Back to Journals » Neuropsychiatric Disease and Treatment » Volume 15
Exploration of Treatment-Resistant Schizophrenia Subtypes Based on a Survey of 204 US Psychiatrists
Authors Correll CU, Brevig T, Brain C
Received 16 October 2019
Accepted for publication 21 November 2019
Published 19 December 2019 Volume 2019:15 Pages 3461—3473
DOI https://doi.org/10.2147/NDT.S234813
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Roger Pinder
Christoph U Correll,1–3 Thomas Brevig,4 Cecilia Brain4
1The Zucker Hillside Hospital, Department of Psychiatry, Glen Oaks, NY, USA; 2The Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, Department of Psychiatry and Molecular Medicine, Hempstead, NY, USA; 3Charité-Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Department of Child and Adolescent Psychiatry, Berlin, Germany; 4H. Lundbeck A/S, Copenhagen, Denmark
Correspondence: Christoph U Correll
The Zucker Hillside Hospital, Psychiatry Research, 75–59 263rd Street, Glen Oaks, NY 11004, USA
Tel +1 718 470-4812
Fax +1 718 343-1659
Email [email protected]
Objective: To explore and describe potential subgroups within the treatment-resistant schizophrenia (TRS) population, using data from a survey of US psychiatrists.
Methods: Psychiatrists completed an online survey of demographic/clinical characteristics and treatment history for two of their patients with TRS. Patients were stratified according to number of suicide attempts, number of hospitalizations, employment status, and TRS onset time frame.
Results: Of the 408 patients with TRS described by psychiatrists, 37.5% had ≥1 suicide attempt, 78.9% had ≥2 hospitalizations, 74.5% were unemployed, 45.0% had TRS onset within 5 years of first treatment (a further 8.0% had TRS from first treatment), and 31.5% had TRS onset after 5 years (15.5% unknown). Patients with ≥1 (vs 0) suicide attempts had statistically significantly more psychiatric (3.6 vs 2.2) and physical (2.2 vs 1.6) comorbidities. Patients with ≥2 (vs ≤1) hospitalizations were statistically significantly more likely to have hallucinations, conceptual disorganization, social withdrawal, and cognitive dysfunction, and had more psychiatric (3.0 vs 1.9) and physical (2.0 vs 1.1) comorbidities. Unemployed (vs employed) patients were statistically significantly more likely to have delusions, hallucinations, blunted affect, social withdrawal, and cognitive dysfunction, and had more psychiatric (2.9 vs 2.3) and physical (2.1 vs 1.2) comorbidities. Patients with TRS onset ≤5 (vs >5) years were statistically significantly younger (35.0 vs 43.7 years), less likely to have hallucinations and social withdrawal, and had fewer psychiatric (2.6 vs 3.3) and physical (1.7 vs 2.3) comorbidities.
Conclusions: Greater clinical burden in TRS is associated with greater illness severity and chronicity markers, suggesting a dimensional gradient from non-TRS to mild–moderate and more severe forms of TRS. Time to onset of TRS may have implications for outcomes, with data indicating greater burden in those with late-onset TRS. Accumulation of illness over time may be more important than time to onset.
Keywords: clinical burden, demography, psychiatry, schizophrenia, surveys and questionnaires, treatment resistance
Introduction
Despite the availability of many antipsychotic drugs, a considerable proportion of patients suffering from schizophrenia do not achieve response with antipsychotic treatment.1,2 Treatment-resistant schizophrenia (TRS) is defined in clinical guidelines as an inadequate response in target schizophrenia symptoms following treatment with two or more antipsychotics of adequate dose and duration.3–6 Patients with schizophrenia and treatment resistance have a higher burden of disease and face poorer outcomes than those without treatment resistance.7 TRS has a high societal and economic burden, and a high caregiver burden.8–11
TRS is itself heterogeneous: some people with schizophrenia experience treatment resistance from the onset of disease, whereas others develop resistance over time.12,13 Studies in first-episode psychosis show that around 13–23% of the patients are treatment resistant from the onset of disease.12–15 Across all patients with schizophrenia, a recent pooled analysis (n=6221) found that non-response rates vary from 20%–87% according to the definition of non-response,16 a higher range than the “up to 30%” of patients having treatment resistance that is commonly quoted (without firm evidence) in scientific literature.3,17 The prevalence of treatment resistance also varies among schizophrenia cohorts, being relatively high in hard-to-treat patients in a rehabilitation service (45%) and in hospitalized patients (49%).18,19
Despite the assumed and apparent heterogeneity of TRS, little research is available examining differences in demographic/clinical characteristics and symptoms among TRS subgroups. One study split patients with TRS (n=147) into those with and without dopamine supersensitivity psychosis (associated with long-term antipsychotic treatment), finding no statistically significant differences in demographic characteristics and only minor differences in clinical characteristics between subgroups.20 Similarly, a study (n=80) that compared early-onset TRS (defined as ≤6 months from first symptoms) with late-onset TRS found no statistically significant differences between subgroups in terms of sociodemographic or clinical characteristics, other than a greater proportion of males in the early-onset TRS subgroup.12 In contrast, a multivariable regression analysis (n=74) found that greater severity of negative symptoms, younger age at symptom onset, and longer duration of untreated psychosis (DUP) predicted treatment resistance from illness onset over delayed-onset treatment resistance.13
It is important to investigate and describe possible subtypes of TRS in order to optimize the diagnosis and treatment of these patients. Using data from a survey of psychiatrists in the US, the aim of this analysis was to explore and describe potential subgroups within the TRS population from the viewpoint of psychiatrists treating such patients.
