Back to Journals » Patient Preference and Adherence » Volume 16
Exploring Stem Cell Transplanted Patients’ Perspectives on Medication Self-Management and Electronic Monitoring Devices Measuring Medication Adherence: A Qualitative Sub-Study of the Swiss SMILe Implementation Science Project
Authors Ribaut J , De Geest S, Leppla L , Gerull S, Teynor A, Valenta S
Received 17 September 2021
Accepted for publication 22 November 2021
Published 6 January 2022 Volume 2022:16 Pages 11—22
DOI https://doi.org/10.2147/PPA.S337117
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Johnny Chen
Janette Ribaut,1,2 Sabina De Geest,1,3 Lynn Leppla,1,4 Sabine Gerull,2,5 Alexandra Teynor,6 Sabine Valenta1,2 On behalf of the SMILe study team
1Institute of Nursing Science, Department Public Health, Faculty of Medicine, University of Basel, Basel, Switzerland; 2Department of Theragnostic, Hematology, University Hospital of Basel, Basel, Switzerland; 3Academic Center for Nursing and Midwifery, Department of Public Health and Primary Care, KU Leuven, Leuven, Belgium; 4Departments of Hematology, Oncology and Stem Cell Transplantation, Medical Center University of Freiburg, Freiburg, Germany; 5Department of Hematology, Cantonal Hospital of Aarau, Aarau, Switzerland; 6Department of Computer Science, University of Applied Sciences, Augsburg, Germany
Correspondence: Sabine Valenta
Institute of Nursing Science, Department Public Health, Faculty of Medicine, University of Basel, Bernoullistrasse 28, Basel, CH-4056, Switzerland
Tel +41 61 32 85275
Email [email protected]
Purpose: Little is known about allogeneic stem cell transplant (alloSCT) patients’ medication adherence strategies. Acceptability and preferences regarding electronic monitoring (EM) systems to assess all three phases of medication adherence (ie, initiation, implementation, persistence) are crucial to allow their successful implementation in clinical or research settings but have not yet been evaluated. We therefore aimed to explore: 1) alloSCT patients’ medication adherence and self-management strategies; and 2) their acceptability and preferences of three different EM systems (MEMS Cap, Helping Hand, Button) as part of the Swiss SMILe study.
Patients and Methods: Respecting anti-pandemic measures, we used a purposive sample of six adult alloSCT patients from the University Hospital Basel, Switzerland (USB)— 6 weeks to 2 years post-alloSCT—to conduct three focus group sessions with two patients each. Using a semi-structured outline, we explored 1) patients’ medication adherence strategies and medication self-management; and 2) their acceptance and preferences regarding EM use. The three tested EM systems were available for testing during each session. Discussions were audio-recorded, visualized using mind-mapping and analyzed using Mayring’s qualitative content analysis.
Results: Patients (33% females; mean age 54.6± 16.3 years; 10.4± 8.4 months post-alloSCT) used medication adherence enhancing strategies (eg, preparing pillbox, linking intake to a habit). Still, they indicated that post-alloSCT medication management was challenging (eg, frequent schedule changes). All participants preferred the MEMS Button. Participants said its small size and the possibility to combine it with existing pillboxes (eg, putting it into/next to them) made them more confident about implementing it in their daily lives.
Conclusion: Regarding EM systems for medication adherence, end-user preferences and acceptability influence adoption and fidelity. Of the three systems tested, our sample found the MEMS Button most acceptable and most preferable. Therefore, we will use it for our USB SMILe study.
