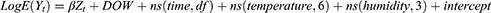Back to Journals » Risk Management and Healthcare Policy » Volume 16
Exploring Associations Between Short-Term Air Pollution and Daily Outpatient Visits for Allergic Rhinitis
Authors Tang W, Sun L, Wang J, Li K, Liu S, Wang M, Cheng Y, Dai L
Received 4 May 2023
Accepted for publication 25 July 2023
Published 7 August 2023 Volume 2023:16 Pages 1455—1465
DOI https://doi.org/10.2147/RMHP.S416365
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jongwha Chang
Wei Tang,1,2 Lixia Sun,3 Jie Wang,4 Kaijie Li,5 Shuhan Liu,5 Mingwei Wang,6 Yongran Cheng,7 Lili Dai1
1Department of Otolaryngology, Affiliated Hospital of Hangzhou Normal University, Hangzhou, Zhejiang, People’s Republic of China; 2Department of Otolaryngology, Hangzhou Xixi Hospital, Hangzhou, Zhejiang, People’s Republic of China; 3Mathematics Teaching and Research Office of the Ministry of Basic Education of Zhejiang University of Water Resources and Electric Power, Hangzhou, Zhejiang, People’s Republic of China; 4Hangzhou Zhenqi Technology Co., Ltd, Hangzhou, Zhejiang, People’s Republic of China; 5Clinical Medicine Department of Hangzhou Normal University, Hangzhou, Zhejiang, People’s Republic of China; 6Metabolic Disease Center, Affiliated Hospital of Hangzhou Normal University, Hangzhou, Zhejiang, People’s Republic of China; 7School of Public Health, Hangzhou Medical College, Hangzhou, Zhejiang, People’s Republic of China
Correspondence: Lili Dai, Department of Otolaryngology, Affiliated Hospital of Hangzhou Normal University, Hangzhou, 310015, People’s Republic of China, Tel +86 15 152 803 386, Fax +8657188021730, Email [email protected] Mingwei Wang, Metabolic Disease Center, Affiliated Hospital of Hangzhou Normal University, Hangzhou, Zhejiang, People’s Republic of China, Email [email protected]
Purpose: Many studies have reported that exposure to air pollution increases the likelihood of acquiring allergic rhinitis (AR). This study investigated associations between short-term air pollution exposure and AR outpatient visits.
Patients and Methods: The Department of Otorhinolaryngology, Affiliated Hospital of Hangzhou Normal University provided AR outpatient data from January 1, 2019 to December 31, 2021. Daily air quality information for that period was gathered from the Hangzhou Air Quality Inspection Station. We used the Poisson’s generalized additive model (GAM) to investigate relationships between daily outpatient AR visits and air pollution, and investigated lag-exposure relationships across days. Subgroup analyses were performed by age (adult (> 18 years) and non-adult (< 18 years)) and sex (male and female).
Results: We recorded 20,653 instances of AR during the study period. Each 10 g/m3 increase in fine particulate matter (PM10 and PM2.5) and carbon monoxide (CO) concentrations was associated with significant increases in AR outpatient Visits. The relative risks (RR) were: 1.007 (95% confidence interval (CI): 1.001– 1.013), 1.026 (95% CI: 1.008– 1.413), and 1.019 (95% CI: 1.008– 1.047). AR visits were more likely due to elevated PM2.5, PM10, and CO levels. Additionally, children were more affected than adults.
Conclusion: To better understand the possible effects of air pollution on AR, short-term exposure to ambient air pollution (PM2.5, PM10, and CO) may be linked to increased daily outpatient AR visits.