Materials and Methods
Survey Design and Participants
This study comprised a 45-min online survey with psychiatrists in the US. The survey was conducted in accordance with Market Research Society (MRS) and Council of American Survey Research Organizations (CASRO) guidelines,21,22 and in accordance with the principles of the Declaration of Helsinki. Formal ethics approval was not required for this US market research study.23 Participating psychiatrists gave digital written informed consent prior to starting the questionnaire. De-identified patient data were used and the need for patient consent was waived.
The survey methodology has been previously published.7 In brief, a mix of hospital-/office-based and public/private psychiatrists were approached. Psychiatrists were eligible if they had been qualified for ≥3 years, were actively treating patients with TRS, were seeing ≥50 patients with schizophrenia per month, had ≥5 patients with TRS in their current caseload, prescribed atypical antipsychotics, were not employed by a pharmaceutical company, and had not participated in any schizophrenia market research in the last month. Eligible psychiatrists self-selected patient records and completed the survey for two of their patients with TRS (and one with non-TRS, not included in the present analysis). Half of the psychiatrists were asked to select patient records based on their own “spontaneous” definition of TRS, whereas the other half were asked to select patient records based on a “prompted” definition of TRS, adapted from treatment guidelines.3–6 As described previously,7 the two TRS subpopulations (spontaneous and prompted definitions) were similar and thus pooled into a single TRS group.
The survey collected demographic and clinical characteristics, and treatment history, for each patient.
Statistical Analyses
The following three stratifiers, chosen to reflect important outcomes that have strong face validity and clinical relevance, were investigated in the present analysis: 1) number of suicide attempts: “≥1” versus “0”; 2) number of hospitalizations: “≥2” versus “≤1”; 3) employed/in education (including part- and full-time work, studying, and volunteering): “no” versus “yes”. Additionally, a fourth stratifier was investigated to test the hypothesis that the timing of onset of TRS may have relevance for outcomes: 4) TRS onset time frame: “from first treatment” (patients classified as having TRS from their first treatment trial), “≤5 years” (patient classified as having TRS from their first relapse or within the first 5 years of treatment), versus “>5 years” (patient classified as having TRS after more than 5 years of treatment). Full subgroup definitions are given in Table S1 of the online supplement to this article.
Statistical analyses were performed using Quantum v5.8 (IBM). Outcomes were summarized as means (with standard deviations) or percentages. Time between symptom onset and schizophrenia diagnosis (a proxy for DUP) was summarized by the median and interquartile range (IQR). Pairwise comparisons between subgroups were calculated using the column means test, except DUP, where the Mann–Whitney U-test was used. All tests were two-sided, and alpha was set at 0.05 without correction for multiple testing.
In addition, a stepwise, backward elimination, multivariable logistic regression analysis was conducted, entering all demographic and clinical characteristic variables that were statistically significant in univariable analyses (p<0.05) into the initial model, and removing sequentially each least statistically significant variable until in the final model only independently statistically significant variables related to the outcome of interest were retained. The Wald chi-square test was used to determine if explanatory variables were statistically significant. Finally, to determine if differences in age and illness duration were key drivers, the logistic regression analysis was repeated with “age” and “age at symptom onset” as forced variables.
Results
Demographic and Clinical Characteristics
Overall, 204 psychiatrists completed a total of 408 TRS patient reports. On average, the psychiatrists had a time in practice of 16.3 ± 7.3 years and, over the past 6 months, a caseload of 229.0 ± 179.0 patients with schizophrenia including 67.6 ± 76.8 patients with TRS. The mean estimated proportion of schizophrenia patients that the psychiatrists saw in an outpatient setting was 82.1%.
Demographic and clinical characteristics for the total TRS population (N=408) have been previously published.7 The following text describes patient demographic and clinical characteristics by TRS subgroup.