Keywords: allogeneic hematopoietic stem cell transplantation, medication adherence, electronic monitoring, patient empowerment, focus groups
Introduction
Medication adherence—“the process by which patients take their medications as prescribed”1 (p. 697)—is an essential factor of treatment success. It consists of three phases: initiation (first intake of a prescribed medication); implementation (how well a patient’s actual dosage matches the prescription in terms of taking, timing and dosing); and persistence (the timespan between first to last intake of the prescribed medication).1
In allogeneic stem cell transplanted (alloSCT) patients, very close immunosuppressant medication adherence is vital to clinical outcomes.2,3 This group’s adherence initiation phase is not an issue, as it takes place in the hospital setting.4,5 However, implementation and persistence of intake require support: failure to adhere to immunosuppressants can lead to or inhibit treatment of major complications (especially acute and chronic Graft-versus-Host Disease) that typically arise in the first six to eighteen months post-transplant.5 Previous research using self-report questionnaires revealed that up to 64.6% of adult alloSCT patients have problems with immunosuppressant implementation and up to 3.1% are nonadherent regarding persistence.2,6 Behavioral, educational and integrated care interventions can support chronically ill patients in their medication adherence.7 However, little is known about the strategies alloSCT patients use to increase their medication adherence.8,9
According to few studies with adult, adolescent and young adults after alloSCT and their caregivers, all age groups reported experiencing medication self-management as challenging.10,11 Derived from Barlow et al's12 general definition of self-management, medication self-management can be defined as a person’s capability to cope with medication treatment for a chronic condition and its physical and psychosocial effects and changes in daily life. Medication self-management was facilitated by social support, organization and information, but hindered by difficulties with medication regimens, isolation, and physical and psychological symptoms.11 Therefore, patients expressed a need for medication self-management support, effective communication, and holistic treatment from healthcare professionals.10,11 Although medication adherence was also considered by healthcare professionals to be one of the most important health behaviors in self-management, clinicians generally did not use structured interventions such as reminder system for medication intake to support medication self-management.10
Several studies from the field of solid organ transplantation (heart, lung, liver, kidney) have shown that many patients underestimated medication self-management tasks and reported insufficient awareness and knowledge of their therapy: Important information and instructions were often not clear and some patients were even unfamiliar with the name and indication of their immunosuppressants.13,14 In addition, healthcare professionals often advised self-management strategies that were circuitous or even not helpful for their individual strategies.14 Establishing a daily routine and coping with hectic schedules were reported as essential for their medication adherence and for the implementation of a successful strategy to handle the medication self-management tasks.14,15 Accordingly, intervention studies found that behavior change techniques (ie, active components of an intervention) such as prompts and alarm cues, time cues, social support and restructuring of the physical environment could improve medication adherence in solid organ transplantation.16,17
Similar, liver transplanted patients reported to be challenged by their medication self-management and perceived medication intake as difficult to implement in their daily life.18 Therefore, they developed several strategies such as self-regulation and self-care. In view of self-regulations, patients developed strategies such as intentional change of their daily life and information seeking. With regard to self-care, patients and their family caregivers focused on different activities such as shifting to independence, monitoring of changes and supporting self-care.18
Electronic Monitoring (EM) is considered the richest and most reliable assessment method for medication adherence.19–21 Various EM devices measure the date and time of each medication intake. Of these, three types of Medication Event Monitoring System (MEMS) are best known (see Figure 1): (1) the MEMS Cap, a bottle top that records each opening of the bottle; (2) the MEMS Helping Hand, a slider into which individual medication blisters can be inserted and which measures the date and time of each removal. As blister sizes match specific medications, the MEMS Helping Hand is produced individually according to blister size; and (3) the MEMS Button, a small device that must be pressed by the patient at each medication intake (Aardex Group, Seraing, Belgium).22 All three devices provide detailed information on adherence patterns,23 but need to be brought to the hospital for care personnel to read the data.22
 |
Figure 1 The three different MEMS devices (MEMS Cap, Helping Hand, Button). (A). MEMS Cap in different sizes and shapes; (B) MEMS Helping Hand with a plastic example blister; (C) MEMS Button. |
While EM devices have been used in kidney transplantation24–26 and in a small sample of adolescent alloSCT patients,27 they have not yet been used in adult alloSCT patients.9 For this group, usability and acceptability issues have been identified as barriers to EM use.24–27 Therefore, a full usability/acceptability assessment is crucial before the start of EM, whether in clinical or research settings. Having patients evaluate EM systems and provide preferences for one system or the other can help ensure its adoption and continued use. Therefore, the aims of this study were 1) to explore alloSCT patients’ experiences and current strategies related to medication adherence and medication self-management; and 2) to learn their perspectives and preferences regarding EM devices.
Materials and Methods
Design
This is an exploratory, qualitative sub-study design with focus groups. Focus groups are a qualitative research approach to illuminate a specific topic by conducting a group interview to reach a discussion guided by a trained moderator.28
This is a sub-study of the international multi-center SMILe study, which focusses on developing, implementing and testing an Integrated Care Model (ICM) in allogeneic SteM cell transplantatIon faciLitated by eHealth (SMILe-ICM).8,29,30 Specifically, this sub-study is an offspring of the Swiss SMILe project, which involved adapting the SMILe–ICM to the Swiss setting (Valenta et al, in preparation, 2022). The SMILe–ICM is currently being tested in first-year post-alloSCT groups at the University Hospital Basel, Switzerland (USB), (ClinicalTrials.gov: NCT04789863) (De Geest et al, in preparation, 2022).
In brief, the SMILe-ICM integrates four intervention modules—monitoring and follow-up, infection prevention, physical activity and medication adherence—targeting different health behaviors. The resulting interventions were developed based in theory and are delivered by a combination of eHealth media (ie, the SMILeApp) and purpose-trained Advanced Practice Nurses (APNs).8,29
The SMILe study’s medication adherence module aims at optimizing the post-alloSCT implementation and persistence phases of immunosuppressive drug therapies. As an EM system would be used to monitor medication adherence, it was necessary to identify the optimal system (ie, MEMS Cap, Helping Hand, Button) through focus group interviews.
