Back to Journals » Clinical Ophthalmology » Volume 17
Exploration of Hippocampal Functional Connectivity Alterations in Patients with High Myopia via Seed-Based Functional Connectivity Analysis
Authors Wei B , Fu WW, Ji Y, Cheng Q , Shu BL, Huang QY, Wu XR
Received 5 September 2023
Accepted for publication 2 November 2023
Published 14 November 2023 Volume 2023:17 Pages 3443—3451
DOI https://doi.org/10.2147/OPTH.S434797
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Bin Wei,* Wen-Wen Fu,* Yu Ji, Qi Cheng, Ben-Liang Shu, Qin-Yi Huang, Xiao-Rong Wu
Department of Ophthalmology, The First Affiliated Hospital of Nanchang University, Nanchang, Jiangxi, 330000, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiao-Rong Wu, Department of Ophthalmology, The First Affiliated Hospital of Nanchang University, Nanchang, Jiangxi, 330000, People’s Republic of China, Email [email protected]
Aim: The objective of this study was to examine changes in functional connectivity (FC) in the hippocampus among patients with high myopia (HM) compared to healthy controls (HCs) through the utilization of seed-based functional connectivity (FC) analysis.
Methods: Resting-state functional magnetic resonance imaging (fMRI) was conducted on a sample of 82 patients diagnosed with high myopia (HM) and 59 HCs. The two groups were matched based on age, weight and other relevant variables. Using seed-based FC analysis to detect alterations in hippocampal FC patterns in HM patients and HCs. Furthermore, a correlation analysis was performed to examine the associations between the mean functional connectivity (FC) signals in various brain regions of patients with HM and their corresponding clinical manifestations.
Results: The FC values in the left temporal pole-temporal gyrus (L-TPOsup), right hippocampus (R-HIP), left medial temporal gyrus (L-MTG) and left hippocampus in HM patients were significantly lower than those of healthy subjects. In the left temporal pole-superior temporal gyrus (L-TPOsup), right orbital part of middle frontal gyrus (RO-MFG), left fusiform gyrus (L-FG), left cerebellum superior (L-Cbe6), left middle temporal gyrus (L-MTG), right thalamus (R-THA), and right hippocampus, FC values were also significantly lower.
Conclusion: Brain dysfunction was observed in various regions of the HM patients, suggesting the existence of neurobiological alterations that could lead to impairments in visual cognition, movement, emotional processing, and visual memory.
Keywords: high myopia, hippocampus, resting state, brain function, brain region, functional connectivity
Introduction
High myopia (HM) refers to a form of visual impairment characterized by a spherical equivalent refractive error of an eye ≤ −6.00 D or an axial length measuring ≥ 26 mm, and its clinical manifestations mainly include decreased visual acuity and anterior dark shadow.1,2 The prevalence of nearsightedness in East Asia, particularly in China, has experienced an unparalleled increase, skyrocketing from 15% to 90% among teenagers and young adults over a span of six decades. It is projected that by 2050, individuals with myopia will make up 9.8% of the worldwide population.3,4 Besides genetic factors, prolonged near work and insufficient sleep are also significant contributors to HM.5 Pathological myopia poses a significant risk to HM, as it can readily result in severe complications like glaucoma, retinal detachment, and myopic macular degeneration.6 Despite extensive research on these complications, there is currently limited understanding of the impact of neural activity in the brain that causes these harms with HM.
Recently, resting state functional magnetic resonance imaging (rs-fMRI) has become a swiftly evolving neuroimaging technique. By using this technique, one can observe alterations in the flow of blood and oxygenation in the brain that take place while performing motor, sensory, and cognitive activities. The alterations indirectly suggest the extent of regional neural function in the brain, rendering rs-fMRI extremely valuable for various purposes.7,8 Prior to this, Yao found that the functional connectivity (FC) changes between the hippocampus and other brain regions could serve as potential neuroimaging biomarkers for cognitive or mental disorders associated with Sepsis-associated encephalopathy (SAE).9 In our prior investigation, we demonstrated the existence of disturbed FC in individuals with HM, particularly between the primary visual cortex (V1) and various other regions of the brain. This interference suggests that HM patients may be impaired in processing visual information.10 To the best of our knowledge, no prior studies have utilized FC to investigate and analyze the functional alterations in both the hippocampus and various brain regions in patients with HM. This research has the potential to enhance our understanding of the etiology and neural mechanisms underlying HM. For this investigation, we employed the hippocampus the seed region and analyzed the directed FC between the hippocampus and other brain regions.