One hundred and fifty-three patients with TRS (37.5%) had ≥1 suicide attempt, while 204 patients (50.0%) had 0 suicide attempts (a further 51 patients had an unknown number of suicide attempts). Patients with TRS and ≥1 suicide attempt, compared with 0 suicide attempts (Table 1, Figure 1), were statistically significantly more likely to live in a sheltered home and to have had contact with a social or case worker. Patients with ≥1 suicide attempt had a statistically significantly different DUP based on Mann–Whitney U-test rankings (although median values were the same), and were statistically significantly more likely to have psychiatric and physical comorbidities.
 |
Table 1 Patient Demographic and Clinical Characteristics by TRS Subgroup |
The majority of patients with TRS (n=322; 78.9%) had ≥2 hospitalizations, while 79 patients (19.4%) had ≤1 hospitalization (a further 7 patients had an unknown number of hospitalizations). Patients with TRS and ≥2 hospitalizations, compared with ≤1 hospitalization (Table 1, Figure 1), were statistically significantly more likely to be unemployed, to be single, and to live in a sheltered home. Patients with ≥2 hospitalizations were statistically significantly younger at symptom onset and diagnosis, had a longer time between schizophrenia diagnosis and TRS onset, and were more likely to have psychiatric and physical comorbidities.
Approximately three-quarters of patients with TRS were not employed/in education (n=304; 74.5%), while one-quarter (n=96; 23.5%) were employed/in education (a further 8 patients had unknown employment status). Patients with TRS who were unemployed, compared with patients who were employed (Table 1, Figure 1), were statistically significantly older, more likely to be single, and more likely to live in a sheltered home. Patients who were unemployed were statistically significantly younger at diagnosis, had a longer time between schizophrenia diagnosis and TRS onset, had more hospitalizations, and were more likely to have psychiatric and physical comorbidities.
The survey question on TRS onset was not asked in relation to 21 patient records, giving a sample size of 387 patients. Thirty-one patients (8.0%) had TRS from their first treatment trial, 174 patients (45.0%) had TRS onset within the first 5 years of treatment, and 122 patients (31.5%) had TRS onset after more than 5 years of treatment (a further 60 patients had unknown timing of TRS onset). Patients classified as having TRS from their first treatment trial, compared with onset after more than 5 years of treatment (Table 1, Figure 1), were statistically significantly younger, less likely to be unemployed, were older at symptom onset, and were less likely to have psychiatric comorbidities. Patients classified as having TRS from their first treatment trial were also statistically significantly older at symptom onset than patients classified as having TRS within the first 5 years of treatment (Table 1). Patients classified as having TRS within the first 5 years of treatment, compared with onset after more than 5 years of treatment (Table 1, Figure 1), were statistically significantly younger, less likely to be unemployed, more likely to live with a partner/family, less likely to live in a sheltered home, had fewer hospitalizations, and had fewer psychiatric and physical comorbidities.
Current Schizophrenia Symptoms
Patients with TRS and ≥1 suicide attempt showed few differences in symptomatology compared with patients with 0 suicide attempts (Table 2); however, cognitive dysfunction was statistically significantly less severe among patients with ≥1 suicide attempt.
 |
Table 2 Prevalence, Severity, and Frequency of Current Schizophrenia Symptoms by TRS Subgroup |
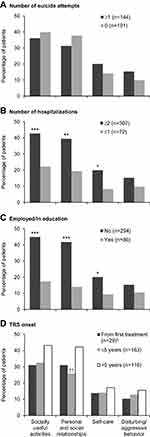
Patients with TRS and ≥2 hospitalizations, compared with ≤1 hospitalization, were statistically significantly more likely to have hallucinations, conceptual disorganization, social withdrawal, and cognitive dysfunction, and had more severe and more frequent positive and negative symptoms (Table 2). Symptoms were statistically significantly more likely to have had a marked to very severe impact on socially useful activities, personal and social relationships, and self-care (Figure 2).
Patients with TRS who were unemployed, compared with patients who were employed, were statistically significantly more likely to have delusions, hallucinations, blunted affect, social withdrawal, and cognitive dysfunction, and had more severe and more frequent positive and negative symptoms (Table 2). Symptoms were statistically significantly more likely to have had a marked to very severe impact on socially useful activities, personal and social relationships, and self-care (Figure 2).