Setting and Sample
We recruited a purposive sample of alloSCT patients using the USB hematological outpatient clinic. The USB is the largest alloSCT center in Switzerland and performs about 100–120 alloSCTs per year. Patients from all over Switzerland can be treated at the USB, but predominantly from German- and Italian-speaking parts of Switzerland. Patients are usually admitted to the USB seven to ten days pre-alloSCT and are discharged approximately 20 to 30 days post-alloSCT, depending on their state of health. Afterwards, patients have follow-up appointments at the USB twice a week at the beginning with decreasing frequency. Inpatients and outpatients are cared for by an interdisciplinary team consisting of physicians, nurses and psycho-oncologists. Other services such as nutritionist, physiotherapy and social services are included mainly on an inpatient basis. Before discharge, most patients receive an educational session by a hospital pharmacist on how to prepare the medication at home without discussing any specific strategies to enhance their medication adherence. In the follow-up, nurses regularly assess adherence to immunosuppressant. However, there is no structured medication adherence support.
Potential participants were selected based on age, gender and time since alloSCT. Consistent with the reported gender distribution in alloSCT of 30–45% females,31–33 we aimed for a similar female proportion in our sample. Inclusion criteria were 1) transplantation and follow-up at the USB; 2) age≥18 years; 3) between six weeks and two years since most recent (first or subsequent) alloSCT; 4) ability to communicate in German. Patients with any cognitive or physical condition impairing adequate communication were excluded. Due to the Covid-19-pandemic, we restricted the number of participants to two per focus group to follow hygiene requirements.
This study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee Northwest and Central Switzerland (EKNZ 2019–00307). All participants participated voluntarily and provided written informed consent before participating in the focus groups. Informed consent included publication of anonymized responses.
Data Collection Procedures
The first and last authors (JR and SV) screened the USB outpatient clinic’s electronic health records to identify possible participants. Patients who met the inclusion criteria were telephoned by the first author (JR). None of the screened patients had to be excluded due to cognitive or physical limitations. The contacted patients received oral and written information about the study. All contacted patients agreed to participate. For descriptive information on the sample, we extracted demographic characteristics (age, gender, time since alloSCT, education, employment and marital status) from medical records and patient information.
The focus group sessions were conducted in October 2020. They were led and audio-recorded by the first author (JR), while the last author (SV) mind-mapped key themes on a flip chart to help memorize previous thoughts and summarize all of the focus groups’ input.34 The participants had the opportunity to reflect on the maps and to add or change keywords. No transcripts were made.
During each focus group interview, the first author asked open-ended, semi-structured questions to explore this study’s two main areas of interest: 1) participants’ experiences and current strategies related to medication management and adherence (eg, How do you manage your medication at home after alloSCT?); and 2) patients’ perspectives and preferences related to using an EM device to measure medication adherence (eg, Could you imagine using an EM device in your daily life?). Depending on the answers, further deepening and reflection questions were asked to broaden our understanding.
For the second topic, the three EM devices (MEMS Cap, MEMS Helping Hand and MEMS Button (see Figure 1) were presented and explained, with the possibility to try them out on-site.
Data Analysis
Demographic characteristics were analyzed descriptively using means and standard deviations (SD), medians and interquartile ranges (IQRs) or frequencies as appropriate. After the final focus group session, all mind maps were combined into a single meta-map using the Microsoft Visio Professional 2019 software.35 Each step was discussed by the research team. For the qualitative analysis, we applied Mayring’s approach to qualitative content analysis.36 This method enabled us to quickly and concretely explore the matter of which MEMS device would fit patients’ preferences and could be used within the Swiss SMILe RCT. The recordings were deleted after data analysis.
Results
Three focus groups—involving six alloSCT patients in total—were interviewed at the USB hematological outpatient clinic. The sessions’ mean duration was 35 minutes (SD 10 min, range 25–45 minutes). Patient characteristics are shown in Table 1. During data collection, the Covid-19-pandemic continued to be present. We therefore did not want to expose the patients to further infection risks and kept the number of participants as small as possible. Additionally, we had overlapping and repeating results across the focus groups, so that the research questions could be answered.
 |
Table 1 Patient Demographics of Focus Group Participants |
Regarding our first aim—to explore the participants’ experiences and current strategies in relation to medication management and adherence—two themes emerged: 1) being challenged by medication management at home due to lack of support before hospital discharge; and 2) implementing one’s own strategies. Related to the second topic—patients’ perspectives and preferences regarding using an EM device to measure medication adherence—one further theme appeared: openness to implement (preferably) the MEMS Button system to measure medication adherence in daily life. Figures 2–5 show the results of all three focus groups combined in meta-maps: Figure 2 summarizes the overall main topics; Figures 3–5 show detailed reflections on each topic separately. As might be expected, discussions and main topics overlapped considerably across the three focus groups.