The hippocampus is a pair of subcortical structures situated on the medial side of the temporal lobe. It is part of the limbic system of the brain and plays an integral role in cognitive functions such as spatial guidance and memory. We know that the guiding navigation is an important role of vision, and strong vision can effectively drive navigation. In fact, the brain’s vision and navigation systems interact. Extensive neural networks spanning from the retina to the hippocampus play a crucial role in enabling us to perceive and recognize visual objects within our environment. The ventral pathway of visual transmission projects from the retina to the V1 region and then to the infratemporal cortex. Visual information reaches and is further projected to the hippocampus through the perinasal cortex and entorhinal cortex, contributing to information processing and facilitating the formation of spatial memory.11–14 Ji discovered that patients with HM experience neural activity dysfunction, particularly in the default mode network (DMN) and cerebellar network (CER). Consequently, patients with HM may exhibit deficits in visual, motor, and cognitive functions.15 Zhang demonstrated that patients with HM show variations in amplitude of low-frequency fluctuations (ALFF) and DMN across different brain regions, which might signify cognitive changes associated with HM.16 Moreover, other studies have indicated that myopia patients are nearly twice as likely to develop cognitive impairment compared to individuals with refractive errors.17 Therefore, in this study, we propose the hypothesis that patients with HM will exhibit characteristic FC pattern alterations in the hippocampus, which could be linked to cognitive, motor, and visual changes induced by HM.
Participants and Methods
Participants
The First Affiliated Hospital of Nanchang University recruited 141 participants from the ophthalmology department from August to December 2021. Of these participants, 82 had high myopia (HM) while 59 were healthy controls (HCs). Both groups were paired based on their educational background, age, and gender. Exclusion criteria encompassed a history of brain illnesses, a clinical examination and evaluation. All eligible study volunteers were provided with detailed information regarding the research methods and potential risks before their participation, and evaluations were carried out in a standardized clinical environment. All methods of this study followed the guiding principles of the Declaration of Helsinki, and the study protocol was approved by the Medical Ethics Committee of the First Affiliated Hospital of Nanchang University. Table 1 displays the original sample size.
 |
Table 1 The Original Sample Size of HM Patients and HCs |
The criteria for selecting the subjects are as follows: (1) visual acuity greater than 600 degrees in both eyes, visual acuity greater than 1.0 with glasses; (2) fulfillment of MRI-related tests; (3) perform optical coherence tomography, ultrasound examination, and other ophthalmic examinations; (5) no contraindications to MRI.
The exclusion criteria for HM patients are as follows: (1) a history of eye trauma or previous ophthalmic surgery exists; (2) existence of retinal detachment, macular edema, retinal neovascularization, and other fundus diseases; (3) Other neurological disorders, such as cerebral infarction, etc.
The inclusion criteria for HCs are as follows: (1) no eye diseases or systemic illnesses (such as stroke or other cerebral disease); (2) uncorrected visual acuity is greater than 1.0; (3) the relevant MRI examination and ophthalmic examination are normal; (4) no contraindications to MRI.
Acquisition of fMRI Data
Rs-fMRI data were collected using a 3T MR scanner (Siemens, Erlangen, Germany) equipped with an 8-channel phased-array head coil for data acquisition. We used the following parameters to capture 240 resting-state volumes over 8 minutes: field of view of 240 mm × 240 mm, repetition time of 2000 ms, echo time of 30 ms, flip angle of 90°, matrix of 64 × 64, slice thickness of 4 mm, and a gap of 1.2 mm. Everyone were given instructions to limit any movement, shut their eyes, and stay awake. All participants were provided with noise-canceling headphone to mitigate other effects. In this study, the imaging parameters used for the high-resolution T1-weighted imaging were an echo time of 2.26 ms, a thickness of 1 with no intersection gap, an acquisition matrix of 256 × 256, a repetition time of 1900 ms, a field of view measuring 240×240 mm2, and a flip angle of 12°.