Patients classified as having TRS from their first treatment trial, compared with patients classified as having TRS after more than 5 years of treatment, were statistically significantly less likely to have hostility or aggression and blunted affect (Table 2). Patients classified as having TRS from their first treatment trial were also statistically significantly less likely to have blunted affect than patients classified as having TRS within the first 5 years of treatment (Table 2). Patients classified as having TRS within the first 5 years of treatment were statistically significantly less likely to have hallucinations and social withdrawal compared with patients classified as having TRS after more than 5 years of treatment (Table 2), and their symptoms were statistically significantly less likely to have had a marked to very severe impact on personal and social relationships (Figure 2).
Treatment Patterns
Patients with TRS and ≥1 suicide attempt, compared with 0 suicide attempts (Table 3), were statistically significantly more likely to have received a long-acting injectable (LAI) antipsychotic, a mood stabilizer, and an anxiolytic. Patients with ≥1 suicide attempt were perceived to be less medication adherent, psychiatrists were more satisfied with their progress, and they had failed on more treatments before treatment resistance was identified.
 |
Table 3 Treatment Regimens by TRS Subgroup |
Patients with TRS and ≥2 hospitalizations, compared with ≤1 hospitalizations (Table 3), were statistically significantly more likely to be currently receiving antipsychotic combination therapy, and clozapine; more likely to have received (currently or previously) a typical antipsychotic, an LAI, a mood stabilizer, an antidepressant, and an anxiolytic; and were less likely to be currently receiving antipsychotic monotherapy, and aripiprazole. Patients with ≥2 hospitalizations were perceived to be more medication adherent, psychiatrists were less satisfied with their progress, and they had failed on more treatments before treatment resistance was identified.
Patients with TRS who were unemployed, compared with patients who were employed (Table 3), were statistically significantly more likely to be currently receiving clozapine; more likely to have received (currently or previously) an LAI, and an anxiolytic; and were less likely to be currently receiving aripiprazole. Psychiatrists were less satisfied with the progress of patients who were unemployed, and these patients had failed on more treatments before treatment resistance was identified.
Patients classified as having TRS from their first treatment trial were statistically significantly more likely to be currently receiving aripiprazole than patients classified as having TRS within the first 5 years or after more than 5 years of treatment (Table 3). Patients classified as having TRS within the first 5 years of treatment, compared with those classified as having TRS after more than 5 years of treatment (Table 3), were statistically significantly less likely to have received (currently or previously) a typical antipsychotic, were perceived to be less medication adherent, psychiatrists were more satisfied with their progress, and they had failed on fewer treatments before treatment resistance was identified.
Multivariable Logistic Regression Analysis Results
Multivariable logistic regression was conducted for the patient demographic and clinical characteristic variables in Table 1.
A history of ≥1 suicide attempt was independently associated with higher likelihood of living in a sheltered home (df=1, Wald χ2=11.3, p=0.0008) and a higher number of psychiatric comorbidities (df=1, Wald χ2=29.2, p<0.0001).
A history of ≥2 hospitalizations was independently associated with higher likelihood of being unemployed (df=1, Wald χ2=6.1, p=0.013), younger age of schizophrenia onset (df=1, Wald χ2=7.1, p=0.0078), and a higher number of psychiatric comorbidities (df=1, Wald χ2=11.9, p=0.0005).
Current unemployment was independently associated with higher likelihood of being single (df=1, Wald χ2=7.2, p=0.0072) and of living in a sheltered home (df=1, Wald χ2=9.7, p=0.0019), a higher number of hospitalizations (df=1, Wald χ2=5.2, p=0.023), and a higher number of physical comorbidities (df=1, Wald χ2=4.9, p=0.027).
Earlier TRS onset was independently associated with younger age (df=1, Wald χ2=32.7, p<0.0001), older age of schizophrenia onset (df=1, Wald χ2=17.8, p<0.0001), and a lower number of psychiatric comorbidities (df=1, Wald χ2=4.4, p=0.035).
In general, when the multivariable logistic regression analyses were repeated with “age” and “age at symptom onset” as forced variables, the same variables were statistically significant as described above. In the ≥1 suicide attempt subgroup, “never had contact with a social or case worker” and “DUP” were also statistically significant.
Discussion
This online survey revealed that, in general, patients with TRS with greater clinical burden (ie, more suicide attempts, more hospitalizations, unemployed) had greater illness severity and chronicity markers. This finding suggests that, not only is TRS a more severe form of schizophrenia,7 but that there is a gradient within the TRS population whereby patients with poorer outcomes have greater illness severity and chronicity. Whether this gradient will also reflect response or non-response to treatments with the strongest evidence-base for TRS, ie, clozapine (where the pooled non-response rate is as high as 60% according to the most recent meta-analysis24) and electroconvulsive therapy adjunct to clozapine (where the pooled non-response rate is 32–46%25), requires further study.