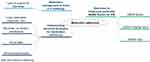 |
Figure 2 Focus groups meta-map with main topics. |
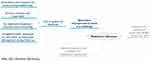 |
Figure 3 Focus groups meta-map on being challenged by medication management at home. |
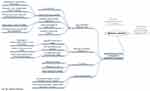 |
Figure 4 Focus groups meta-map on implementing own strategies for medication adherence. |
 |
Figure 5 Focus groups meta-map on openness to implement preferably MEMS Button for EM. |
Medication Management at Home is a Challenge
In view of medication self-management (Figure 3), all participants mentioned that being suddenly responsibility for their medication management (eg, organizing medication, preparing tablets, taking the medication at the right time) at home was a serious challenge. They associated this with inadequate training support before discharge. During their inpatient stay, these tasks were performed or shared by nurses. Some patients (n = 3) received a medication training session before discharge; however, many did not receive any specific training sessions before discharge; two did not even receive a medication box or a medication plan overview for home.
Once home, many patients were discouraged by the high number of different medications as well as the complication of their changing intake schedules (especially in the first weeks after alloSCT):
At the hospital, the nurses took care of the whole medication management: they prepared everything and told me when to take it. But at home, I suddenly had to learn how to do it on my own. Although the nurses explained everything briefly before discharge, it was difficult to deal with it. (patient 1, male, 60-70 years)
This account indicates deficits in the study hospital’s transplant program regarding preparation for discharge, self-management support and transitional care.
Another patient reported severe difficulties with the number of different drug packages. This was because no-one on her care team had given her a pillbox before discharge. In fact, no-one had even told her about such boxes:
This pillbox you (other participant of the focus group) are using looks very practical. I don’t know them. I didn’t receive one at the hospital and nobody told me that this could be helpful. I think this would have made medication management much easier. (patient 2, female, 60-70 years)
Implementing Personal Strategies
To manage medication intake at home, all patients implemented strategies in their daily lives. Most used different sizes of medication dispensers (n = 5) to prepare their drugs in advance; a number used electronic alarms (n = 2) or maintained their own list of medications (n = 4) (Figure 4). They needed to develop these strategies because the support they had received in hospital for medication management after discharge was inadequate.
All patients carefully applied different strategies to manage and monitor changes in their medication prescriptions. These included using self-developed medication charts to track changes in their prescriptions, or adapting their medication plans by hand after each outpatient clinic visit. Some patients also tried to link the medication intake with other regular habits such as taking meals:
It’s quite easy to remember the morning dosage because I take it with breakfast. It’s more difficult not to forget the dosage at 8 p.m. because that’s not dinner time. (patient 3, male, 30-40 years)
All participants emphasized both, that they knew immunosuppressants were their most important medications and that they gave special attention to their correct intake, dosing and timing:
It doesn’t matter to take most medications a little earlier or later. Only not with the immunosuppressants. It is very important to take them at the right time, which is why I give special attention to them and use an alarm clock for them. (patient 4, male, 50-60 years)
Openness to Implement Preferably MEMS Button to Measure Medication Adherence in Daily Lives
Regarding EM use (Figure 5), all participants (100%) said they would be willing to use an electronic system to measure their medication adherence. One patient said, “I can well imagine using such a device. This can be easily combined with medication intake” (patient 5, male, 30–40 year). The participants tried the offered MEMS Cap, MEMS Helping Hand and MEMS Button systems during their focus group sessions. Because the immunosuppressants must remain in the blister until they are taken, the blisters would have to be cut to put the tablets into the bottles when using the MEMS Cap. This was seen as impractical, because first loading the blisters into bottles, then taking the out would be difficult. Moreover, different sizes of bottle would be needed depending on different situations, which the participants deemed unnecessarily complicated:
At home, I would prefer the big bottle in order to store more of the big tablets. However, I cannot take that huge bottle with me when I come to the hospital and need to take the medication here. (….) But I really don’t want to use two different bottles. (patient 1, male, 60-70 years)
The MEMS Helping Hand was seen as handier than a bottle-based system. However, after alloSCT the dosages of immunosuppressants often change, along with the blister sizes. Therefore, after each dosage change, the patients would need a new MEMS Helping Hand device. Again, all participants considered this impractical.
Moreover, as patients sometimes need to take several different dosages, they would need to have a different blister size for each. That meant each patient would need several MEMS Helping Hand devices simultaneously. This was also seen as inconvenient. To cope with this second difficulty, patients could see that one simple solution would be to use the device to record only one of the necessary dosages. As this would require any different-sized dosages to be recorded some other way, eg, with a pen in a notebook, it could easily lead to incomplete or overly-complicated records. This would defeat the purpose of an EM system.
All participants (100%) preferred the MEMS Button as the most practical option. It is convenient, can be combined with existing dispensers and is small enough to carry when leaving home: “This device looks very nice and discreet. It is small enough to put into my pillbox or my bag when I go out” (patient 6, female, 60–70 years).