Keywords: air pollution, daily outpatient visits, allergic rhinitis, the Poisson’s generalized additive model, GAM, PM10, PM2.5
Introduction
In atopic individuals exposed to allergens, allergic rhinitis (AR) can develop, which is a non-infectious chronic inflammatory illness of the nasal mucosa.1 The condition is characterized by paroxysmal sneezing, clear watery mucus, nasal itching, and nasal congestion.1 Although AR is not a fatal condition, it significantly impairs individuals’ quality of life, imposes a substantial economic burden on both patients and society, and has emerged as a significant public health concern.2,3 It is estimated that AR affects between 10% and 40% of the global population.4 The direct health expenditure associated with AR is estimated to range from $2 billion to $5 billion annually, with additional productivity losses of up to $2 billion to $4 billion per year.2 Moreover, the incidence of AR is alarmingly increasing over time.5 AR is characterized by a propensity for recurrence and is closely associated with other diseases such as asthma, chronic rhinosinusitis, and allergic conjunctivitis.6 The coexistence of AR, chronic rhinosinusitis, and asthma is common, with up to 40% of AR patients reporting symptoms of asthma and up to 80% of individuals with asthma experiencing symptomatic AR.7 Additionally, 34–50% of patients with sinusitis suffer from asthma, while 84% of asthma patients experience sinusitis, particularly during asthma onset.8 Furthermore, a remarkable 88.4% of patients with allergic conjunctivitis develop AR, and the severity and duration of conjunctivitis are significantly correlated with the severity and duration of rhinitis across both children and adults.9 The mechanism behind these comorbidities can be explained by the single airway theory and upper airway remodeling.10
It is now widely accepted that the development of AR is influenced by interactions between genetic factors and the environment.1 Among various environmental factors, air pollution is one of the most prevalent forms of pollution.11,12 Particulate matter 10 (PM10) are coarse particles of diameter 10 micrometers or less, and fine particles with a diameter of 2.5 micrometers or less are particulate matter 2.5 (PM2.5). The World Health Organization (WHO, 2014) estimated that 7 million people died in 2012 due to air pollution, primarily due to cardiovascular and respiratory ailments. Air pollution has been proven to be associated with both acute and chronic respiratory diseases. Hospital admissions or emergency room visits for pneumonia were increased by PM2.5 levels in East Asia.13 Asthma exacerbations were associated with air pollution.14,15 Lung function, emphysema prevalence, chronic obstructive pulmonary disease (COPD) progression, and lung cancer incidence rates were all negatively impacted by long-term exposure to air pollution.16–20
Although air quality in China has improved significantly in recent years, the annual concentration of inhalable particulates greatly exceeds WHO guidelines.21,22 Critically, Recent research suggests that air pollution can exacerbate the occurrence and development of AR.23,24 Air pollution exposure over an extended period, due to transportation and industrial activity, can impair the immune system, promote inflammation, and increase the chances of developing AR and asthma.25 However, there is still no unified conclusion on which components have an impact on the incidence rate of AR. Therefore, we aim to investigate the correlation between short-term air pollution exposure in Hangzhou and the number of outpatient AR patients to identify the specific components of air pollution that contribute to changes in the short-term incidence rate of AR.
Materials and Methods
We conducted a retrospective study in Hangzhou, in which we matched air pollution data from Hangzhou with the number of patients diagnosed with AR at Hangzhou Normal University Affiliated Hospital. The aim of the study was to investigate the relative risk factors of air pollution components and the incidence of AR, as well as to identify which specific components have an impact on the number of AR cases. This study was approved by the Ethics Committee of Affiliated Hospital of Hangzhou Normal University (no. 2022-(E2)-KS-104) and was granted an exemption from obtaining informed consent from the patients involved.
Air Pollution and Meteorological Data
From January 1, 2019 to December 31, 2021, the Hangzhou Meteorological Bureau released daily air quality data for PM2.5, PM10, and carbon monoxide (CO) concentrations. Also, daily average temperature and humidity data were downloaded from the China Meteorological Administration website (http://data.cma.cn/). The daily concentrations are represented as 24 h averages (Supplementary Table 1).
AR Visits per Day
Between 2019 and 2021, we obtained the number of AR-related outpatient visits from the Department of Otolaryngology, Affiliated Hospital of Hangzhou Normal University. This tertiary care general hospital is located in the central city of Hangzhou. The study population comprised all outpatients with AR who were examined by an otolaryngologist during the study period. Records provided visit date, age, gender, and diagnosis code information (Supplementary Table 2). Only one visit/patient/day was gathered to prevent double counting. According to the latest diagnostic standards, the diagnostic program was written in R (The R Foundation R Foundation for Statistical Computing, Vienna, Austria) to re-diagnose outpatient information recorded in the hospital outpatient database. The process of incorporating daily medical data of patients with AR, as well as corresponding air pollution and meteorological data, into the study is shown in Figure 1.