An exception to this “greater clinical burden equals greater illness severity” observation was that a history of suicide attempts was associated with statistically significantly less severe cognitive dysfunction. This observation has been previously reported in the literature, hypothesized to be because cognitive dysfunction impairs insight into the disorder.26–29
Another exception to the “greater clinical burden equals greater illness severity” observation occurred in the TRS onset analyses. Due to the small number of patients classified as having TRS from their first treatment trial, this subgroup is not discussed in detail, as the data are hard to interpret. Clearly, this subgroup of TRS requires further study and attention in studies with more patients. Considering the larger subgroups, patients with early-onset TRS (within 5 years) were found to have lower illness severity and chronicity compared with patients with late-onset TRS (after 5 years). This finding is counterintuitive, since patients with early-onset TRS might be expected to have poorer social skills, education, etc., because the non-responsive illness stage occurred earlier in the illness and in a period of life when developmental and social/educational milestones were still to be achieved. While this result was not explained by sex (other studies have shown that men have worse outcomes30), age at onset of schizophrenia, medication non-adherence (patients with late-onset TRS were perceived to be more adherent to their antipsychotic medication), or use of clozapine or LAIs (which did not statistically significantly differ between the two groups), the potential disadvantaging effect of earlier illness onset seems to be counteracted and, even, overridden, by adverse disease-modifying effects and the cumulative burden of longer illness duration in the late-onset group.
There are several possible explanations for the early- vs late-onset TRS results. First, patients with early-onset TRS were statistically significantly younger (35.0 vs 43.7 years), and therefore had a shorter illness duration (calculated as “age” minus “age at symptom onset”, approximately 13 vs 22 years) and fewer relapses (hospitalizations: 5.7 vs 9.0). Obviously, a longer illness duration would disadvantage the late-onset TRS group, allowing their illness time to develop and accumulate poor outcomes such as suicide attempts and hospitalizations. While unemployment might be expected to be higher when people have not been able to achieve certain educational and vocational milestones due to early-onset TRS, being younger may drive greater rehabilitation efforts and increase chances of being placed in an educational or vocational setting. This is reflected by a statistically significantly lower proportion of patients with early-onset TRS being unemployed compared with late-onset TRS (73.0% vs 83.6%), although the proportion who had never been in contact with a social or case worker did not significantly differ between subgroups (17.8% vs 14.8%). There may also have been a subtle shift in the treatment of schizophrenia in the decade between the two subgroups entering treatment, with a greater choice of antipsychotics, slightly reduced stigma, and changes to commercial health insurance over time. Moreover, duration of relapse has been associated with generalized and frontal lobe brain tissue loss,31 and relapses may reduce the chance for subsequent treatment response and rehabilitation.32–34
Second, patients with early-onset TRS were more likely to be living with a partner or family member (50.0% vs 36.1%), who could potentially facilitate earlier treatment for TRS (patients had failed fewer antipsychotic trials before treatment resistance was identified), as well as better, more targeted, or possibly more continuous care.
Third, although age at diagnosis did not differ between subgroups, patients with early-onset TRS had a shorter DUP (using the proxy of median time between symptom onset and schizophrenia diagnosis: 1 vs 2 years). Longer DUP is a consistent correlate of poor outcomes in schizophrenia.35–37
Fourth, patients with early-onset TRS had fewer comorbidities, both psychiatric (eg, substance abuse) and physical (eg, dyslipidemia), although this difference may be explained by the fact that the early-onset TRS subgroup was statistically significantly younger than the late-onset TRS subgroup and that older age is a well-known risk factor for physical illness, especially cardiovascular disease. Both psychiatric and medical comorbidities can adversely affect psychiatric outcomes, for example, metabolic syndrome predicts relapse in schizophrenia,38 which could further worsen the outcome in the late-onset TRS subgroup. In the multivariable logistic regression analysis of TRS onset type, only age, age at schizophrenia onset, and number of psychiatric comorbidities were independent statistically significant variables.
Fifth, early-onset TRS could be a biologically different – and milder – form of TRS. Previous work has identified subtypes of TRS: some patients meet the criteria for TRS from illness onset, whereas others show an initial response to antipsychotics but develop resistance over time.12,13 It has been suggested that these subtypes of TRS have a different underlying neurobiology, with late-onset TRS arising due to upregulation of striatal D2 receptor number and function as a consequence of long-term antipsychotic administration and dopamine D2 blockade.39,40 Overall, further studies are necessary to better understand the differences between early- and late-onset TRS subgroups.