Of the three devices tested, the MEMS Button is the only one that requires a conscious action from the patients—pressing a button at the time of medication intake. However, this did not worry the participants:
I am very confident that I would not forget to press the button. If I put it next to the immunosuppressants, I always see it and cannot forget to press it. It is the same as taking the tablet, if you take it, you can also quickly press the device. (patient 3, male, 30-40 years)
Discussion
In this exploratory study, we found that, when our participating alloSCT patients returned home after their transplantation, they found themselves seriously challenged regarding medication self-management. To implement medication intake behavior in their daily lives, then, all developed personal strategies to ease the implementation process. These included preparing pillboxes for several days in advance or linking their medication intake with other routine activities (eg, taking meals).
We found that all were open to using an EM device to measure their medication adherence, preferring the MEMS Button to either of the other options suggested. When we examined the alloSCT program where this study was conducted, we also found major gaps in daily clinical practice regarding self-management support and discharge planning.
In studies including solid organ transplanted patients, results have shown that many patients underestimate the importance of their medication regimen, highly contributing to nonadherence.13,14 In contrast, a systematic review of qualitative studies in kidney transplant recipients37 found that patients are generally aware of how important medication adherence is. In our study group, this was true of all participants: all found ways to support their timing and taking adherence. However, they reported difficulties fulfilling the many tasks involved in medication management and scheduling. This finding is consistent with other study results indicating that medication self-management was viewed as challenging by alloSCT patients.10,11 Their experience echoed the findings of a qualitative study in the parents of pediatric alloSCT patients.38 When interviewed, participants generally described medication management as very difficult, stressful, and tiring.38 This has been confirmed also by solid organ transplant patients such as liver or kidney transplant recipients, who as well described medication self-management as a challenge.13,18 They highlighted the need to develop effective strategies to prevent medication nonadherence for all transplant populations because of the high risk for poor outcomes.
Previous studies also reported that their participants developed strategies to establish routines for mediation intake. As with our group, these included linking medication intake with meals or brushing teeth.37–39 Patients reported that taking immunosuppressants every day at the same time and under similar circumstances facilitated to develop a habit of medication taking.15
In line with our results, Jamieson et al37 found, for example, that by carefully tracking prescription changes, his patients not only increased control over their health-related processes, but also built up confidence in their abilities. Gaining control over one’s own life and health was also an important theme in the study including liver transplant patients.18 The participants of this study emphasized the importance of being proactive as a patient to request necessary information and support services. To ensure that patients’ health is not solely dependent on their ability to actively request support, the authors concluded that patients should receive more self-management support from healthcare professionals.18 Through our focus group discussions, we identified gaps in the transplant program’s discharge planning and self-management support—gaps that left patients struggling to work out medication management strategies on their own.
In our study, all participants were open to the idea of using an EM device to monitor and support their self-management efforts. Surprisingly, when asked to choose between the three candidate devices, all preferred the MEMS Button, which requires the most active user involvement. Even after the participants were explicitly asked about this point, none considered it a serious problem. They saw pressing the button at the time of medication intake as a relatively simple behavior to learn. Poor implementation of this behavior would lead to inaccurate adherence assessments. To prevent this from becoming an issue, our participants suggested possible strategies to implement the MEMS Button into everyday life. For example, in order to facilitate the button-pressing behavior, several suggested putting the button either directly next to or even into the pillbox.
To date, the MEMS Cap has been used far more often in research projects than the MEMS Button.40–42 However, our participants found the form, size and use procedures of both the MEMS Cap and the Helping Hand impractical. With this observation, they highlighted an unexpected barrier to implementation in daily life. This supports Hayes et al43 finding in older patients that they need to be easily able to take their pillboxes with them when they leave their homes. Even for our participants, whose mean age was slightly below 55, the large size of the MEMS Cap represented a barrier when going out for appointments. And De Bleser et al evaluation25 of eleven kidney transplant recipients and ten healthy volunteers found the MEMS Helping Hand impractical in two ways: it can be used for only one medication at a time; and it is too large to fit easily in a handbag or pocket. Considering the MEMS Button’s small size and the associated convenience of carrying it, our participants saw it as easier to implement in their daily lives.
Our findings highlight the need to select adherence measures based on their usability, acceptability and, of course, how well they fit the needs and preferences of the end-users—the patients. Continuous patient and stakeholder involvement to evaluate EM system-user fit is crucial. Whether in trial or clinical settings, understanding not only patients’ needs but their preferences will increase acceptance and prevent many usability issues, maximizing both adoption and fidelity.25,44–46
Limitations
Our study has several notable limitations. First, the sample size of six patients is rather small. This was necessary because of Covid-19 pandemic regulations. Therefore, our results need to be interpreted with caution. However, even with this small sample, the discussions and opinions largely overlapped. Second, the mind-mapping method34 could not reach the depth or interpretive level of a traditional qualitative transcript analytic methodology such as Grounded Theory. In our situation, however, it was a stable method for quick analysis of the focus groups, and supported rapid decision-making. These characteristics made it suitable to choose adherence measurement methods used in the SMILe RCT.
