Back to Journals » Drug Design, Development and Therapy » Volume 18
Exploration of the in vitro Antiviral Effects and the Active Components of Changyanning Tablets Against Enterovirus 71
Authors Ge Q, Zhang Z , Cao Z, Wu D, Xu C, Yao J, Gao J, Feng Y
Received 13 November 2023
Accepted for publication 15 February 2024
Published 2 March 2024 Volume 2024:18 Pages 651—665
DOI https://doi.org/10.2147/DDDT.S444625
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Qiongyu Guo
Qiong Ge,1,* Zhewen Zhang,2,* Zhiming Cao,2 Dan Wu,3 Changping Xu,1 Jianbiao Yao,3 Jian Gao,1 Yan Feng1
1Key Laboratory of Public Health Detection and Etiological Research of Zhejiang Province, Department of Microbiology, Zhejiang Provincial Center for Disease Control and Prevention, Hangzhou, Zhejiang, 310051, People’s Republic of China; 2College of Pharmaceutical Sciences, Zhejiang Chinese Medical University, Hangzhou, Zhejiang, 310053, People’s Republic of China; 3Zhejiang Provincial Key Laboratory of Traditional Chinese Medicine Pharmaceutical Technology, Zhejiang Conba Pharmaceutical Co., Ltd, Hangzhou, Zhejiang, 310057, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yan Feng, Key Laboratory of Public Health Detection and Etiological Research of Zhejiang Province, Department of Microbiology, Zhejiang Provincial Center for Disease Control and Prevention, Hangzhou, Zhejiang, 310051, People’s Republic of China, Tel/Fax +86-571-87115204, Email [email protected]
Purpose: This study aims to investigate the in vitro antiviral effects of the aqueous solution of Changyanning (CYN) tablets on Enterovirus 71 (EV71), and to analyze its active components.
Methods: The in vitro anti-EV71 effects of CYN solution and its herbal ingredients were assessed by testing the relative viral RNA (vRNA) expression level and the cell viability rates. Material basis analysis was performed using HPLC-Q-TOF-MS/MS detection. Potential targets and active components were identified by network pharmacology and molecular docking. The screened components were verified by in vitro antiviral experiments.
Results: CYN solution exerted anti-EV71 activities as the vRNA is markedly reduced after treatment, with a half maximal inhibitory concentration (IC50) of 996.85 μg/mL. Of its five herbal ingredients, aqueous extract of Mosla chinensis (AEMC) and leaves of Liquidambar formosana Hance (AELLF) significantly inhibited the intracellular replication of EV71, and the IC50 was tested as 202.57 μg/mL and 174.77 μg/mL, respectively. Based on HPLC-Q-TOF-MS/MS results, as well as the comparison with the material basis of CYN solution, a total of 44 components were identified from AEMC and AELLF. Through network pharmacology, AKT1, ALB, and SRC were identified as core targets. Molecular docking performed between core targets and the components indicated that 21 components may have anti-EV71 effects. Of these, nine were selected for in vitro pharmacodynamic verification, and only rosmarinic acid manifested in vitro anti-EV71 activity, with an IC50 of 11.90 μg/mL. Moreover, rosmarinic acid can stably bind with three core targets by forming hydrogen bonds.
Conclusion: CYN solution has inhibitory effects on EV71 replication in vitro, and its active component was identified as rosmarinic acid. Our study provides a new approach for screening and confirmation of the effective components in Chinese herbal preparation.
Keywords: antiviral effects, material basis analysis, component-target-pathway-disease network, protein–protein interaction network, core targets, rosmarinic acid
Introduction
Enterovirus 71 (EV71) is the main pathogen causing hand, foot, and mouth disease (HFMD).1 Since 1997, HFMD has emerged as a serious childhood infection in the Asia-Pacific region.2 From 2008 to July 2022, up to 26.1 million cases of HFMD and 3727 deaths had been reported in China. The disease became a serious public health problem.3 Although the main manifestations of HFMD are low-grade fever, maculopapular or papular blistering rash on the hands, feet, and mouth ulcers,4 there were growing evidences that a large number of patients with HFMD have atypical presentations, especially those infected with the EV71, and may rapidly develop serious complications such as encephalitis, pulmonary edema, and myocarditis.5,6 With the use of EV71 vaccine,1,7 severe HFMD cases had significantly decreased in China.8 However, vaccination does not completely prevent viral infection, and some children who were vaccinated may still be infected and develop complications such as encephalitis.9 At present, no antiviral drug was approved for the treatment of EV71 infection. Moreover, as enterovirus belongs to a non-envelope virus, it is difficult to be killed in the host environment.10 Therefore, the constant search for effective medicine is essential for the control of the infection caused by EV71.
The therapeutic effects of Traditional Chinese medicine (TCM) on infectious diseases, such as influenza, dengue, and SARS-CoV, have been proven by long-term historical practice.11 Generally, the treatment of HFMD with TCM is based on clearing heat and relieving dampness and detoxification. The TCM preparations which had been clinically used for the treatment of HFMD included heat poisoning injection, Xiyanping injection, Pudilan anti-inflammatory oral solution, Shuanghuanglian oral solution, and anti-viral oral solution.12,13
Changyanning (CYN) tablets are composed of five herbal ingredients: Mosla chinensis (Chinese name: Jiangxiangru, MC), leaves of Liquidambar formosana (Chinese name: Fengxiangshuye, LLF), Euphorbia humifusa (Chinese name: Dijincao, EH), roots of Cinnamomum camphora (Chinese name: Zhangshugen, RCC), and Hedyotis chrysotricha (Chinese name: Jingmaoercao, HC). Clinically, CYN tablets have been proven effective for the treatment of diarrhea, acute gastroenteritis, and ulcerative colitis.14–16 When we screened the anti-EV71 medicines, we found by chance that the aqueous solution of CYN tablets has in vitro anti-EV71 effects. The 2020 edition of the Chinese Pharmacopoeia records that CYN tablets have the effects of clearing heat and relieving dampness, which is consistent with the therapeutic principle of HFMD. Therefore, whether CYN tablets could be used for the treatment of EV71 infection needs further study.
