure
Luis Seijo reports grants from Menarini and personal fees from Sabartech, Astra Zeneca, Esteve, Roche, Medtronic, and Chiesi, outside the submitted work. The authors report no other possible conflicts of interest in this work.
References
1. Law MR, Hackshaw AK, Daniell H, et al. A meta-analysis of cigarette smoking, bone mineral density and risk of hip fracture: recognition of a major effect. BMJ. 1997;315(7112):841–846. doi:10.1136/bmj.315.7112.841
2. Pompe E, Bartstra J, Verhaar HJ, et al. Bone density loss on computed tomography at 3-year follow-up in current compared to former male smokers. Eur J Radiol. 2017;89:177–181. doi:10.1016/j.ejrad.2017.02.011
3. Sin DD, Man JP, Man SFP. The risk of osteoporosis in Caucasian men and women with obstructive airways disease. Am J Med. 2003;114(1):10–14. doi:10.1016/S0002-9343(02)01297-4
4. de Vries F, van Staa TP, Bracke MSGM, Cooper C, Leufkens HGM, Lammers J-WJ. Severity of obstructive airway disease and risk of osteoporotic fracture. Eur Respir J. 2005;25(5):879–884. doi:10.1183/09031936.05.00058204
5. Bolton CE, Ionescu AA, Shiels KM, et al. Associated loss of fat-free mass and bone mineral density in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2004;170(12):1286–1293. doi:10.1164/rccm.200406-754OC
6. Ohara T, Hirai T, Muro S, et al. Relationship between pulmonary emphysema and osteoporosis assessed by CT in patients with COPD. Chest. 2008;134(6):1244–1249. doi:10.1378/chest.07-3054
7. Bon J, Fuhrman CR, Weissfeld JL, et al. Radiographic emphysema predicts low bone mineral density in a tobacco-exposed cohort. Am J Respir Crit Care Med. 2011;183(7):885–890.
8. Churg A, Dai J, Tai H, Xie C, Wright JL. Tumor necrosis factor-α is central to acute cigarette smoke–induced inflammation and connective tissue breakdown. Am J Respir Crit Care Med. 2002;166(6):849–854. doi:10.1164/rccm.200202-097OC
9. Churg A, Wang RD, Tai H, Wang X, Xie C, Wright JL. Tumor necrosis factor-α drives 70% of cigarette smoke–induced emphysema in the mouse. Am J Respir Crit Care Med. 2004;170(5):492–498. doi:10.1164/rccm.200404-511OC
10. Pompe E, de Jong PA, van Rikxoort EM, et al. Smokers with emphysema and small airway disease on computed tomography have lower bone density. Int J Chron Obstruct Pulmon Dis. 2016;11:1207–1216. doi:10.2147/COPD.S103680
11. Bon J, Zhang Y, Leader JK, et al. Radiographic emphysema, circulating bone biomarkers, and progressive bone mineral density loss in smokers. Ann Am Thorac Soc. 2018;15:615–621. doi:10.1513/AnnalsATS.201709-743OC
12. Smith BM, Austin JHM, Newell JD, et al. Pulmonary emphysema subtypes on computed tomography: the MESA COPD study. Am J Med. 2014;127(1):
13. Copley SJ, Wells AU, Müller NL, et al. Thin-section CT in obstructive pulmonary disease: discriminatory value. Radiology. 2002;223(3):812–819. doi:10.1148/radiol.2233010760
14. Kukkonen MK, Tiili E, Vehmas T, Oksa P, Piirilä P, Hirvonen A. Association of genes of protease-antiprotease balance pathway to lung function and emphysema subtypes. BMC Pulm Med. 2013;13:1. doi:10.1186/1471-2466-13-36
15. Engsig MT, Chen QJ, Vu TH, et al. Matrix metalloproteinase 9 and vascular endothelial growth factor are essential for osteoclast recruitment into developing long bones. J Cell Biol. 2000;151(4):879–889. doi:10.1083/jcb.151.4.879
16. Bolton CE, Stone MD, Edwards PH, Duckers JM, Evans WD, Shale DJ. Circulating matrix metalloproteinase-9 and osteoporosis in patients with chronic obstructive pulmonary disease. Chron Respir Dis. 2009;6(2):81–87. doi:10.1177/1479972309103131
17. Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Bourbeau J, et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report. GOLD Executive Summary. Am J Respir Crit Care Med. 2017;195(5):557–82.
18. Wedzicha JA, Seemungal TA. COPD exacerbations: defining their cause and prevention. Lancet. 2007;370:786–796. doi:10.1016/S0140-6736(07)61382-8
19. Celli BR, Macnee W. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23:932–946.
20. Roca J, Burgos F, Sunyer J, et al. References values for forced spirometry. Group of the European Community Respiratory Health Survey. Eur Respir J. 1998;11(6):1354–1362. doi:10.1183/09031936.98.11061354
21. Crapo RO, Casaburi R, Coates AL, et al. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–117.
22. Kanis JA. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: synopsis of a WHO report. WHO Study Group. Osteoporos Int. 1994;4(6):368–381. doi:10.1007/BF01622200
23. Henschke CI, Mccauley DI, Yankelevitz DF, et al. Early lung cancer action project: overall design and findings from baseline screening. Lancet. 1999;354:99–105.
24. Society F, Austin JHM, Hogg JC, et al. Chronic obstructive pulmonary disease: a statement of the Fleischner Society. Radiology. 2015;277(1):192–205.