Analysis of fMRI Data Preprocessing
The data preprocessing process employed Data Processing and Analysis for Brain Imaging (DPABI) toolboxes and MATLAB 2013b and utilized the Statistical Parametric Mapping (SPM12). The acquired data underwent various preprocessing steps, including: (1) obtain and categorize the data, (2) converting the DICOM format to NIFTI format, (3) remove the first ten images, (4) analyzing the functional volumes, (5) correcting for time, (6) correcting for head motion, (7) normalizing spatially, (8) smoothing spatially, and (9) eliminating linear data, interference noise, and low-frequency filtering.
Definition of Region of Interest (ROI)
The focus of this research was the hippocampus, which was identified as the region of interest (ROI) based on Brodmann 28. The calculation of FC was performed using the hippocampus as the starting point. Table 2 provides detailed information on the coordinates of the right and left hippocampus, which were (36, −21, −9) and (−36, −21, −9) respectively, as coordinated by the Montreal Neurological Institute (MNI). The radius of the ROI was adjusted to 6 millimeters. We calculated the average time series of all voxels within the ROI and performed Pearson correlation calculation between each voxel and the whole-brain voxel time series. In the end, we obtained a correlation coefficient between the voxel and the pre-selected ROI, as well as a whole-brain functional connectivity map (FC map). After normalization, they were subjected to statistical analysis. (By applying Fisher-Z transformation to convert the distribution of Pearson correlation coefficients from its original skewness to a normal distribution, in order to meet the assumptions of hypothesis testing.)
 |
Table 2 Montreal Neurological Institute Coordinates for Region of Interest |
Functional Connectivity Analysis Based on Seed
To evaluate the FC of the hippocampus, we utilized seed-based FC analysis. Initially, we established the variables for the FC examination. We used the DPABI toolbox to build a sphere with a diameter of 12 mm. Afterwards, we computed Pearson correlation coefficients between the initial region and every voxel in the brain for every individual. Next, Fisher’s z-transform analysis was applied to these coefficients in order to enhance normality. The resulting outcomes were utilized for the FC analysis.
Statistical Analysis
Aggregated clinical and demographic data were analyzed using SPSS 8.0 software. Proportions were analyzed using the chi-squared test, and continuous variables were assessed using independent two-sample t-tests (P < 0.05). The SPM12 software was used to conduct one-sample t-tests for examining patterns of FC intragroup z-values. Furthermore, a comparison was made between the two groups using two-sample t-tests to examine disparities in z-value FC patterns (voxel-level P < 0.01, Gaussian random field [GRF] correction, cluster-level P < 0.01). Furthermore, we utilized Pearson correlation coefficients to investigate the connections between mean FC signal values in various brain regions and clinical features in HM patients (P < 0.05).
Results
Basic Information
The study comprised 82 individuals diagnosed with HM (43 males and 39 females; average age, 26.53 ± 5.291 years) and 59 healthy controls (24 males and 35 females; average age, 25.67 ± 3.102 years). Table 3 displays the demographic attributes.
 |
Table 3 Demographic Characteristics of HM Patients and HCs |
Group Differences in FC
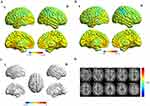
HM patients and HCs are compared in Figures 1 and 2, illustrating the distribution of FC values in the left and right hippocampus. Significantly reduced functional connectivity (FC) values were observed in HM patients compared to HCs in the L-TPOsup, R-HIP, L-MTG, and left hippocampus (Figure 1 and Table 4) (P < 0.01 at voxel-level, GRF correction, P < 0.01 at cluster-level). Additionally, HM patients also had lower FC values in L-TPOsup, L-Cbe6, L-FG, RO-MFG, L-MTG, R-THA, and the right hippocampus (Figure 2 and Table 5) (P < 0.01 at voxel-level, GRF correction, P < 0.01 at cluster-level).