 |
Figure 1 Flow chart showing outpatient visit inclusion criteria and daily air pollution information. |
Statistical Methods
A GAM was used for statistical analysis to examine all data. The GAM used quasi-Poisson regression as daily outpatient visits generally followed an over-dispersed Poisson distribution. To account for potential confounding effects, a number of covariates, such as mean daily temperature and mean daily humidity, were included. Firstly, unmeasured long-term and seasonal trends of > 2 months and a natural cubic regression smoothing function with seven degrees of freedom (df) per year for calendar time, were excluded. Secondly, according to minimum Akaike information criterion (AIC) model principles, nonlinear confounding effects for weather were controlled by natural smoothing functions for mean temperature (6 df) and relative humidity (4 df). Thirdly, public holidays and “day of the week” indicator variables were implemented. Briefly, the estimated log relative rate of pollution for the city was obtained by fitting the following log-linear GAM:
Where E(Yt) is the expected number of outpatients with AR on day t; β is the log correlation rate of AR associated with a unit increase in air pollutants; Zt is the pollutant concentration on day t; the DOW Jones index is a dummy variable for a day of the week; and ns is a natural cubic regression smoothing function.
After basic model development, a single pollutant model was initially used and introduced, and the concentrations of each pollutant on the same day (lag0) were presented in turn. To validate model stability, and considering that the health effects of ambient air pollutants may have lasted for several days, additional single lag days were used (lag0, lag1, lag2, lag3, lag4, lag5, lag6, and lag7) and moving average exposures were obtained for multiple days (lag0–1, lag0–2, lag0–3, lag0–4, lag0–5, lag0–6, and lag0–7). The stability of impact estimations following changes for shared contaminants was then examined using two pollutant models.
All outpatients were stratified by age (< 18 years and ≥ 18 years) and sex as behavioral patterns and common diseases may have differed.26,27 Results were expressed as relative risks (RR) and 95% confidence intervals (CI) associated with a 10 μg/m3 increase in PM2.5. Statistical tests were two-sided, and effects with a p value < 0.05 were 95% statistically significant. All statistical models were constructed using R software version 3.6.0 (R Foundation for Statistical Computing, version 3.6.1; http://www.Rproject.org) in the MGCV package.
Results
Basic Research Data
Basic information on outpatient visits, pollutants, and weather data are provided (Table 1). In total, 20,653 AR outpatient visits were recorded between January 1, 2019 and December 31, 2021, with 10,998 male and 9655 female patients. Overall, the mean number of daily outpatient visits was 16.34, with minors (< 18 years) accounting for 35% (5.73 visits) and males for 55% (8.93 visits). The daily mean air PM2.5, PM10, and CO concentrations during the study period were 42.21 µg/m3, 61.69 µg/m3, and 24.39 µg/m3, respectively. Daily mean PM2.5 levels were greater than the national secondary air pollution limit (35 µg/m3). Also, the daily average temperature in Hangzhou was 18.2°C and the average relative humidity was 72.84%.
 |
Table 1 Descriptive Statistics Showing Daily Allergic Rhinitis Outpatient Visits, Air Pollutant Concentrations, and Weather Conditions |
Analysis results of Single Day Lag Model
The single-day lagged model findings for age and gender are shown (Table 2). We accounted for the fact that effects were delayed when compared with exposure. The model included a delay (lag). For instance, lag1 indicated an exposure on a day prior to the resulting incident. Overall, in terms of AR, for PM10, the whole group lag was 0, 2–4, male lag was 0, 4, female lag was 1, 2, adult lag was 0, 2, and non-adult lag was 1–4. For PM2.5, a lag between 0–1, 4 was identified for the whole group, a lag between 0–1 was identified for males, a lag between 1–2, 6–7 was identified for females, a lag of 1, 7 was identified for adults, and a lag of 1, 4 was identified for non-adults. For CO, the whole group lag was 0, 2, the male lag was 4, the female lag was 4, the adult lag was 2, and the non-adult lag was 4.
 |
Table 2 Relative Risks of Allergic Rhinitis-Associated Outpatient Visits for PM10, PM2.5, and CO at Different Single Lag Days, with 95th Percentiles |
Analysis Results of Cumulative Exposure Model
Age and gender subgroup analysis results in cumulative exposure models (lag00–lag07) are shown (Figure 2). Statistically significant results were observed at lag 01–07 for PM10 and PM2.5 and lag 03–07 for carbon monoxide (CO). The impact increased as the lag time increased. Connections between male and female groupings did not differ significantly. Associations for PM10, PM2.5, and CO were stronger in minors (< 18 years) at different lag days when compared with adults.