A prior analysis of this same dataset compared the total group of patients with TRS (n=408) to a group of patients with non-TRS (n=204).7 The patients with TRS had a greater clinical burden, with a higher rate of unemployment (74.5% vs 45.1%, p<0.001), of hospitalization (93.4% vs 74.0%, p<0.001), and of having physical/psychiatric comorbidities including obesity (40.2% vs 23.5%, p<0.001) and depression (38.7% vs 25.0%, p=0.001).7 Additionally, psychiatric symptoms were more frequent and severe in TRS and interfered more with social and functioning domains.7
The results of the present study need to be interpreted within its limitations. Questionnaire-based data may be associated with recall bias and, furthermore, only the last three treatments were captured. The study had a low response rate,7 and the selection of psychiatrists and reported cases may not be generalizable. In particular, the psychiatrists’ choice of patients to include may not be representative of their TRS caseload because, for ease of identification, they may have chosen patients who were more obviously treatment resistant or who were receiving clozapine (the only currently approved medication for TRS). Nonetheless, the characteristics of the TRS patients did not differ substantially between those who were included based on the psychiatrist’s own judgment of TRS and those who were selected based on a provided definition of TRS (as discussed previously7). The survey did not collect objective medication non-adherence and psychopathology data, patient- or caregiver-reported outcomes, or healthy lifestyle behavior information. Moreover, the variables that were included in this study and used to characterize TRS and TRS subgroups were limited to certain demographic, illness and treatment variables that are intrinsically associated with schizophrenia, that clinicians would have assessed as part of regular care, and that were relatively easily and reliably accessible from patient charts. There was a lack of biological markers, and future studies should consider a broader clinical and biological set of variables. There was no correction for multiple comparisons. Finally, in using stratifiers to generate TRS subgroups, there is a risk of introducing confounding effects.
Conclusion
Greater clinical burden in TRS is associated with greater illness severity and chronicity markers, suggesting a dimensional gradient from non-TRS to mild–moderate and more severe forms of TRS. Time to onset of TRS may have implications for outcomes, with data indicating greater burden in those with late-onset TRS. Accumulation of illness over time may be more important than time to onset, but patients with early-onset TRS may be helped by faster identification of TRS and availability of caregivers to aid appropriate care. There is a need for a better understanding of TRS and its subgroups, including underlying biology and clinical characteristics. Finally, there is a need for new effective and tolerable treatment options for patients with TRS and its subgroups, to improve patient outcomes.
Data Availability
The datasets generated and/or analyzed during the current study are not publicly available because no suitable repository for these data exists, but are available from the corresponding author on reasonable request.
Acknowledgments
Funding for this study was provided by H. Lundbeck A/S (Valby, Denmark). H. Lundbeck A/S was involved in the design of the survey, and aided in the interpretation of data and the writing of the manuscript. Writing support was provided by Chris Watling, PhD, assisted by his colleagues at Cambridge Medical Communication Ltd (Cambridge, UK), and funded by H. Lundbeck A/S.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
CUC has been a consultant and/or advisor to or has received honoraria from: Alkermes, Allergan, Angelini, Boehringer Ingelheim, Gedeon Richter, Gerson Lehrman Group, Indivior, IntraCellular Therapies, Janssen/J&J, LB Pharma, Lundbeck, MedAvante-ProPhase, Medscape, Merck, Neurocrine, Noven, Otsuka, Pfizer, Recordati, Rovi, Servier, Sumitomo Dainippon, Sunovion, Supernus, Takeda, and Teva. He has provided expert testimony for Bristol-Myers Squibb, Janssen, and Otsuka. He served on a Data Safety Monitoring Board for Boehringer Ingelheim, Lundbeck, Rovi, Supernus, and Teva. He received royalties from UpToDate and grant support from Janssen, and Takeda. He is a shareholder of LB Pharma. TB and CB are employees of H. Lundbeck A/S. The authors report no other conflicts of interest in this work.
References
1. Carbon M, Correll CU. Clinical predictors of therapeutic response to antipsychotics in schizophrenia. Dialogues Clin Neurosci. 2014;16(4):505–524.
2. Lally J, MacCabe JH. Antipsychotic medication in schizophrenia: a review. Br Med Bull. 2015;114(1):169–179. doi:10.1093/bmb/ldv017
3. Lehman AF, Lieberman JA, Dixon LB, et al; American Psychiatric Association; Steering Committee on Practice Guidelines. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004;161(2 Suppl):1–56.
4. Hasan A, Falkai P, Wobrock T, et al. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of schizophrenia, part 1: update 2012 on the acute treatment of schizophrenia and the management of treatment resistance. World J Biol Psychiatry. 2012;13(5):318–378. doi:10.3109/15622975.2012.696143
5. National Institute for Health and Care Excellence. Psychosis and schizophrenia in adults: prevention and management. Clinical guideline [CG178]. 2014. Available from: https://www.nice.org.uk/guidance/cg178.