 |
Table 4 Significant Differences in FC Values of the Left Hippocampus Between HM Patients and HCs |
 |
Table 5 Significant Differences in FC Values of the Right Hippocampus Between HM Patients and HCs |
Discussion
In the present investigation, a method based on seed-based functional connectivity (FC), recognized for its dependability and effectiveness, was employed to evaluate information transmission and coordination among different brain regions and the hippocampus. Table 6 demonstrates the prior utilization of this approach in examining individuals with ophthalmic or alternative systemic conditions. Based on our current understanding, this research is the initial exploration of the FC between the hippocampus and other relevant brain regions, focusing specifically on individuals with HM. This study has the potential to enhance our comprehension of the hippocampal connectivity associated with HM. Compared with HCs, FC values in l-tpossup, R-HIP, L-MTG, and the left hippocampus were significantly reduced in HM patients, as were FC values in L-TPOsup, L-Cbe6, L-FG, RO-MFG, L-MTG, R-THA, and the right hippocampus. These findings may offer valuable information about the neural processes implicated in HM and may help to understand the underlying pathogenesis of spatial vision and cognitive impairment in patients with HM.
 |
Table 6 Use of Seed-Based FC Technique for Analysis of Individuals with Ophthalmic or Other Systemic Diseases |
FC Alterations and Their Significance
Patients with HM showed a notable decrease in FC of the left and right hippocampus and the L-TPOsup/L-MTG. Likewise, diminished FC was observed in the bilateral hippocampus. L-TPOsup, L-MTG, R-HIP are all part of the temporal lobe. The temporal pole is unique to primates and is associated with higher brain functions. The hippocampus is widely regarded as a crucial area in the creation and retention of memories that last for a long time, and it plays a role in various aspects including emotional and spatial memory. MTG is situated between the superior temporal gyrus and the inferior temporal gyrus. It contributes to enhancing the speed of word identification.24 Huang observed that the FC between SN and MTG is reduced in depressed patients, which presumably reflects an active downregulation or inhibition in depression.25,26 According to the research carried out by Brueggen K, it was found that changes in tone result in increased stimulation of the temporal pole on the left side of the brain, suggesting its possible involvement in combining the melodic/harmonic background and emotional meaning of music.27 In another research, it was found that individuals with presbycusis showed reduced directed functional links of the hippocampus, which were observed to be associated with particular cognitive abilities.28 To support these findings, we noticed a decrease in both sides of the hippocampus and L-TPOsup/L-MTG, along with a decrease in FC in the hippocampus on both the left and right sides in individuals with HM. This indicates that HM patients may impairments in memory, the transmission and processing of semantic information, as well as visual and social-emotional functioning.
The RO-MFG is a region of the cerebral cortex located between the supraorbital bone (orbital bone) and the frontal lobe, primarily participates in the processing of conditioned emotions and the regulation of cognitive functions.29,30 A study conducted in 2019 discovered a reduction in ALFF within the RO-MFG region among individuals diagnosed with anxious depression (AD), indicating a potential neural alteration underlying AD.31 A different investigation found that individuals experiencing their initial bout of depression displayed decreased ALFF in the right orbitofrontal cortex when compared to individuals without depression, suggesting impaired control of the reward system in those with the first depressive episode.32 Similarly, our study found significantly lower FC values in the RO-MFG among HM patients, indicating impaired functioning of the RO-MFG. We believe that individuals with HM might undergo a deterioration in cognitive abilities and encounter difficulties in processing emotions.