Response curves for outpatient visits, air pollutants (PM2.5, PM10, and CO), and single-day lag times for patients with AR are shown (Figure 3). From graphs, we identified a positive correlation between RR of AR and pollutant concentrations; no positive correlation was observed with a single-day lag time. Different air pollutants produced different irritation peaks in patients. PM10 had the greatest impact on outpatient visits for AR after 1 day of generation; PM2.5 had the highest RR on the day of generation and after 7 days of generation; while CO displayed the greatest irritation in patients with AR at 4 days of cumulative exposure.
 |
Figure 3 Ratio risks of daily outpatient visits for allergic rhinitis with the concentration of air pollutants and lag days ((A) for PM10, (B) for PM2.5, and (C) for CO). |
Results of Two-Pollutant Model
Table 3 presents the results of the two-pollutant models at lag 0. It was observed that the effects of all three pollutants substantially attenuated and became less statistically significant when adjusted for co-pollutants. The association between outpatient visits and PM10, as well as CO, slightly decreased but remained significant after adjustment for PM10 (1.317, 95% CI: 1.118–1.717) and CO (1.209, 95% CI: 1.018–1.347) respectively. However, the associations of PM10 and CO lost statistical significance after controlling for CO and PM2.5, respectively.
 |
Table 3 Relative Risks of Allergic Rhinitis Outpatient Visits Associated with a 10 µg/m3 (PM10, PM2.5, and CO) Increase in Pollutant Concentrations in Two-Pollutant Models |
Discussion
Our study presents evidence of the detrimental impact of background air pollution on AR in the Hangzhou region. We investigated the lag effects of air pollutants and potential differences in gender and age among daily outpatients receiving AR treatment at a hospital over a 3-year period. Our results revealed a positive and delayed correlation between air pollution and the frequency of AR outpatient visits. As air pollution levels increased, the RR of AR in outpatients also increased.
The effects of PM on respiratory disorders have been investigated by several studies.23,24,28–30 PM2.5 are airborne particles with an aerodynamic equivalent diameter of 2.5 microns or smaller. They reside for a long time in the atmosphere and are transported over vast distances. Because particle sizes are small enough to pass through fine bronchi and alveoli, PM2.5 has become one of the major causes of lower respiratory disease. PM10 only reaches the upper respiratory tract due to its larger diameter. From animal studies, PM2.5 exposure was shown to cause ciliary epithelial destruction, cupped cell proliferation, and increased mucin MUC5AC secretion from the nasal mucosa of AR rats. PM2.5 also inhibited interferon-γ (IFN-γ) production, suppressed Th1 responses, but enhanced Th2 cell-dominated immune responses, such as increased interleukin-4 (IL-4), IL-13, IL-6 levels, and other pro-inflammatory factor release.31,32 In human nasal epithelial cells, PM2.5 impaired epithelium barrier function and caused cellular oxidative stress and inflammation. Epigenetically, PM2.5 upregulated GATA-3 mRNA expression in AR rat nasal mucosa, whereas T-bet was significantly downregulated.31 Prolonged exposure to PM2.5 levels positively correlated with IFN-γ promoter methylation levels, possibly through the ERK-DNMT pathway.33 PM2.5 also caused significant changes in nasal mucosal miRNA expression in AR rats;32 the expression of eight microRNAs were consistent with microRNA gene microarray results and were shown to have crucial roles regulating PM2.5 effects on the biological behavior and mucosal inflammation in AR rats.32 PM may also enhance organism sensitivity to airborne allergens. A Japanese study confirmed that PM2.5 increased organism sensitivity to pollen.34 A Hangzhou study reported that PM2.5 and PM10 were aggravating factors for dust mite sensitization.35 This may have been due to increased allergens deposited in airways via particle transport, increased epithelial permeability by oxidative damage, and increased antigenicity and adjuvant actions of chemically modified proteins.36
We observed that PM2.5 had stronger pathogenic effects on AR when compared with PM10. When compared with PM10, PM2.5 had a higher surface area ratio, was more active, and carried more poisonous and hazardous materials, such as heavy metals and germs. Also, PM2.5 could enter deeper into the lower respiratory tract and alveoli, making it more difficult to excrete, and causing more intense and sustained oxidative stress when compared with PM10. From previous toxicological studies, when compared with PM10, PM2.5 induced M1 inflammatory phenotypes, which decreased phagocytosis and increased macrophage apoptosis. This was manifested as increased tumor necrosis factor- and M1-related gene expression (inducible nitric oxide synthase) and decreased IL-10 and M2 phenotype gene expression (arginase).37