Although TCM has been used to treat infectious diseases for many years,17 the complexity of the components led to the ambiguity of its mechanism of action, therefore hindering the research and application of TCM.18 Identification and search for active components are crucial for clarifying the mechanism of TCM. With the advantages of high resolution, high efficiency and sensitivity, Liquid Chromatography-Mass Spectrometry (LC-MS) has become one of the most powerful tools for the analysis of chemical components of TCM.19 Hence, the HPLC-Q-TOF-MS/MS method was used to identify the active components after confirming the in vitro antiviral activities of CYN and its herbal ingredients. Network pharmacology uses a variety of cutting-edge technologies such as omics, high-throughput screening, and network analysis to reveal the complex network relationship between “component-gene-target-disease”, which helps to understand the molecular basis from multiple dimensions and predict the potential pharmacological mechanism of drugs.20–22 Molecular docking is a method for studying the interaction and recognition between protein receptors and small-molecule ligands22. It predicts the binding ability between different molecules and receptors when two molecules bind to each other to form a stable complex based on the 3D structure of the molecules.23 Its operating procedures include the search for receptor and ligand structures, the search for binding sites, the selection of computational tools and algorithms, and the selection of scoring methods.24 Because it relies heavily on computer operations, it can save a lot of experimental materials and reduce the time and energy of repeated experiments.25 Therefore, molecular docking has become an important tool for drug discovery and molecular simulation.26–28
CYN tablet has been approved for the treatment of bacillary dysentery by the Chinese Pharmacopoeia Commission since 2015, but its antiviral activities, especially if it could be used for the treatment of infection caused by enterovirus, are unclear. In addition, as CYN is a Chinese herbal prescription and its compositions are very complicated, the effective components that could play an essential role in resisting viruses or bacteria have not been well studied. Therefore, this study aims to verify whether CYN tablets have antiviral effects on EV71 by in vitro pharmacodynamic experiments and also to find its active herbal ingredients. Furthermore, the antiviral mechanism and the effective components with anti-EV71 activity were analyzed by integrating network pharmacology with HPLC-Q-TOF-MS/MS and molecular docking techniques.
Materials and Methods
Ethics Statement
This study was exempted from ethics review and approval by the Ethics committee of Zhejiang provincial Center for Disease Control and Prevention (Zhejiang CDC) as it does not involve the use of any clinical samples, human or animals. The EV71 strain used was a virus stock in laboratory of Zhejiang CDC, hence there was no operation of sampling from patients as well.
Medicines and Chemical Components
CYN tablets (Lot number: N211010), provided by Zhejiang CONBA Pharmaceutical Co., Ltd., were prepared into a solution by dissolving it with pure water, making the initial concentration of 100 mg/mL. Five herbal ingredients (MC, LLF, EH, RCC, and HC) that constitute CYN tablets were also provided by Zhejiang CONBA Pharmaceutical Co., Ltd. and were authenticated by Dr. Jianbiao Yao, Zhejiang CONBA Pharmaceutical Co., Ltd. The herbal ingredients were stored at the Zhejiang Provincial Key Laboratory of Traditional Chinese Medicine Pharmaceutical Technology. The numbers of MC, LLF, EH, RCC, and HC were YZ004-200,801, YZ005-190,601, YZ001-191,001, YZ003-200,904, and YZ002-200,814, respectively. To maintain consistency, all of the crude slices of these medicines conformed to quality standards in Chinese Pharmacopeia (2020 edition) or the Standards of Chinese Medicinal Materials in Jiangxi Province (2014 edition). The aqueous extracts of five herbal ingredients were prepared with the same method. Briefly, the herb was boiled and refluxed in 30 volumes of water for 1h. The extraction process was repeated twice followed by filtration. The aqueous extracts were concentrated under reduced pressure to 50 mL and were determined to contain 1 g/mL of dried herbs.
Astragalin (CAS: 480–10-4), gallic acid (CAS: 149–91-7), cryptochlorogenic acid (CAS: 905–99-7), neochlorogenic acid (CAS: 906–33-2), rosmarinic acid (CAS: 20283–92-5), chlorogenic acid (CAS: 327–97-9) standards were purchased from Chengdu Desite Biotech Co., Ltd., China. Hyperoside (CAS: 482–36-0) standard was purchased from Shanghai High-Tech Chemical Technology Co. Caffeic acid (CAS: 331–39-5) and rutin (CAS: 153–18-4) standard were purchased from National Institutes for Food and Drug Control. These standards were dissolved with dimethyl sulfoxide, making the initial concentration 10 mg/mL and stored at −20°C. Ribavirin (Lot number: 181202) purchased from Biokin Pharmaceutical Co. Ltd. and used as a positive control, was dissolved in phosphate buffer saline (PBS) at a concentration of 5 mg/mL and stored at −20°C.
Virus and Cells
EV71 strain EV71/Zhejiang. CHN/195/2016 (GenBank accession no. OR198186) was an isolate stored at −80°C in the Zhejiang CDC. The human rhabdomyosarcoma (RD) cell line was provided by the Poliomyelitis Laboratory of the Chinese Center for Disease Control and Prevention. RD cells were cultured in minimum essential medium supplemented with 10% fetal bovine serum, 1% L-glutamine, and 1% penicillin-streptomycin in an incubator at 37°C and 5% CO2.