The L-FG plays a crucial role in the ventral visual pathway and is strongly linked to different visual cognitive processes, aiding in the integration of pertinent multisensory data.33 A study conducted in 2018 found that migraine patients with aura (MA) experienced a decrease in L-FG volume, suggesting a connection between migraines and disturbances in the integration of multiple senses and the processing of memory.34 According to Jung et al, the study conducted in 2021 discovered reduced sizes of gray matter in the hippocampus on the left side and the Fusiform Gyrus among individuals diagnosed with schizophrenia spectrum disorders. This indicates a potential connection between atypical gray matter in the L-FG and difficulties in recognizing social-emotional cues as well as the intensity of negative symptoms.35 Disruptions in visual function may have led to a decrease in FC in the L-FG of HM patients, as revealed by our study. These changes could potentially result in modifications to the brain’s structure, leading to subsequent impairments in visual perception, recognition of social-emotional cues, and other cognitive functions.
The L-Cbe6 is situated in the posterior region of the brain and is responsible for coordinating movements, balance, and posture. Motor control and various cognitive functions are heavily reliant on its pivotal role. Niu identified reduced functional connectivity in the L-Cbe6 among smokers during a seed-based functional connectivity analysis. This may contribute to the dysfunction associated with chronic smokers.36 During our study, we noticed a notable decrease in FC within the L-Cbe6 region among individuals with HM. We hypothesize that HM could lead to impaired functioning in the L-Cbe6, potentially causing difficulties in movement, balance, and posture in individuals with HM.
The thalamus, positioned between the brainstem and the cerebral cortex, serves a crucial function in the transmission of information. Multiple nuclei within the thalamus are responsible for processing tactile information, motor control, and regulating emotions. Xia found that individuals with vestibular migraine (VM) had a notable reduction in the thalamus’ amplitude of low-frequency fluctuations (ALFF) when compared to the HCs. A negative correlation was observed between ALFF and the frequency of VM episodes in the left thalamus within the VM group. This implies that irregularities in the connection of the thalamocortical area are involved in the atypical handling, control, transfer, and merging of pain data in individuals with VM.37 In addition to these findings, a reduction in FC was also noted in the left hippocampus and thalamus among individuals diagnosed with high myopia (HM). We hypothesized that patients with high myopia may have dysfunction in coordination and integration for various brain functions.
Nonetheless, our study does have certain limitations. These include a relatively modest sample size and variations in refractive diopter among patients with HM. Moreover, variations in the timing of HM onset could potentially affect the precision of the outcome.
Conclusion
The results of our inquiry indicate that individuals with HM exhibit significant alterations in the FC of various brain regions when compared to HCs. This suggests that HM causes significant alterations in brain region activity, which may present relevant clinical symptoms. These findings provide new viewpoints on the origin and neural processes of HM, which could potentially provide valuable understanding in diagnostic considerations and facilitating early-stage treatment for patients with HM to prevent the development of neurological diseases, and enable necessary preventive measures.
Prospect
Recently, static and dynamic fMRI studies have consistently shown that high myopia and associated eye diseases can result in abnormalities in the brain areas involved in visual information processing, spatial vision, emotion, and cognitive processing. These abnormalities primarily manifest as decreased performance in the relevant brain areas along with compensatory functional mechanisms. These specific functional abnormalities, as well as the compensatory mechanisms in the corresponding brain areas, are expected to be significant focal points for future studies on high myopia.
Data Sharing Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by the First Affiliated Hospital of Nanchang University (Jiangxi Province, China). The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Funding
This study received support from the National Natural Science Foundation of China (Grant No. 82160207), the Technology Plan of Jiangxi Provincial Health and Health Commission (202130156), and the Science and Key Projects of Jiangxi Youth Science Fund (No. 20202ACBL216008).