Our study suggested that children were more affected by air pollution. AR pathogenesis in children is not significantly different to adults. Studies have reported that air pollutants are the main pollutants in the respiratory systems of children.30 Firstly, children weigh less than adults, they have smaller airway calibers, and smaller respiratory surface areas. Also, children breathe 50% more air per kilogram of body weight. A study shows that 3-month old infants exhibited 4-fold higher inhalation (PM2.5 and PM10) doses than their mothers.38 Also, the number of particles deposited per unit of frontal surface area was 4–5 times higher in children. Secondly, children’s lungs are immature at birth and do not develop until approximately 6 years old. Such lung epithelium immaturity during infancy facilitates toxins and other substances from atmospheric pollution crossing the epithelial barrier. Thirdly, the immune system is immature at birth. Studies have shown that PM disrupt intrapulmonary homeostasis in the innate immune system by affecting epithelial functions in the lung, macrophages, pattern recognition receptors, and the microbiota.37 Fourthly, distinct to adults, a child’s respiratory zone is located closer to the ground and is limited by height. Environmental pollutants from transportation are emitted near the ground and then diffused. Air pollutant concentrations associated with traffic pollution are highest closer to the ground and this increases children’s exposure to ambient air pollutants.39 Combined, these factors may explain why PM made children more prone to developing AR when compared with adults.
CO had a significant effect on increased AR outpatient visits; these effects were greater in preadolescent children when compared with adults. Currently, CO relevance to AR is unclear. So far, CO does not appear to have a significant impact on the incidence rate of AR.24,40 However, a 100 parts per billion increase in daily CO levels in children within a year of birth had a lifetime RR of 1.10 for AR. A previous study supported this view and suggested that children under 15 years of age may be more vulnerable to CO.41 This concurred with our data. Therefore, the relevance of CO to AR remains to be fully characterized.
In urban environments, the main CO sources are derived from the incomplete combustion of carbon-containing fuels and photochemical atmospheric reactions. CO, which is frequently found in indoor and outdoor settings, significantly contributes to air pollution via transportation, tobacco smoke, and gas stove emissions. Due to CO poisoning, reactive oxygen species are produced, different organs receive less oxygen, and cardiac ion channels are impacted. Critically, even at non-toxic levels, the impact is still evident. A recent study proposed a connection between reactive oxygen species formation and childhood allergy illnesses such as asthma and AR, and identified higher malondialdehyde levels in nasal and oral samples from children with asthma or AR when compared with healthy controls. This could be why CO-related AR is getting worse.31 Further studies are required to unravel how CO and AR interact.
When compared with previous studies, firstly, our study period was up to 3 years and included the time since the outbreak of the COVID-19 pandemic, which reflected a new situation in terms of the impact of air pollution on AR; and secondly, we selected Hangzhou, a typical tourist city, as the study location, as it provided a good sampling area for observing air pollution at low concentrations.
Additionally, our study had several drawbacks. Potential mixed factors, such as family allergy history, smoking habits, pet-breeding behaviors, and drug-use history may have affected relationships between air pollutants and AR. Secondly, the number of outpatient clinics at a single hospital does not represent levels in a large city. Then, in the context of the COVID-19 pandemic, people pay more attention to personal protection such as wearing masks, which can reduce the impact of air pollutants on AR to a certain extent. In the end, the chemical composition of PM was not analyzed in this study, which makes it challenging to precisely identify a single emission source. If specific public health actions are required to regulate emission sources, further comprehensive investigations on chemical composition (ions, elements, black carbon, and organic carbon) and its impact on AR are required.