Cytotoxicity of Medicines
Cytotoxicity of medicines was determined using Cell Counting Kit-8 (CCK-8) (Beyotime, Shanghai, China). RD cells seeded in 96-well plates were cultivated at 37°C for 48 h. Subsequently, they were divided into blank group, cell control group, and different concentrations of medicine-treated groups (n=5). The growth medium was discarded and replaced with different concentrations of medicines diluted in maintenance medium (MM) (100 μL/well). After incubation at 37°C for 48 h, the MM-containing medicines were removed and the cells were washed twice with PBS. Then, 100 μL MM containing 10 μL CCK-8 was added to the cells. Cell plates were reincubated for 1.5 h at 37°C, and the optical density of each well was measured at 450 nm. The maximum concentration with a cell survival rate ≥ 90% was recognized as the maximum non-toxic concentration (MNTC) of the medicines. And the 50% cytotoxic concentration (CC50) was calculated according to the equation.29
Antiviral Activity Evaluation of Medicines
RD cells were seeded in 24-well plates. After the monolayer was formed, the cells were inoculated with 0.01 MOI of EV71 (100 µL/well), followed by incubation at 37°C for 1 h. The virus was discarded, and the cells were treated with different concentrations of medicines (1 mL/well). Cells were divided into base group, virus control (VC) group, cell control group, and different concentrations of medicine-treated groups (n=6). For VC, 1 mL/well MM was added to cells after infection. Cell control was cells without infection. After 24 h incubation, the culture plates were freeze-thawed twice, and the anti-EV71 activity was assessed by comparing the viral RNA (vRNA) levels between the VC and medicine-treated groups. Ribavirin (1 mg/mL) was used as a positive control in the same way as for the medicine groups.
To further investigate the intracellular inhibitory effect of medicine on EV71, the half maximal inhibitory concentration (IC50) of medicine was determined by testing cell viability.30 RD cells were infected with 0.01 MOI of EV71 in 96-well plates for 1 h at 37°C. After infection, the virus was discarded, and 100 µL MM containing various concentrations of medicine was added to the cells. For the VC group, the cells were covered with 100 µL MM without medicine after infection. Cells were divided into cell control group, VC group, and different concentrations of medicine-treated groups (n=5). After 48 h of incubation, the cell viability of each well was measured by CCK-8, and the IC50 of medicine was calculated.29
RNA Extraction and Quantitative Real-Time PCR (qRT-PCR)
qRT-PCR targeting the VP1 gene of EV71 was performed to test vRNA level. Total RNA was extracted from 300 µL mixture of cells and culture fluid and then eluted into 60 µL RNase-free water using Nucleic Acid (DNA/RNA) Extraction or Purification Kit (SANSURE BIOTECH INC, China). The extracted RNA was detected by One-Step TB Green™ PrimeScript PLUS RT-PCR Kit (Perfect Real-time) (TaKaRa, Kyoto, Japan). The relative RNA expression level was calculated using a classical 2−ΔΔCt method after normalizing against the expression level of β-actin. Sequences of primer pairs are shown in Table 1.
 |
Table 1 Primer Pairs Used for Detection of EV71 and Host Cells |
Material Basis Analyzed by HPLC-Q-TOF-MS/MS
The HPLC was carried out using an Agilent 1260 Infinity liquid chromatography system. Chromatographic separation was performed on an Agilent ZORBAX Eclipse XDB-C18 column (4.6×250 mm, 5 µm) at a column temperature of 30°C. The mobile phase flow rate was kept constant at 1.0 mL/min. The mobile phase consisted of methanol (solvent A) and water containing 0.05% acetic acid (v/v) (solvent B) with elution by a linear gradient at a flow rate of 1.0 mL/min. A simple gradient elution system is established as shown in Table 2. Detection was 320 nm wavelength, and the injection volume was 10 μL. The MS was carried out using Agilent 6530 Accurate-Mass Q-TOF LC/MS with an ESI source operated in the negative ion mode. The source parameters were set as follows: capillary voltage, 3500 V; nebulizer, 35 psig; fragmentor, 65 V; skimmer, 65 V; dring gas, 10 L/min; collision energy, 20 V. Mass spectra were recorded across the range m/z 120–1000.
 |
Table 2 Details of HPLC Assay |
Network Pharmacology
Firstly, the 44 components identified by HPLC-Q-TOF-MS/MS were imported to PubChem (https://pubchem.ncbi.nlm.nih.gov/), and only 40 can be retrieved. Then, the 40 components were downloaded in SDF format in PubChem and imported into Pharmmapper (http://www.lilab-ecust.cn/pharmmapper/) for the prediction of the related targets of each component. Based on the criteria with Norm Fit > 0.80, only 34 components could be used for target prediction. Subsequently, the EV71-related targets were obtained through retrieving three databases, including GeneCards (https://www.genecards.org/), DisGeNET (https://www.disgenet.org/), and OMIM (https://www.omim.org) using keywords EV71, EV-A7, or Human enterovirus 71. The component-related targets and the EV71-related targets were imported into R 4.2.0 software to obtain the intersection targets and plot Venn diagram. The component-gene-target-disease network diagram was drawn using Cytoscape 3.9.1 software. Finally, the intersection targets were imported into the String database (http://string.embl.de/), and the free targets were removed. The PPI network diagram was drawn using Cytoscape 3.9.1 software.