Implication for Clinical Practice and Future Research
Our findings highlight the need for improvements in discharge planning and medication adherence support during alloSCT follow-up. Patients found medication management at home challenging; therefore, they implemented their own adherence strategies. In clinical practice, such strategies should be a part of patients’ discharge planning. Further medication adherence interventions should be incorporated in routine follow-up care.
For future research, careful assessment of the MEMS Button’s acceptability and usability is essential, preferably with larger and more varied samples and multiple centers. This sub-study’s results were included in the basis of the recently-started SMILe RCT at the USB. This use is described elsewhere (De Geest et al, in preparation, 2022). In the proposed RCT, we will use the MEMS Button to monitor medication adherence as recommended by the current sub-study’s end-users.
Conclusion
Following alloSCT, participants were aware that medication adherence was very essential. However, their hospital discharge preparation had included little or no training in implementing and managing it at home. Therefore, they developed various strategies to self-manage their medication. These included habit formation or using special pillboxes. As an EM system, they considered the MEMS Button a feasible device to monitor medication adherence in everyday life.
Therefore, we selected the MEMS Button for use in the Swiss SMILe RCT at the USB. Exploring patients’ views and preferences regarding proposed EM systems’ acceptability is crucial to maximize adoption and fidelity. We will continue to gather patient feedback on these systems in our Swiss SMILe study as well as in other trials and clinical settings.
Abbreviations
AlloSCT, allogeneic stem cell transplantation; EM, electronic monitoring; MEMS, Medication Event Monitoring Systems; RCT, randomized controlled trial; SMILe Development, implementation and testing of an integrated care model in allogeneic SteM cell transplantatIon faciLitated by eHealth (study acronym); SMILe–ICM, SMILe–integrated care model; USB, University Hospital of Basel.
Acknowledgments
We gratefully acknowledge all or our focus group participants, as well as the SMILe study team, especially Sonja Beckmann, Juliane Mielke and Anja Schmid of the Institute of Nursing Science, University of Basel, Switzerland. We are also grateful to Fabienne Dobbels and Nathalie Duerinckx of the Academic Centre for Nursing and Midwifery, University of Leuven, Belgium, as well as to the SMILe software development team of the University of Applied Sciences Augsburg, Germany, led by Alexandra Teynor. On the clinical side, we owe many thanks to Dora Bolliger, Sabine Degen, Katharina Koehly, Yuliya Senft, Sandra Schönfeld, Anja Hermann, Florian Grossmann, Birgit Maier, Roby Mathew and Jakob Passweg, of the USB, Switzerland, as well as Robert Zeiser, Monika Engelhardt, Monika Hasemann and Klaus Kaier of the University Medical Center Freiburg, Germany. We also thank Chris Shultis for the editing of this paper.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
The overall Swiss SMILe research project is funded by the Swiss Cancer League, the Werner-and-Hedy-Berger-Janser Stiftung and Nursing Science Foundation, Switzerland. The funders had no role in study design, data collection, analysis, interpretation or the writing of the report.
Disclosure
The authors declare that they have no competing interests.
References
1. Vrijens B, De Geest S, Hughes DA, et al. A new taxonomy for describing and defining adherence to medications. Br J Clin Pharmacol. 2012;73(5):691–705. doi:10.1111/j.1365-2125.2012.04167.x
2. Gresch B, Kirsch M, Fierz K, et al. Medication nonadherence to immunosuppressants after adult allogeneic haematopoietic stem cell transplantation: a multicentre cross-sectional study. Bone Marrow Transplant. 2017;52(2):304–306. doi:10.1038/bmt.2016.262
3. Kirsch M, Götz A, Halter J, et al. Differences in health behaviour between recipients of allogeneic haematopoietic SCT and the general population: a matched control study. Bone Marrow Transplant. 2014;49(9):1223–1230. doi:10.1038/bmt.2014.142
4. Smith A, Wisloff F, Samson D; UK Myeloma Forum NMSG, Haematology BCfSi. Guidelines on the diagnosis and management of multiple myeloma 2005. Br J Haematol. 2006;132(4):410–451. doi:10.1111/j.1365-2141.2005.05867.x
5. Tomblyn M, Chiller T, Einsele H, et al. Guidelines for preventing infectious complications among hematopoietic cell transplant recipients: a global perspective. Bone Marrow Transplant. 2009;44(8):453–455. doi:10.1038/bmt.2009.254
6. Ice LL, Bartoo GT, McCullough KB, et al. A prospective survey of outpatient medication adherence in adult allogeneic hematopoietic stem cell transplantation patients. Biol Blood Marrow Transplant. 2020;26(9):1627–1634. doi:10.1016/j.bbmt.2020.05.020
7. Costa E, Giardini A, Savin M, et al. Interventional tools to improve medication adherence: review of literature. Patient Prefer Adherence. 2015;9:1303. doi:10.2147/PPA.S87551