There are some limitations to this study. Firstly, it is a retrospective study that can only establish a correlation between air pollution and the number of outpatient visits for AR based on data analysis, but it cannot determine a causal relationship between the two factors. Secondly, this study was conducted at a single center, and the data included may not fully represent outpatient visits for AR in Hangzhou. Finally, the study period coincided with the outbreak of a new epidemic, during which public health measures such as travel restrictions and mask-wearing were implemented, which may have impacted the incidence rate of AR.
Conclusion
We suggest that air pollution, specifically PM2.5, PM10, and O3, is correlated with the number of outpatient visits for AR in Hangzhou, particularly among children. We recommend implementing environmental control measures and public health strategies to address this growing issue.
Abbreviations
AR, allergic rhinitis; PM10, Particulate matter 10; PM2.5, particulate matter 2.5; WHO, The World Health Organization; NO2, Nitrogen dioxide; SO2, sulfur dioxide; O3, ozone; COPD, chronic obstructive pulmonary disease; FVC, forced vital capacity; FEV1, forced expiratory volume in the first second; PEF, peak expiratory flow; GAM, generalized additive model; CO, carbon monoxide; AIC, Akaike information criterion; CI, confidence intervals.
Data Sharing Statement
The data for this study can be made available from the corresponding author on reasonable request.
Ethics Approval
The study was approved by the Ethics Committee of Affiliated Hospital of Hangzhou Normal University (no. 2022-(E2)-KS-104) and complied with the Declaration of Helsinki. The study was granted exemption from informed patient consent. Because this study was retrospective in nature, many patients could not be contacted. We declare that the patient’s name, gender, age, address, and other information will be kept confidential. We have processed the data for privacy, and any identifiable information would not appear in the records of images, text, or photos.
Acknowledgments
We acknowledge the Affiliated Hospital of Hangzhou Normal University and Hangzhou Zhenqi Technology Co., Ltd. for providing us with necessary data to realize this research. The research work was funded by the Affiliated Hospital of Hangzhou Normal University (no. 2021YN2021121).
Disclosure
The authors report no potential conflicts of interest in this work.
References
1. Seidman MD, Gurgel RK, Lin SY, et al. Clinical practice guideline: allergic rhinitis. Otolaryngol Head Neck Surg. 2015;152(1 Suppl):S1–S43. doi:10.1177/0194599814561600
2. Blaiss MS. Allergic rhinitis: direct and indirect costs. Allergy Asthma Proc. 2010;31(5):375–380. doi:10.2500/aap.2010.31.3329
3. Li X, Xu X, Li J, et al. Direct and indirect costs of allergic and non-allergic rhinitis to adults in Beijing, China. Clin Transl Allergy. 2022;12(4):e12148. doi:10.1002/clt2.12148
4. Brożek JL, Bousquet J, Agache I, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines-2016 revision. J Allergy Clin Immunol. 2017;140(4):950–958. doi:10.1016/j.jaci.2017.03.050
5. Bousquet J, Anto JM, Bachert C, et al. Allergic rhinitis. Nat Rev Dis Primers. 2020;6(1):95. doi:10.1038/s41572-020-00227-0
6. Greiner AN, Hellings PW, Rotiroti G, Scadding GK. Allergic rhinitis. Lancet. 2011;378(9809):2112–2122. doi:10.1016/s0140-6736(11)60130-x