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Flitcroft DI, He M, Jonas JB, et al. IMI - defining and classifying myopia: a proposed set of standards for clinical and epidemiologic studies. Invest Ophthalmol Vis Sci. 2019;60(3):M20–M30. PMID: 30817826; PMCID: PMC6735818. doi:10.1167/iovs.18-25957
2. Ikuno Y. Overview of the complications of high myopia. Retina. 2017;37(12):2347–2351. PMID: 28590964. doi:10.1097/IAE.0000000000001489
3. Dolgin E. The myopia boom. Nature. 2015;519(7543):276–278. PMID: 25788077. doi:10.1038/519276a
4. Holden BA, Fricke TR, Wilson DA, et al. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology. 2016;123(5):1036–1042. PMID: 26875007. doi:10.1016/j.ophtha.2016.01.006
5. Zhuang M, Xie H, Zhang Y, et al. Prevalence and influence factors for myopia and high myopia in schoolchildren in Shandong, China. Cent Eur J Public Health. 2022;30(3):190–195. PMID: 36239368. doi:10.21101/cejph.a7158
6. Wong TY, Ferreira A, Hughes R, Carter G, Mitchell P. Epidemiology and disease burden of pathologic myopia and myopic choroidal neovascularization: an evidence-based systematic review. Am J Ophthalmol. 2014;157(1):9–25.e12. PMID: 24099276. doi:10.1016/j.ajo.2013.08.010
7. DeYoe EA, Bandettini P, Neitz J, Miller D, Winans P. Functional magnetic resonance imaging (FMRI) of the human brain. J Neurosci Methods. 1994;54(2):171–187. PMID: 7869750. doi:10.1016/0165-0270(94)90191-0
8. Glover GH. Overview of functional magnetic resonance imaging. Neurosurg Clin N Am. 2011;22(2):133–9, vii. PMID: 21435566; PMCID: PMC3073717. doi:10.1016/j.nec.2010.11.001
9. Yao Y, Lu C, Chen J, et al. Increased resting-state functional connectivity of the hippocampus in rats with sepsis-associated encephalopathy. Front Neurosci. 2022;16:894720. PMID: 35720716; PMCID: PMC9201098. doi:10.3389/fnins.2022.894720
10. Ji Y, Huang SQ, Cheng Q, et al. Exploration of static functional connectivity and dynamic functional connectivity alterations in the primary visual cortex among patients with high myopia via seed-based functional connectivity analysis. Front Neurosci. 2023;17:1126262. PMID: 36816124; PMCID: PMC9932907. doi:10.3389/fnins.2023.1126262
11. Suzuki WA, Amaral DG. Topographic organization of the reciprocal connections between the monkey entorhinal cortex and the perirhinal and parahippocampal cortices. J Neurosci. 1994;14(3 Pt 2):1856–1877. PMID: 8126576; PMCID: PMC6577578. doi:10.1523/JNEUROSCI.14-03-01856.1994
12. Mock VL, Luke KL, Hembrook-Short JR, Briggs F. Dynamic communication of attention signals between the LGN and V1. J Neurophysiol. 2018;120(4):1625–1639. PMID: 29975169; PMCID: PMC6230788. doi:10.1152/jn.00224.2018
13. Yamasaki T, Tobimatsu S. Driving ability in Alzheimer disease spectrum: neural basis, assessment, and potential use of optic flow event-related potentials. Front Neurol. 2018;9:750. PMID: 30245666; PMCID: PMC6137098. doi:10.3389/fneur.2018.00750
14. Tsanov M, Manahan-Vaughan D. Synaptic plasticity from visual cortex to hippocampus: systems integration in spatial information processing. Neuroscientist. 2008;14(6):584–597. PMID: 18612086. doi:10.1177/1073858408315655
15. Ji Y, Shi L, Cheng Q, et al. Abnormal large-scale neuronal network in high myopia. Front Hum Neurosci. 2022;16:870350. PMID: 35496062; PMCID: PMC9051506. doi:10.3389/fnhum.2022.870350
16. Zhang XW, Dai RP, Cheng GW, Zhang WH, Long Q. Altered amplitude of low-frequency fluctuations and default mode network connectivity in high myopia: a resting-state fMRI study. Int J Ophthalmol. 2020;13(10):1629–1636. PMID: 33078115; PMCID: PMC7511385. doi:10.18240/ijo.2020.10.18