Molecular Docking
Three core targets obtained in PPI were used for molecular docking with the 34 components. The PDB structures of AKT1 (PDB ID: 3mvh), ALB (PDB ID: 6yg9), and SRC (PDB ID: 1o4i) were downloaded from the RSCB PDB database (http://www.rcsb.org/). All proteins were processed by pymol 2.3 software, including the removal of ligands and water, the addition of hydrogen, and the setting of active centers. Then, 34 components identified from HPLC-Q-TOF-MS/MS were imported into the ChemDraw 3D 20.0 for energy minimum processing and saved in pdb format. The docking scores are shown in images using the OmicStudio tools at https://www.omicstudio.cn/tool. The proteins and components were converted to pdbqt format by AutodockTool 1.5.6. Docking was performed with AutoDock vina software and visualized with Pymol 2.5.4 and LigPlot 2.2.
Statistical Analysis
Experimental results were expressed as mean ± standard deviation. Data were tested for normality and homogeneity of variance using the one-way ANOVA test. If the variances were homogeneous, the least significant difference test was selected. If the variances were not uniform, the Games-Howell test was selected. P < 0.05 indicated that the difference was significant.
Results
In vitro Anti-EV71 Activity of CYN Solution
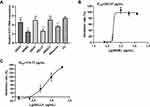
To avoid cytotoxicity, the MNTC of CYN solution was first determined on RD cells. Cell viability was tested as 103.39% when the cells were treated with 5 mg/mL of CYN solution, while it dropped to 23.90% when the concentration of CYN solution increased to 10 mg/mL (Supplementary Table S1). Hence, the MNTC of CYN solution was identified as 5 mg/mL and the CC50 was calculated as 7.96 mg/mL (Figure 1A). The MNTC and CC50 of the positive control ribavirin were 1 mg/mL and 2.43 mg/mL, respectively (Supplementary Table S2). To investigate the antiviral effects, RD cells were treated with various concentrations (5, 2.5, 1.25, 0.63 mg/mL) of CYN solution after EV71 infection. Figure 1B shows that CYN solution treatments efficiently inhibited the replication of EV71 as the vRNA levels of CYN treatment groups were significantly reduced compared to the vRNA of VC (P<0.01) (Supplementary Table S3). Also, CYN solution presented dose-dependent inhibitory activities on cell death caused by EV71 infection and the IC50 was tested as 996.85 μg/mL (Figure 1C and Supplementary Table S4). The IC50 of ribavirin was tested as 289.68 μg/mL (Figure 1D and Supplementary Table S5).
In vitro Anti-EV71 Activity of Five Herbal Medicines Which Compose CYN Tablet
To clarify the active herbal ingredients, we tested the in vitro anti-EV71 effects of the aqueous extracts of five herbal ingredients that compose CYN tablet by comparing the relative vRNA expression level between VC and medicine-treated groups. The MNTC of aqueous extract of MC (AEMC), aqueous extract of LLF (AELLF), aqueous extract of EH (AEEH), aqueous extract of RCC (AERCC), and aqueous extract of HC (AEHC) were determined as 10, 2.5, 1.25, 80, and 20 mg/mL, respectively (Supplementary Table S6–S10). In order to compare the antiviral effects at the same concentration, the minimum MNTC among the five ingredients (1.25 mg/mL) was used in the subsequent experiments.
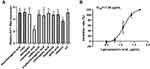
When the infected cells were treated with five herbal ingredients, a marked drop in EV71 RNA level was only observed in AEMC and AELLF treated groups (P < 0.01). AEMC and AELLF at a concentration of 1.25 mg/mL resulted in >99% reduction in the expression of EV71 VP1 transcripts, indicating that AEMC and AELLF inhibited EV71 replication intracellularly (Figure 2A and Supplementary Table S11). However, the EV71 RNA levels between other three medicine-treated (AEEH, AEHC, and AERCC) groups and the VC group were not different significantly, indicating that the EV71 replication was not inhibited by treatments with these herbal ingredients. Furthermore, to clarify the inhibitory effects of AEMC and AELLF on EV71, the infected cells were treated with various concentrations of the two herbs. Cell viability rates proved the anti-EV71 activities of AEMC and AELLF. Also, the inhibitory rates showed a dose–effect relationship with the concentration of the medicines. The calculated IC50 of AEMC and AELLF were 202.57 μg/mL and 174.77 μg/mL, respectively (Figure 2B and C and Supplementary Table S12 and S13).
Material Basis of AEMC and AELLF
To explore the active components of CYN solution, the material basis of two active herbal ingredients (AEMC and AELLF) which possess in vitro anti-EV71 activities were analyzed by HPLC-Q-TOF-MS/MS. Total ion chromatograms are shown in Figure 3A and B. Through comparison with the material basis of CYN solution,31 a total of 44 components were identified. Of these, 23 were contained in both AEMC and CYN solutions, while 32 were contained in both AELLF and CYN solutions (Supplementary Table S14). Eleven components were identified in all three. Seven of the 11 are organic acids, including quinic acid, malic acid, citric acid, gallic acid, hydroxybenzoic acid-glucoside, protocatechuic acid, and vanillic acid. Their structural formulas are shown in Figure 3C. Two of the 11 belong to flavonoids. They are rutin and astragalin (Figure 3D). The remaining two are amino acids N-acetyl-DL-glutamic acid and tannins ellagic acid (Figure 3E and F).