8. Ribaut J, Leppla L, Teynor A, et al. Theory-driven development of a medication adherence intervention delivered by eHealth and transplant team in allogeneic stem cell transplantation: the SMILe implementation science project. BMC Health Serv Res. 2020;20(1):1–22. doi:10.1186/s12913-020-05636-1
9. Morrison CF, Martsolf DM, Wehrkamp N, Tehan R, Pai ALH. Medication adherence in hematopoietic stem cell transplantation: a review of the literature. Biol Blood Marrow Transplant. 2017;23(4):562–568. doi:10.1016/j.bbmt.2017.01.008
10. Leppla L, Mielke J, Kunze M, et al. Clinicians and patients perspectives on follow-up care and eHealth support after allogeneic hematopoietic stem cell transplantation: a mixed-methods contextual analysis as part of the SMILe study. Eur J Oncol Nurs. 2020;45:101723. doi:10.1016/j.ejon.2020.101723
11. Morrison CF, Pai AL, Martsolf D. Facilitators and barriers to self-management for adolescents and young adults following a hematopoietic stem cell transplant. J Ped Oncol Nurs. 2018;35(1):36–42. doi:10.1177/1043454217723864
12. Barlow J, Wright C, Sheasby J, Turner A, Hainsworth J. Self-management approaches for people with chronic conditions: a review. Patient Educ Couns. 2002;48(2):177–187. doi:10.1016/S0738-3991(02)00032-0
13. Vankova B, Mala-Ladova K, Kubena AA, Maly J, Sulkova SD. Immunosuppressive therapy related adherence, beliefs and self-management in kidney transplant outpatients. Patient Prefer Adherence. 2018;12:2605–2613. doi:10.2147/ppa.S184166
14. Vanhoof JM, Vandenberghe B, Geerts D, et al. Shedding light on an unknown reality in solid organ transplant patients’ self‐management: a contextual inquiry study. Clin Transplant. 2018;32(8):e13314. doi:10.1111/ctr.13314
15. Côté J, Fortin M-C, Auger P, et al. Web-based tailored nursing intervention to support medication self-management: a qualitative study of the experience of kidney transplant recipients. Comp Informatics Nurs. 2019;37(11):564–572. doi:10.1097/CIN.0000000000000572
16. Andrews AM, Cheng A-L, Bartlett Ellis RJ, Emerson AM, O’Brien T, Russell CL. SystemCHANGE™ solutions to improve medication adherence in kidney transplant recipients: a secondary data analysis. Nephrol Nurs J. 2021;48:4. doi:10.37526/1526-744X.2021.48.4.389
17. Dobbels F, De Bleser L, Berben L, et al. Efficacy of a medication adherence enhancing intervention in transplantation: the MAESTRO-Tx trial. J Heart Lung Transplant. 2017. doi:10.1016/j.healun.2017.01.007
18. Moayed MS, Ebadi A, Khodaveisi M, Toosi MN, Soltanian AR, Khatiban M. Factors influencing health self-management in adherence to care and treatment among the recipients of liver transplantation. Patient Prefer Adherence. 2018;12:2425. doi:10.2147/PPA.S180341
19. Dunbar-Jacob J, Sereika SM, Houze M, Luyster FS, Callan JA. Accuracy of measures of medication adherence in a cholesterol-lowering regimen. West J Nurs Res. 2012;34(5):578–597. doi:10.1177/0193945912439251
20. Vrijens B, Urquhart J. Patient adherence to prescribed antimicrobial drug dosing regimens. J Antimicrob Chemother. 2005;55(5):616–627. doi:10.1093/jac/dki066
21. El Alili M, Vrijens B, Demonceau J, Evers SM, Hiligsmann M. A scoping review of studies comparing the medication event monitoring system (MEMS) with alternative methods for measuring medication adherence. Br J Clin Pharmacol. 2016;82(1):268–279. doi:10.1111/bcp.12942
22. Aardex Group. MEMS® Adherence Hardware. 2019 [Internet]. Available from: https://www.aardexgroup.com/solutions/mems-adherence-hardware/.
23. Riekert KA, Rand CS. Electronic monitoring of medication adherence: when is high-tech best? J Clin Psychol Med Settings. 2002;9(1):25–34. doi:10.1023/A:1014131928789
24. Foster BJ, Pai ALH, Zelikovsky N, et al. A randomized trial of a multicomponent intervention to promote medication adherence: the Teen Adherence in Kidney Transplant Effectiveness of Intervention Trial (TAKE-IT). Am J Kidney Dis. 2018;72(1):
25. De Bleser L, Vincke B, Dobbels F, et al. A new electronic monitoring device to measure medication adherence: usability of the helping HandTM. Sensors (Basel, Switzerland). 2010;10(3):1535–1552. doi:10.3390/s100301535
26. Eisenberger U, Wüthrich RP, Bock A, et al. Medication adherence assessment: high accuracy of the new Ingestible Sensor System in kidney transplants. Transplantation. 2013;96(3):245. doi:10.1097/TP.0b013e31829b7571
27. McGrady ME, Williams SN, Davies SM, Pai AL. Adherence to outpatient oral medication regimens in adolescent hematopoietic stem cell transplant recipients. Eur J Oncol Nurs. 2014;18(2):140–144. doi:10.1016/j.ejon.2013.11.007
28. Sim J, Waterfield J. Focus group methodology: some ethical challenges. Qual Quant. 2019;53(6):3003–3022. doi:10.1007/s11135-019-00914-5
29. Leppla L, Schmid A, Valenta S, et al. Development of an integrated model of care for allogeneic stem cell transplantation facilitated by eHealth—the SMILe study. Support Care Cancer. 2021;29:1–13.