7. Leynaert B, Neukirch C, Kony S, et al. Association between asthma and rhinitis according to atopic sensitization in a population-based study. J Allergy Clin Immunol. 2004;113(1):86–93. doi:10.1016/j.jaci.2003.10.010
8. Marseglia GL, Caimmi S, Marseglia A, et al. Rhinosinusitis and asthma. Int J Immunopathol Pharmacol. 2010;23(1 Suppl):29–31. doi:10.1177/03946320100230S108
9. Sánchez-Hernández MC, Dordal MT, Navarro AM, et al. Severity and duration of allergic conjunctivitis: are they associated with severity and duration of allergic rhinitis and asthma?. Eur Ann Allergy Clin Immunol. 2022;54(6):277–283. doi:10.23822/EurAnnACI.1764-1489.231
10. Samitas K, Carter A, Kariyawasam HH, Xanthou G. Upper and lower airway remodelling mechanisms in asthma, allergic rhinitis and chronic rhinosinusitis: the one airway concept revisited. Allergy. 2018;73(5):993–1002. doi:10.1111/all.13373
11. Hassan Bhat T, Jiawen G, Farzaneh H. Air Pollution Health Risk Assessment (AP-HRA), principles and applications. Int J Environ Res Public Health. 2021;18(4):1935. doi:10.3390/ijerph18041935
12. Santos UP, Arbex MA, Braga ALF, et al. Environmental air pollution: respiratory effects. J Bras Pneumol. 2021;47(1):e20200267. doi:10.36416/1806-3756/e20200267
13. Yee J, Cho YA, Yoo HJ, Yun H, Gwak HS. Short-term exposure to air pollution and hospital admission for pneumonia: a systematic review and meta-analysis. Environ Health. 2021;20(1):6. doi:10.1186/s12940-020-00687-7
14. Khreis H, Kelly C, Tate J, Parslow R, Lucas K, Nieuwenhuijsen M. Exposure to traffic-related air pollution and risk of development of childhood asthma: a systematic review and meta-analysis. Environ Int. 2017;100:1–31. doi:10.1016/j.envint.2016.11.012
15. Nejjari C, Marfak A, Rguig A, et al. Ambient air pollution and emergency department visits among children and adults in Casablanca, Morocco. AIMS Public Health. 2021;8(2):285–302. doi:10.3934/publichealth.2021022
16. Liu S, Jørgensen JT, Ljungman P, et al. Long-term exposure to low-level air pollution and incidence of chronic obstructive pulmonary disease: the ELAPSE project. Environ Int. 2021;146:106267. doi:10.1016/j.envint.2020.106267
17. Wang M, Aaron CP, Madrigano J, et al. Association between long-term exposure to ambient air pollution and change in quantitatively assessed emphysema and lung function. JAMA. 2019;322(6):546–556. doi:10.1001/jama.2019.10255
18. Hvidtfeldt UA, Severi G, Andersen ZJ, et al. Long-term low-level ambient air pollution exposure and risk of lung cancer - A pooled analysis of 7 European cohorts. Environ Int. 2021;146:106249. doi:10.1016/j.envint.2020.106249
19. Park J, Kim HJ, Lee CH, Lee CH, Lee HW. Impact of long-term exposure to ambient air pollution on the incidence of chronic obstructive pulmonary disease: a systematic review and meta-analysis. Environ Res. 2021;194:110703. doi:10.1016/j.envres.2020.110703
20. Zhang W, Ma R, Wang Y, Jiang N, Zhang Y, Li T. The relationship between particulate matter and lung function of children: a systematic review and meta-analysis. Environ Pollut. 2022;309:119735. doi:10.1016/j.envpol.2022.119735
21. Zhang Q, Zheng Y, Tong D, et al. Drivers of improved PM(2.5) air quality in China from 2013 to 2017. Proc Natl Acad Sci U S A. 2019;116(49):24463–24469. D - 7505876. (- 1091-6490 (Electronic)):T - ppublish.
22. Liu S, Yang X, Duan F, Zhao W. Changes in air quality and drivers for the heavy PM(2.5) pollution on the North China Plain Pre-to Post-COVID-19. Int J Environ Res Public Health. 2022;19(19):12904. D - 101238455. (- 1660-4601 (Electronic)):T - epublish.