Network Pharmacology
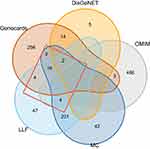
Although we obtained 44 components by material basis analysis, there are only 40 which could be successfully retrieved from PubChem. After screening with the criteria, Norm Fit > 0.80, 34 components could be used for target prediction. A total of 349 component-related targets were screened. Of these targets, 298 were related to MC and 304 were related to LLF. Then, EV71-related targets were retrieved from three databases. Figure 4 shows that 473 targets were screened from OMIM, 298 were screened from Genecards and 21 were screened from DisGeNET database. The component-related targets and the EV71-related targets were imported to plot Venn diagram using R 4.2.0 software, and 29 targets were obtained in the intersection.
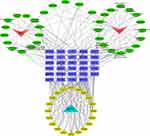
The component-target-pathway-disease network was composed of two herbal ingredients, 34 components (used for target prediction), 29 intersection targets, and 20 signaling pathways, showing the potential relationship between herbal ingredients and EV71 infection (Figure 5). The 29 intersection targets were imported into the String platform to construct a PPI network, and the results were imported into Cytoscape 3.9.1 software for visualization. Figure 6 shows there are 25 associated target proteins and 214 edges obtained. The top three targets associated with other proteins were RAC-alpha serine/threonine-protein kinase (AKT1), albumin (ALB), and proto-oncogene tyrosine-protein kinase Src (SRC). Hence, they were regarded as core targets.
 |
Figure 6 Protein–Protein Interaction (PPI) network diagram. The redder the color, the larger the circle, indicating that the target correlation is stronger. |
Molecular Docking and Verification of in vitro Anti-EV71 Activity
For seeking the active components of CYN solution, the 34 components were docked with three core target proteins, respectively. The binding energies between components and proteins are shown in Figure 7. The binding energy ≤-7.0 kcal/mol indicates the stable binding between components and targets.32 Hence, 21 out of the 34 components may bind tightly with the core targets and play an anti-EV71 role as the average binding energy with the three targets was ≤ −7.0 kcal/mol. As not all the components were available, only nine including rutin, gallic acid, astragalin, rosmarinic acid, caffeic acid, neochlorogenic acid, cryptochlorogenic acid, chlorogenic acid, and hyperoside were selected for pharmacodynamic verification in vitro. The MNTC of the nine components are shown in Table 3 (Supplementary Table S15–S23). After EV71 infection, cells were treated with each component of the MNTC. Compared to VC, the relative vRNA level markedly dropped only when the cells were treated with 50 μg/mL rosmarinic acid after infection (P<0.01) (Figure 8A, Supplementary Table S24). The results experimentally verified that rosmarinic acid had anti-EV71 activities in vitro. The inhibitory activity of the rosmarinic acid is dose-dependent, with an IC50 of 11.90 μg/mL (Figure 8B, Supplementary Table S25).
 |
Table 3 The MNTC of the Nine Components Selected for Pharmacodynamic Verification |
 |
Figure 7 The docking scores of components and core targets. The redder the color, the lower the binding energy between the component and the target, and the component may bind tightly to the target. |
To further understand the possible mechanism of rosmarinic acid, we analyzed the interaction between rosmarinic acid and three core targets. The docking score of rosmarinic acid was −9.2 kcal/mol with AKT1, −9.9 kcal/mol with ALB, and −5.5 kcal/mol with SRC (Figure 7). Figure 9 shows that rosmarinic acid could form hydrogen bonds with residues ALA-230, GLY-159, GLY-162, and ASP-292 in AKT1 (PDB: ID 3mvh), and residues ARG-10, LEU-66, HIS-67, ASN-99, and TYR-30 in ALB (PDB: 6yg9), as well as residues HIS-60, SER-36, THR-38, and GLU-37 in SRC (PDB: ID 1o4i).
Discussion
CYN tablet, a Chinese proprietary medicine used for the treatment of enteritis and diarrhea, was found to have in vitro antiviral effects against EV71 in this study. As CYN tablets consist of five herbal ingredients, the anti-EV71 activities of the aqueous extracts of the five herbal ingredients were also analyzed. Of the five, only AEMC and AELLF exerted inhibitory effects on the replication of EV71 intracellularly.
Due to the ingredients of TCM being complex, we first analyzed the material basis of the two herbal ingredients (AEMC and AELLF) which have in vitro anti-EV71 activities by HPLC-Q-TOF-MS/MS and then compared the results with the material basis of CYN solution reported previously.31 In total of 11 components were identified in all three medicines. Two of them, namely, rutin and the intestinal metabolites of ellagic acid, were considered to have inhibitory effects on the replication of EV71.33,34 Among the components shared by AEMC and CYN solution, AELLF and CYN solution, rosmarinic acid, chlorogenic acid, caffeic acid, and hyperoside were reported to have antiviral effects,35–38 especially rosmarinic acid, which was proved to inhibit the proliferation of EV71 virus.39
In order to know which components played an essential role in in vitro anti-EV71 process, we explored the anti-EV71 mechanism of CYN using network pharmacology and molecular docking.
Firstly, the component-related targets and the disease-related targets were screened. Based on Venn diagram, we obtained 29 targets of components and EV71 in the intersection. Then, the 29 intersection targets were used to construct a PPI network. AKT1, ALB, and SRC were considered core targets as they are closely related to other proteins. In terms of AKT1, the expression of AKT1 was significantly down-regulated in the early stage of EV71 virus infection, which indicates the correlation between AKT1 and EV71 infection.40 SRC is a typical member of the non-receptor protein tyrosine kinase family, which is involved in a variety of basic functions to maintain cell homeostasis, including regulating cell cycle progression, proliferation, differentiation, survival, and other cellular processes. Studies have found that Src tyrosine kinase inhibitors (STKIs) can be used for the treatment of HIV-1.41 ALB is produced in the liver and is associated with cell death in the liver.42 After the identification of core targets, molecular docking was performed to seek the active components of CYN. Combining previous reports and results of molecular docking, nine components were selected for in vitro pharmacodynamic validation. It was found that only rosmarinic acid showed a significant inhibitory effect on EV71 proliferation, which indicated that rosmarinic acid might be the chemical compound mainly responsible for the anti-EV71 activity of CYN tablet.