30. Leppla L, Hobelsberger S, Rockstein D, et al. Implementation science meets software development to create ehealth components for an integrated care model for allogeneic stem cell transplantation facilitated by eHealth: the SMILe study as an example. J Nurs Scholarship. 2020;53(1):35–45. doi:10.1111/jnu.12621
31. Takekiyo T, Dozono K, Nara S, et al. Gender differences in physical function and muscle mass change in patients undergoing allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2017;52(10):1460–1462. doi:10.1038/bmt.2017.156
32. Morishita S, Kaida K, Yamauchi S, et al. Gender differences in health‐related quality of life, physical function and psychological status among patients in the early phase following allogeneic haematopoietic stem cell transplantation. Psycho‐Oncology. 2013;22(5):1159–1166. doi:10.1002/pon.3128
33. Gahrton G, Iacobelli S, Apperley J, et al. The impact of donor gender on outcome of allogeneic hematopoietic stem cell transplantation for multiple myeloma: reduced relapse risk in female to male transplants. Bone Marrow Transplant. 2005;35(6):609–617. doi:10.1038/sj.bmt.1704861
34. Burgess‐Allen J, Owen‐Smith V. Using mind mapping techniques for rapid qualitative data analysis in public participation processes. Health Expectations. 2010;13(4):406–415. doi:10.1111/j.1369-7625.2010.00594.x
35. Microsoft. Visio Professional 2019; 2021. Available from: https://www.microsoft.com/de-lu/microsoft-365/p/visio-professional-2019/cfq7ttc0k7cg.
36. Mayring P. Qualitative content analysis: theoretical background and procedures. In: Bikner-Ahsbahs A, Knipping C, Presmeg N (eds). Approaches to Qualitative Research in Mathematics Education. Springer; 2015:365–380.
37. Jamieson NJ, Hanson CS, Josephson MA, et al. Motivations, challenges, and attitudes to self-management in kidney transplant recipients: a systematic review of qualitative studies. Am J Kidney Dis. 2016;67(3):461–478. doi:10.1053/j.ajkd.2015.07.030
38. Hoegy D, Bleyzac N, Rochet C, et al. Medication adherence after pediatric allogeneic stem cell transplantation: barriers and facilitators. Eur J Oncol Nurs. 2019;38:1–7. doi:10.1016/j.ejon.2018.11.006
39. Sanders MJ, Van Oss T. Using daily routines to promote medication adherence in older adults. Am J Occup Ther. 2013;67(1):91–99. doi:10.5014/ajot.2013.005033
40. Yaegashi H, Kirino S, Remington G, Misawa F, Takeuchi H. Adherence to oral antipsychotics measured by electronic adherence monitoring in schizophrenia: a systematic review and meta-analysis. CNS Drugs. 2020;34(6):579–598. doi:10.1007/s40263-020-00713-9
41. Forbes CA, Deshpande S, Sorio-Vilela F, et al. A systematic literature review comparing methods for the measurement of patient persistence and adherence. Curr Med Res Opin. 2018;34(9):1613–1625. doi:10.1080/03007995.2018.1477747
42. Malta M, Strathdee SA, Magnanini MM, Bastos FI. Adherence to antiretroviral therapy for human immunodeficiency virus/acquired immune deficiency syndrome among drug users: a systematic review. Addiction. 2008;103(8):1242–1257. doi:10.1111/j.1360-0443.2008.02269.x
43. Hayes TL, Hunt JM, Adami A, Kaye JA, editors. An electronic pillbox for continuous monitoring of medication adherence. 2006 International Conference of the IEEE Engineering in Medicine and Biology Society; 2006: IEEE.
44. De Geest SM, Ribaut J, Denhaerynck K, Dobbels F. Adherence management in transplantation. In: Cukor D, Cohen S, Kimmel PL (eds). Psychosocial Aspects of Chronic Kidney Disease. Elsevier; 2020:409–448.
45. De Geest S, Zúñiga F, Brunkert T, et al. Powering Swiss health care for the future: implementation science to bridge “The Valley of death”. Swiss Med Wkly. 2020;150:3738.
46. Peters DH, Adam T, Alonge O, Agyepong IA, Tran N. Implementation research: what it is and how to do it. BMJ. 2013;347:6753.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