6. Howes OD, McCutcheon R, Agid O, et al. Treatment-resistant schizophrenia: Treatment Response and Resistance in Psychosis (TRRIP) working group consensus guidelines on diagnosis and terminology. Am J Psychiatry. 2017;174(3):216–229. doi:10.1176/appi.ajp.2016.16050503
7. Correll CU, Brevig T, Brain C. Patient characteristics, burden and pharmacotherapy of treatment-resistant schizophrenia: results from a survey of 204 US psychiatrists. BMC Psychiatry. 2019;19:362. doi:10.1186/s12888-019-2318-x
8. Kennedy JL, Altar CA, Taylor DL, Degtiar I, Hornberger JC. The social and economic burden of treatment-resistant schizophrenia: a systematic literature review. Int Clin Psychopharmacol. 2014;29(2):63–76. doi:10.1097/YIC.0b013e32836508e6
9. Kymes S, Sullivan C, Kinon B, Simonsen J, Hartry A. The cost of incident treatment-resistant schizophrenia in commercial insurance beneficiaries.
10. Brain C, Kymes S, DiBenedetti DB, Brevig T, Velligan DI. Experiences, attitudes, and perceptions of caregivers of individuals with treatment-resistant schizophrenia: a qualitative study. BMC Psychiatry. 2018;18(1):253. doi:10.1186/s12888-018-1833-5
11. Velligan DI, Brain C, Duvold LB, Agid O. Caregiver burdens associated with treatment-resistant schizophrenia: a quantitative caregiver survey of experiences, attitudes and perceptions. Front Psychiatry. 2019;10:584. doi:10.3389/fpsyt.2019.00584
12. Lally J, Ajnakina O, Di Forti M, et al. Two distinct patterns of treatment resistance: clinical predictors of treatment resistance in first-episode schizophrenia spectrum psychoses. Psychol Med. 2016;46(15):3231–3240. doi:10.1017/S0033291716002014
13. Demjaha A, Lappin JM, Stahl D, et al. Antipsychotic treatment resistance in first-episode psychosis: prevalence, subtypes and predictors. Psychol Med. 2017;47(11):1981–1989. doi:10.1017/S0033291717000435
14. Agid O, Arenovich T, Sajeev G, et al. An algorithm-based approach to first-episode schizophrenia: response rates over 3 prospective antipsychotic trials with a retrospective data analysis. J Clin Psychiatry. 2011;72(11):1439–1444. doi:10.4088/JCP.09m05785yel
15. Robinson DG, Woerner MG, Alvir JM, et al. Predictors of treatment response from a first episode of schizophrenia or schizoaffective disorder. Am J Psychiatry. 1999;156(4):544–549. doi:10.1176/ajp.156.4.544
16. Samara MT, Nikolakopoulou A, Salanti G, Leucht S. How many patients with schizophrenia do not respond to antipsychotic drugs in the short term? An analysis based on individual patient data from randomized controlled trials. Schizophr Bull. 2019;45(3):639–646. doi:10.1093/schbul/sby095
17. Meltzer HY. Treatment-resistant schizophrenia – the role of clozapine. Curr Med Res Opin. 1997;14(1):1–20. doi:10.1185/03007999709113338
18. Mortimer AM, Singh P, Shepherd CJ, Puthiryackal J. Clozapine for treatment-resistant schizophrenia: National Institute of Clinical Excellence (NICE) guidance in the real world. Clin Schizophr Relat Psychoses. 2010;4(1):49–55. doi:10.3371/CSRP.4.1.4
19. Essock SM, Hargreaves WA, Dohm FA, Goethe J, Carver L, Hipshman L. Clozapine eligibility among state hospital patients. Schizophr Bull. 1996;22(1):15–25. doi:10.1093/schbul/22.1.15
20. Suzuki T, Kanahara N, Yamanaka H, et al. Dopamine supersensitivity psychosis as a pivotal factor in treatment-resistant schizophrenia. Psychiatry Res. 2015;227(2–3):278–282. doi:10.1016/j.psychres.2015.02.021
21. Market Research Society (MRS). Code of conduct. 2014. Available from: https://www.mrs.org.uk/pdf/mrs%20code%20of%20conduct%202014.pdf.
22. Council of American Survey Research Organizations (CASRO). Code of standards and ethics for market, opinion, and social research. Undated. Available from: https://www.insightsassociation.org/sites/default/files/misc_files/casrocode.pdf.
23. EphMRA. Code of conduct. 2018. Available from: https://www.ephmra.org/media/2300/ephmra-code-of-conduct-august-2018-gdpr-update-v5-for-issue.pdf.