Rosmarinic acid is an esterification product of caffeic acid and 3.4-dihydroxyphenyl lactic acid, which is widely distributed in the Compositae and Labiatae families. Rosmarinic acid has a variety of biological activities such as antioxidant, anti-inflammatory, antibacterial, antiviral, antitumor, antidiabetic, and neuroprotective.43 The anti-EV71 effects of rosmarinic acid were recognized in RD cells in previous reports.44,45 Chung et al put forward that rosmarinic acid could reduce EV71 viral particle production and viral RNA expression in RD cells,46 which is similar to the results in this study. Apart from suppressing EV71 replication, rosmarinic acid was considered to inhibit EV71 infection through reducing EV71 viral attachment and entry during the viral absorption stage.46 In vitro and in vivo studies showed that rosmarinic acid has a protective effect against cytopathic and apoptotic cell death in the early stages of EV71 virus infection.39 Our results demonstrate that rosmarinic acid could efficiently decrease cell death caused by EV71 infection. After experimental verification, the anti-EV71 effect of rosmarinic acid has been proven by molecular docking. It is generally considered that binding energy less than −7.0 kcal/mol indicates a strong binding activity between the ligand and the receptor.32 The binding energy between rosmarinic acid and two core targets (AKT1 and ALB) was much lower than −7.0 kcal/mol, indicating it could bind tightly to the target proteins, thereby proving the anti-EV71 activity of rosmarinic acid from the molecular point of view. Besides the anti-EV71 activity, rosmarinic acid was reported to be efficient against a variety of other viruses, including severe acute respiratory syndrome coronavirus, herpes simplex virus, dengue virus, influenza A virus, and hepatitis B virus.36,47–50 Therefore, rosmarinic acid might be a broad-spectrum antiviral compound.
Another component we are concerned with is rutin, which was identified from both AEMC and AELLF by HPLC-Q-TOF-MS/MS, and was also demonstrated to bind tightly with two of the three core targets (AKT1 and ALB) by molecular docking. It gave negative results when we verified the anti-EV71 effects through comparing the vRNA level between VC and rutin treated cells, which indicated that rutin could not inhibit EV71 replication by post-infection treatment. However, rutin has been reported to significantly suppress EV71-induced CPE and viral replication on Vero cells when the cells are pretreated with 200 μM of rutin before infection.33 It could be inferred, therefore, that rutin may exert antiviral effects by reducing viral attachment or entry to cells, but not inhibiting intracellular viral replication.
The limitation of the study is that in vivo anti-EV71 activities of CYN tablets and rosmarinic acid were not assessed because of no animal bio-safety level-2 (ABSL-2) laboratory, which needs to be further studied.
Conclusion
In summary, CYN tablets exerted an in vitro anti-EV71 effect through inhibiting viral replication. Combining the HPLC-Q-TOF-MS/MS with network pharmacology, rosmarinic acid was identified as the effective component with anti-EV71 activities. Our study could provide a reference for the screening and confirmation of antiviral components in Chinese herbal preparation.
Acknowledgments
We are grateful to Zhejiang Conba Pharmaceutical Co., Ltd. for assisting in the HPLC-Q-TOF-MS/MS analysis.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sun J, Li Y, Yang Z, Fang Q, Chen B. Effect of enterovirus 71 vaccination on the epidemiological characteristics and etiology in hospitalized children with hand-foot-and-mouth disease: a retrospective study from a tertiary children’s hospital. Medicine. 2022;101(37):e30356. doi:10.1097/MD.0000000000030356
2. Nguyet LA, Thanh TT, Nhan LNT, et al. Neutralizing antibodies against enteroviruses in patients with hand, foot and mouth disease. Emerg Infect Dis. 2020;26(2):298–306. doi:10.3201/eid2602.190721
3. Liang ZL, Mao QY, Wang YP, et al. Progress on the research and development of inactivated EV71 whole-virus vaccines. Hum Vaccin Immunother. 2013;9(8):1701–1705. doi:10.4161/hv.24949
4. Saguil A, Kane S, Lauters R, Mercado M. Hand-foot-and-mouth disease: rapid evidence review. Am Family Phys. 2019;100(7):408–414.