25. National Emphysema Treatment Trial Research Group, Fishman A, Fessler H, et al. Patients at high risk of death after lung-volume-reduction surgery. N Engl J Med. 2001;345(15):1075–1083. doi:10.1056/NEJMoa11798
26. Cetin A, Gökçe-Kutsal Y, Celiker R. Predictors of bone mineral density in healthy males. Rheumatol Int. 2001;21(3):85–88. doi:10.1007/s00296-001-0142-2
27. Barrera G, Bunout D, Gattás V, De La Maza MP, Leiva L, Hirsch S. A high body mass index protects against femoral neck osteoporosis in healthy elderly subjects. Nutrition. 2004;20(9):769–771. doi:10.1016/j.nut.2004.05.014
28. Kirchengast S, Peterson B, Hauser G, Knogler W. Body composition characteristics are associated with the bone density of the proximal femur end in middle- and old-aged women and men. Maturitas. 2001;39(2):133–145. doi:10.1016/S0378-5122(01)00205-5
29. Kirchengast S, Knogler W, Hauser G. Protective effect of moderate overweight on bone density of the hip joint in elderly and old Austrians. Anthropol Anz. 2002;60(2):187–197. doi:10.1127/anthranz/60/2002/187
30. Wee J, Sng BYJ, Shen L, Lim CT, Singh G, Das De S. The relationship between body mass index and physical activity levels in relation to bone mineral density in premenopausal and postmenopausal women. Arch Osteoporos. 2013;8(1–2). doi:10.1007/s11657-013-0162-z
31. Espallargues M, Sampietro-Colom L, Estrada MD, et al. Identifying bone-mass-related risk factors for fracture to guide bone densitometry measurements: a systematic review of the literature. Osteoporos Int. 2001;12(10):811–822. doi:10.1007/s001980170031
32. Mazess RB, Barden HS. Bone density in premenopausal women: effects of age, dietary intake, physical activity, smoking, and birth-control pills. Am J Clin Nutr. 1991;53(1):132–142. doi:10.1093/ajcn/53.1.132
33. Van der Voort DJM, Geusens PP, Dinant GJ. Risk factors for osteoporosis related to their outcome: fractures. Osteoporos Int. 2001;12(8):630–638. doi:10.1007/s001980170062
34. Cummings SR, Nevitt MC, Browner WS, et al. Risk factors for hip fracture in white women. Study of Osteoporotic Fractures Research Group. N Engl J Med. 1995;332(12):767–773. doi:10.1056/NEJM199503233321202
35. Ensrud KE, Ewing SK, Stone KL, Cauley JA, Bowman PJ, Cummings SR. Intentional and unintentional weight loss increase bone loss and hip fracture risk in older women. J Am Geriatr Soc. 2003;51(12):1740–1747. doi:10.1046/j.1532-5415.2003.51558.x
36. Rutten EPA, Grydeland TB, Pillai SG, et al. Quantitative CT: associations between emphysema, airway wall thickness and body composition in COPD. Pulm Med. 2011;2011:1–6. doi:10.1155/2011/419328
37. Makita H, Nasuhara Y, Nagai K, et al. Characterisation of phenotypes based on severity of emphysema in chronic obstructive pulmonary disease. Thorax. 2007;62(11):932–937. doi:10.1136/thx.2006.072777
38. Celli BR, Locantore N, Tal-Singer R, et al. Emphysema and extrapulmonary tissue loss in COPD: a multi-organ loss of tissue phenotype. Eur Respir J. 2018;51(2):1702146. doi:10.1183/13993003.02146-2017
39. Sambrook P, Cooper C. Osteoporosis. Lancet. 2006;367(9527):2010–2018. doi:10.1016/S0140-6736(06)68891-0
40. Raisz LG. Pathogenesis of osteoporosis: concepts, conflicts, and prospects. J Clin Invest. 2005;115:3318–3325. doi:10.1172/JCI27071
41. Couillin I, Vasseur V, Charron S, et al. IL-1R1/MyD88 signaling is critical for elastase-induced lung inflammation and emphysema. J Immunol. 2009;183(12):8195–8202. doi:10.4049/jimmunol.0803154
42. Churg A, Zhou S, Wang X, Wang R, Wright JL. The role of interleukin-1beta in murine cigarette smoke-induced emphysema and small airway remodeling. Am J Respir Cell Mol Biol. 2009;40(4):482–490. doi:10.1165/rcmb.2008-0038OC
43. Xiong Z, Leme AS, Ray P, Shapiro SD, Lee JS. CX3CR1+ lung mononuclear phagocytes spatially confined to the interstitium produce TNF- and IL-6 and promote cigarette smoke-induced emphysema. J Immunol. 2011;186(5):3206–3214. doi:10.4049/jimmunol.1003221
44. Ruwanpura SM, McLeod L, Miller A, et al. Interleukin-6 promotes pulmonary emphysema associated with apoptosis in mice. Am J Respir Cell Mol Biol. 2011;45(4):720–730. doi:10.1165/rcmb.2010-0462OC
45. Chen K, Pociask DA, McAleer JP, et al. IL-17RA is required for CCL2 expression, macrophage recruitment, and emphysema in response to cigarette smoke. PLoS One. 2011;6(5:)e20333.
46. Bon JM, Zhang Y, Duncan SR, et al. Plasma inflammatory mediators associated with bone metabolism in COPD. Copd. 2010;7:186–191. doi:10.3109/15412555.2010.482114
47. Ito I, Nagai S, Handa T, et al. Matrix metalloproteinase-9 promoter polymorphism associated with upper lung dominant emphysema. Am J Respir Crit Care Med. 2005;172(11):1378–1382. doi:10.1164/rccm.200506-953OC
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