24. Siskind D, Siskind V, Kisely S. Clozapine response rates among people with treatment-resistant schizophrenia: data from a systematic review and meta-analysis. Can J Psychiatry. 2017;62(11):772–777. doi:10.1177/0706743717718167
25. Wang G, Zheng W, Li XB, et al. ECT augmentation of clozapine for clozapine-resistant schizophrenia: a meta-analysis of randomized controlled trials. J Psychiatr Res. 2018;105:23–32. doi:10.1016/j.jpsychires.2018.08.002
26. Long Y, Ouyang X, Liu Z, et al. Associations among suicidal ideation, white matter integrity and cognitive deficit in first-episode schizophrenia. Front Psychiatry. 2018;9:391. doi:10.3389/fpsyt.2018.00391
27. Zhang XY, Du X, Yin G, et al. Prevalence and clinical correlates of and cognitive function at the time of suicide attempts in first-episode and drug-naive patients with schizophrenia. J Clin Psychiatry. 2018;79(4):17m11797. doi:10.4088/JCP.17m11797
28. Villa J, Choi J, Kangas JL, Kaufmann CN, Harvey PD, Depp CA. Associations of suicidality with cognitive ability and cognitive insight in outpatients with schizophrenia. Schizophr Res. 2018;192:340–344. doi:10.1016/j.schres.2017.06.013
29. Delaney C, McGrane J, Cummings E, et al. Preserved cognitive function is associated with suicidal ideation and single suicide attempts in schizophrenia. Schizophr Res. 2012;140(1–3):232–236. doi:10.1016/j.schres.2012.06.017
30. Ochoa S, Usall J, Cobo J, Labad X, Kulkarni J. Gender differences in schizophrenia and first-episode psychosis: a comprehensive literature review. Schizophr Res Treatment. 2012;916198.
31. Andreasen NC, Liu D, Ziebell S, Vora A, Ho BC. Relapse duration, treatment intensity, and brain tissue loss in schizophrenia: a prospective longitudinal MRI study. Am J Psychiatry. 2013;170(6):609–615. doi:10.1176/appi.ajp.2013.12050674
32. Takeuchi H, Siu C, Remington G, et al. Does relapse contribute to treatment resistance? Antipsychotic response in first- vs. second-episode schizophrenia. Neuropsychopharmacology. 2019;44(6):1036–1042. doi:10.1038/s41386-018-0278-3
33. Emsley R, Oosthuizen P, Koen L, Niehaus D, Martinez L. Comparison of treatment response in second-episode versus first-episode schizophrenia. J Clin Psychopharmacol. 2013;33(1):80–83.
34. Wiersma D, Nienhuis FJ, Slooff CJ, Giel R. Natural course of schizophrenic disorders: a 15-year followup of a Dutch incidence cohort. Schizophr Bull. 1998;24(1):75–85. doi:10.1093/oxfordjournals.schbul.a033315
35. Marshall M, Lewis S, Lockwood A, Drake R, Jones P, Croudace T. Association between duration of untreated psychosis and outcome in cohorts of first-episode patients: a systematic review. Arch Gen Psychiatry. 2005;62(9):975–983. doi:10.1001/archpsyc.62.9.975
36. Perkins DO, Gu H, Boteva K, Lieberman JA. Relationship between duration of untreated psychosis and outcome in first-episode schizophrenia: a critical review and meta-analysis. Am J Psychiatry. 2005;162(10):1785–1804. doi:10.1176/appi.ajp.162.10.1785
37. Kane JM, Robinson DG, Schooler NR, et al. Comprehensive versus usual community care for first-episode psychosis: 2-year outcomes from the NIMH RAISE early treatment program. Am J Psychiatry. 2016;173(4):362–372. doi:10.1176/appi.ajp.2015.15050632
38. Godin O, Leboyer M, Schürhoff F, et al.; FACE-SZ (FondaMental Academic Centers of Expertise for schizophrenia) group. Metabolic syndrome and illness severity predict relapse at 1-year follow-up in schizophrenia: the FACE-SZ cohort. J Clin Psychiatry. 2018;79(6):17m12007. doi:10.4088/JCP.17m12007
39. Chouinard G, Samaha AN, Chouinard VA, et al. Antipsychotic-induced dopamine supersensitivity psychosis: pharmacology, criteria, and therapy. Psychother Psychosom. 2017;86(4):189–219. doi:10.1159/000477313
40. Samaha AN, Seeman P, Stewart J, Rajabi H, Kapur S. “Breakthrough” dopamine supersensitivity during ongoing antipsychotic treatment leads to treatment failure over time. J Neurosci. 2007;27(11):2979–2986. doi:10.1523/JNEUROSCI.5416-06.2007
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.