5. Zhang W, Huang Z, Huang M, Zeng J. Predicting severe enterovirus 71-infected hand, foot, and mouth disease: cytokines and Chemokines. Mediators Inflamm. 2020;2020:9273241. doi:10.1155/2020/9273241
6. Esposito S, Principi N. Hand, foot and mouth disease: current knowledge on clinical manifestations, epidemiology, aetiology and prevention. Eur J Clin Microbiol Infect Dis. 2018;37(3):391–398. doi:10.1007/s10096-018-3206-x
7. Mao QY, Wang Y, Bian L, Xu M, Liang Z. EV71 vaccine, a new tool to control outbreaks of hand, foot and mouth disease (HFMD). Expert Rev Vaccines. 2016;15(5):599–606. doi:10.1586/14760584.2016.1138862
8. Du Z, Huang Y, Bloom MS, et al. Assessing the vaccine effectiveness for hand, foot, and mouth disease in Guangzhou, China: a time-series analysis. Hum Vaccin Immunother. 2021;17(1):217–223. doi:10.1080/21645515.2020.1763076
9. Li J, Yin X, Lin A, et al. EV71 vaccination impact on the incidence of encephalitis in patients with hand, foot and mouth disease. Hum Vaccines Immunother. 2021;17(7):2097–2100. doi:10.1080/21645515.2020.1851129
10. Solomon T, Lewthwaite P, Perera D, Cardosa MJ, McMinn P, Ooi MH. Virology, epidemiology, pathogenesis, and control of enterovirus 71. Lancet Infect Dis. 2010;10(11):778–790. doi:10.1016/S1473-3099(10)70194-8
11. Huang K, Zhang P, Zhang Z, et al. Traditional Chinese Medicine (TCM) in the treatment of COVID-19 and other viral infections: efficacies and mechanisms. Pharmacol Ther. 2021;225:107843. doi:10.1016/j.pharmthera.2021.107843
12. Tang J, Zhang G, Xing J, Yu Y, Han T. Network meta-analysis of heat-clearing and detoxifying oral liquid of Chinese medicines in treatment of children’s hand-foot-mouth disease: a protocol for systematic review and meta-analysis. Medicine. 2022;101(5):e28778. doi:10.1097/MD.0000000000028778
13. Li XH, Li SJ, Xu Y, et al. Effect of integrated Chinese and Western medicine therapy on severe hand, foot and mouth disease: a prospective, randomized, controlled trial. Chin J Integr Med. 2017;23(12):887–892. doi:10.1007/s11655-016-2504-3
14. Yu W, Zhang Y, Kang C, et al. The pharmacological evidence of the Chang-yan-ning formula in the treatment of colitis. Front Pharmacol. 2022;13:1029088. doi:10.3389/fphar.2022.1029088
15. Hu X, Yang F. Analysis of the therapeutic effect of changyanning on intestinal flora in inflammatory bowel disease. Contrast Media Mol Imaging. 2022;2022:3757763. doi:10.1155/2022/3757763
16. Guo P, Wang Z, Lv X, et al. Changyanning regulates gut microbiota and metabolism to ameliorate intestinal injury induced by ETEC K88. Front Microbiol. 2023;14:1098818. doi:10.3389/fmicb.2023.1098818
17. Kang N, Gao H, He L, et al. Ginsenoside Rb1 is an immune-stimulatory agent with antiviral activity against enterovirus 71. J Ethnopharmacol. 2021;266:113401. doi:10.1016/j.jep.2020.113401
18. Yu Y, Yao C, Guo DA. Insight into chemical basis of traditional Chinese medicine based on the state-of-the-art techniques of liquid chromatography-mass spectrometry. Acta Pharm Sin B. 2021;11(6):1469–1492. doi:10.1016/j.apsb.2021.02.017
19. Chen K, Liu J, Ma Z, Duan F, Guo Z, Xiao H. Rapid identification of chemical constituents of Rhodiola crenulata using liquid chromatography-mass spectrometry pseudotargeted analysis. J Sep Sci. 2021;44(20):3747–3776. doi:10.1002/jssc.202100342
20. Yuan Z, Pan Y, Leng T, et al. Progress and prospects of research ideas and methods in the network pharmacology of Traditional Chinese Medicine. J Pharm Pharm Sci. 2022;25:218–226. doi:10.18433/jpps32911
21. Liu YY, Yu LH, Zhang J, Xie DJ, Zhang XX, Yu JM. Network pharmacology-based and molecular docking-based analysis of suanzaoren decoction for the treatment of parkinson’s disease with sleep disorder. Biomed Res Int. 2021;2021:1752570. doi:10.1155/2021/1752570
22. Liu J, Liu J, Tong X, et al. Network pharmacology prediction and molecular docking-based strategy to discover the potential pharmacological mechanism of huai hua san against ulcerative colitis. Drug Des Devel Ther. 2021;15:3255–3276. doi:10.2147/DDDT.S319786
23. Crampon K, Giorkallos A, Deldossi M, Baud S, Steffenel LA. Machine-learning methods for ligand-protein molecular docking. Drug Discov Today. 2022;27(1):151–164. doi:10.1016/j.drudis.2021.09.007
24. Das DR, Kumar D, Kumar P, Dash BP. Molecular docking and its application in search of antisickling agent from Carica papaya. J Appl Biol Biotech. 2020;8(01):105–116. doi:10.7324/JABB.2020.80117
25. Bai G, Pan Y, Zhang Y, et al. Research advances of molecular docking and molecular dynamic simulation in recognizing interaction between muscle proteins and exogenous additives. Food Chem. 2023;429:136836. doi:10.1016/j.foodchem.2023.136836
26. Li X, Wei S, Niu S, et al. Network pharmacology prediction and molecular docking-based strategy to explore the potential mechanism of Huanglian Jiedu Decoction against sepsis. Comput Biol Med. 2022;144:105389. doi:10.1016/j.compbiomed.2022.105389
27. Li L, Liu S, Wang B, et al. An updated review on developing small molecule kinase inhibitors using computer-aided drug design approaches. Int J Mol Sci. 2023;24(18):13953. doi:10.3390/ijms241813953
28. Agu PC, Afiukwa CA, Orji OU, et al. Molecular docking as a tool for the discovery of molecular targets of nutraceuticals in diseases management. Sci Rep. 2023;13(1):13398. doi:10.1038/s41598-023-40160-2
29. Wei W, Wan H, Peng X, Zhou H, Lu Y, He Y. Antiviral effects of Ma Huang Tang against H1N1 influenza virus infection in vitro and in an ICR pneumonia mouse model. Biomed Pharmacother. 2018;102:1161–1175. doi:10.1016/j.biopha.2018.03.161
30. Utsunomiya H, Ichinose M, Ikeda K, et al. Inhibition by caffeic acid of the influenza A virus multiplication in vitro. Int J Mol Med. 2014;34(4):1020–1024. doi:10.3892/ijmm.2014.1859
31. Tan L, Jin H, Liu Y, et al. Main chemical constituents of Changyanning Tablets based on HPLC-Q-TOF-MS/MS. Chin Traditional Herbal Drugs. 2020;51(16):4124–4132.