23. Deng Q, Lu C, Yu Y, Li Y, Sundell J, Norbäck D. Early life exposure to traffic-related air pollution and allergic rhinitis in preschool children. Respir Med. 2016;121:67–73. doi:10.1016/j.rmed.2016.10.016
24. Hao S, Yuan F, Pang P, Yang B, Jiang X, Yan A. Early childhood traffic-related air pollution and risk of allergic rhinitis at 2–4 years of age modification by family stress and male gender: a case-control study in Shenyang, China. Environ Health Prev Med. 2021;26(1):48. doi:10.1186/s12199-021-00969-7
25. Vawda S, Mansour R, Takeda A, et al. Associations between inflammatory and immune response genes and adverse respiratory outcomes following exposure to outdoor air pollution: a HuGE systematic review. Am J Epidemiol. 2014;179(4):432–442. doi:10.1093/aje/kwt269
26. Schuler Iv CF, Montejo JM. Allergic rhinitis in children and adolescents. Immunol Allergy Clin North Am. 2021;41(4):613–625. doi:10.1016/j.iac.2021.07.010
27. Hong SN, Won JY, Nam EC, et al. Clinical manifestations of allergic rhinitis by age and gender: a 12-year single-center study. Ann Otol Rhinol Laryngol. 2020;129(9):910–917. doi:10.1177/0003489420921197
28. Huang Y, Zhu M, Ji M, et al. Air pollution, genetic factors, and the risk of lung cancer: a prospective study in the UK Biobank. Am J Respir Crit Care Med. 2021;204(7):817–825. doi:10.1164/rccm.202011-4063OC
29. Bălă GP, Râjnoveanu RM, Tudorache E, Motișan R, Oancea C. Air pollution exposure-the (in)visible risk factor for respiratory diseases. Environ Sci Pollut Res Int. 2021;28(16):19615–19628. doi:10.1007/s11356-021-13208-x
30. Heinrich J, Slama R. Fine particles, a major threat to children. Int J Hyg Environ Health. 2007;210(5):617–622. doi:10.1016/j.ijheh.2007.07.012
31. Guo ZQ, Dong WY, Xu J, et al. T-helper type 1-T-helper type 2 shift and nasal remodeling after fine particulate matter exposure in a rat model of allergic rhinitis. Am J Rhinol Allergy. 2017;31(3):148–155. doi:10.2500/ajra.2017.31.4437
32. Huang Y, Guo ZQ, Zhang RX, et al. Effect of PM2.5 on microRNA expression and function in nasal mucosa of rats with allergic rhinitis. Am J Rhinol Allergy. 2020;34(4):543–553. doi:10.1177/1945892420912367
33. Li Y, Zhou J, Rui X, Zhou L, Mo X. PM2.5 exposure exacerbates allergic rhinitis in mice by increasing DNA methylation in the IFN-γ gene promoter in CD4+T cells via the ERK-DNMT pathway. Toxicol Lett. 2019;301:98–107. doi:10.1016/j.toxlet.2018.11.012
34. Konishi S, Ng CF, Stickley A, et al. Particulate matter modifies the association between airborne pollen and daily medical consultations for pollinosis in Tokyo. Sci Total Environ. 2014;499:125–132. doi:10.1016/j.scitotenv.2014.08.045
35. Ye Q, Zhang T, Mao JH. Haze facilitates sensitization to house dust mites in children. Environ Geochem Health. 2020;42(7):2195–2203. doi:10.1007/s10653-019-00481-6
36. Fukuoka A, Yoshimoto T. Barrier dysfunction in the nasal allergy. Allergol Int. 2018;67(1):18–23. doi:10.1016/j.alit.2017.10.006
37. Rahmani H, Sadeghi S, Taghipour N, et al. The effects of particulate matter on C57BL/6 peritoneal and alveolar macrophages. Iran J Allergy Asthma Immunol. 2020;19(6):647–659. doi:10.18502/ijaai.v19i6.4934
38. Madureira J, Slezakova K, Silva AI, et al. Assessment of indoor air exposure at residential homes: inhalation dose and lung deposition of PM(10), PM(2.5) and ultrafine particles among newborn children and their mothers. Sci Total Environ. 2020;717:137293. doi:10.1016/j.scitotenv.2020.137293
39. Gao J, Qiu Z, Cheng W, Gao HO. Children’s exposure to BC and PM pollution, and respiratory tract deposits during commuting trips to school. Ecotoxicol Environ Saf. 2022;232:113253. doi:10.1016/j.ecoenv.2022.113253
40. Kim SH, Lee J, Oh I, et al. Allergic rhinitis is associated with atmospheric SO2: follow-up study of children from elementary schools in Ulsan, Korea. PLoS One. 2021;16(3):e0248624. doi:10.1371/journal.pone.0248624
41. Wang J, Lu M, An Z, et al. Associations between air pollution and outpatient visits for allergic rhinitis in Xinxiang, China. Environ Sci Pollut Res Int. 2020;27(19):23565–23574. doi:10.1007/s11356-020-08709-0
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.