32. Abduljalil JM, Elfiky AA, Elgohary AM. Exploration of natural compounds against the human mpox virus DNA-dependent RNA polymerase in silico. J Infect Public Health. 2023;16(7):996–1003. doi:10.1016/j.jiph.2023.04.019
33. Wang S, Qiao J, Chen Y, Tian L, Sun X. Urolithin A inhibits enterovirus 71 replication and promotes autophagy and apoptosis of infected cells in vitro. Arch. Virol. 2022;167(10):1989–1997. doi:10.1007/s00705-022-05471-1
34. Wang C, Wang P, Chen X, Wang W, Jin Y. Saururus chinensis (Lour). Baill blocks enterovirus 71 infection by hijacking MEK1-ERK signaling pathway. Antiviral Res. 2015;119:47–56. doi:10.1016/j.antiviral.2015.04.009
35. Wu LL, Yang XB, Huang ZM, Liu HZ, Wu GX. In vivo and in vitro antiviral activity of hyperoside extracted from Abelmoschus manihot (L) medik. Acta Pharmacol Sin. 2007;28(3):404–409. doi:10.1111/j.1745-7254.2007.00510.x
36. Panchal R, Ghosh S, Mehla R, et al. Antiviral activity of rosmarinic acid against four serotypes of dengue virus. Curr Microbiol. 2022;79(7):203. doi:10.1007/s00284-022-02889-3
37. Shirasago Y, Inamori Y, Suzuki T, et al. Inhibition mechanisms of hepatitis C virus infection by caffeic acid and tannic acid. Biol Pharm Bull. 2019;42(5):770–777. doi:10.1248/bpb.b18-00970
38. Ding Y, Cao Z, Cao L, Ding G, Wang Z, Xiao W. Antiviral activity of chlorogenic acid against influenza A (H1N1/H3N2) virus and its inhibition of neuraminidase. Sci Rep. 2017;10(7):45723. doi:10.1038/srep45723
39. Lin WY, Yu YJ, Jinn TR. Evaluation of the virucidal effects of rosmarinic acid against enterovirus 71 infection via in vitro and in vivo study. Virol J. 2019;16(1):94. doi:10.1186/s12985-019-1203-z
40. Shi W, Hou X, Li X, et al. Differential gene expressions of the MAPK signaling pathway in enterovirus 71-infected rhabdomyosarcoma cells. Braz J Infect Dis. 2013;17(4):410–417. doi:10.1016/j.bjid.2012.11.009
41. Rivera-Torres J, San Jose E. Src tyrosine kinase inhibitors: new perspectives on their immune, antiviral, and senotherapeutic potential. Front Pharmacol. 2019;10:1011. doi:10.3389/fphar.2019.01011
42. Pan AN, Xu WW, Luo YL, et al. A novel system for predicting liver histopathology in patients with chronic hepatitis B. Medicine. 2017;96(14):e6465. doi:10.1097/MD.0000000000006465
43. Petersen M, Simmonds M. Rosmarinic acid. Phytochemistry. 2003;62(2):121–125. doi:10.1016/s0031-9422(02)00513-7
44. Hsieh CF, Jheng JR, Lin GH, et al. Rosmarinic acid exhibits broad anti-enterovirus A71 activity by inhibiting the interaction between the five-fold axis of capsid VP1 and cognate sulfated receptors. Emerg Microbes Infect. 2020;9(1):1194–1205. doi:10.1080/22221751.2020.1767512
45. Chen SG, Leu YL, Cheng ML, et al. Anti-enterovirus 71 activities of Melissa officinalis extract and its biologically active constituent rosmarinic acid. Sci Rep. 2017;7(1):12264. doi:10.1038/s41598-017-12388-2
46. Chung YC, Hsieh FC, Lin YJ, et al. Magnesium lithospermate B and rosmarinic acid, two compounds present in Salvia miltiorrhiza, have potent antiviral activity against enterovirus 71 infections. Eur J Pharmacol. 2015;755:127–133. doi:10.1016/j.ejphar.2015.02.046
47. Weng JY, Chen XX, Wang XH, et al. Reducing lipid peroxidation attenuates stress-induced susceptibility to herpes simplex virus type 1. Acta Pharmacol Sin. 2023;44(9):1856–1866. doi:10.1038/s41401-023-01095-6
48. Tsukamoto Y, Ikeda S, Uwai K, et al. Rosmarinic acid is a novel inhibitor for Hepatitis B virus replication targeting viral epsilon RNA-polymerase interaction. PLoS One. 2018;13(5):e0197664. doi:10.1371/journal.pone.0197664
49. Jheng J-R, Hsieh C-F, Chang Y-H, et al. Rosmarinic acid interferes with influenza virus A entry and replication by decreasing GSK3β and phosphorylated AKT expression levels. J Microbiol Immunol Infect. 2022;55(4):598–610. doi:10.1016/j.jmii.2022.04.012
50. Moschovou K, Antoniou M, Chontzopoulou E, et al. Exploring the binding effects of natural products and antihypertensive drugs on SARS-CoV-2: an in silico investigation of main protease and spike protein. Int J Mol Sci. 2023;24(21):15894. doi:10.3390/ijms242115894
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.