17. Ong SY, Ikram MK, Haaland BA, et al. Myopia and cognitive dysfunction: the Singapore Malay eye study. Invest Ophthalmol Vis Sci. 2013;54(1):799–803. PMID: 23307956. doi:10.1167/iovs.12-10460
18. Landelle C, Dahlberg LS, Lungu O, Misic B, De Leener B, Doyon J. Altered spinal cord functional connectivity associated with parkinson’s disease progression. Mov Disord. 2023;38(4):636–645. PMID: 36802374. doi:10.1002/mds.29354
19. Kroemer NB, Opel N, Teckentrup V, et al. Functional connectivity of the nucleus accumbens and changes in appetite in patients with depression. JAMA Psychiatry. 2022;79(10):993–1003. PMID: 36001327; PMCID: PMC9403857. doi:10.1001/jamapsychiatry.2022.2464
20. Xia G, Hu Y, Chai F, Wang Y, Liu X, Teekaraman Y. Abnormalities of the default mode network functional connectivity in patients with insomnia disorder. Contrast Media Mol Imaging. 2022;2022:9197858. PMID: 36101797; PMCID: PMC9440808. doi:10.1155/2022/9197858
21. Qi CX, Huang X, Tong Y, Shen Y. Altered functional connectivity strength of primary visual cortex in subjects with diabetic retinopathy. Diabetes Metab Syndr Obes. 2021;14:3209–3219. PMID: 34285528; PMCID: PMC8286104. doi:10.2147/DMSO.S311009
22. Yu Y, Lan DY, Tang LY, et al. Intrinsic functional connectivity alterations of the primary visual cortex in patients with proliferative diabetic retinopathy: a seed-based resting-state fMRI study. Ther Adv Endocrinol Metab. 2020;11:2042018820960296. PMID: 33149884; PMCID: PMC7580186. doi:10.1177/2042018820960296
23. Han Q, Zhang Y, Liu D, et al. Disrupted local neural activity and functional connectivity in subjective tinnitus patients: evidence from resting-state fMRI study. Neuroradiology. 2018;60(11):1193–1201. PMID: 30159629. doi:10.1007/s00234-018-2087-0
24. Zhu Z, Gold BT, Chang CF, Wang S, Juan CH. Left middle temporal and inferior frontal regions contribute to speed of lexical decision: a TMS study. Brain Cogn. 2015;93:11–17. PMID: 25463244. doi:10.1016/j.bandc.2014.11.002
25. Huang H, Chen C, Rong B, et al. Resting-state functional connectivity of salience network in schizophrenia and depression. Sci Rep. 2022;12(1):11204. PMID: 35778603; PMCID: PMC9249853. doi:10.1038/s41598-022-15489-9
26. Cao X, Liu Z, Xu C, et al. Disrupted resting-state functional connectivity of the hippocampus in medication-naïve patients with major depressive disorder. J Affect Disord. 2012;141(2–3):194–203. PMID: 22460056. doi:10.1016/j.jad.2012.03.002
27. Tsai CG, Li CW. Increased activation in the left ventrolateral prefrontal cortex and temporal pole during tonality change in music. Neurosci Lett. 2019;696:162–167. PMID: 30557595. doi:10.1016/j.neulet.2018.12.019
28. Chen YC, Yong W, Xing C, et al. Directed functional connectivity of the hippocampus in patients with presbycusis. Brain Imaging Behav. 2020;14(3):917–926. PMID: 31270776. doi:10.1007/s11682-019-00162-z
29. Xie J, Li L, Wang L, et al. Homotopy of resting-state functional connectivity correlates with psychological distress in adolescent and young adult cancer patients. Front Biosci. 2021;26(12):1470–1479. PMID: 34994162. doi:10.52586/5041
30. Fleck MS, Daselaar SM, Dobbins IG, Cabeza R. Role of prefrontal and anterior cingulate regions in decision-making processes shared by memory and nonmemory tasks. Cereb Cortex. 2006;16(11):1623–1630. PMID: 16400154. doi:10.1093/cercor/bhj097
31. Zhao P, Yan R, Wang X, et al. Reduced resting state neural activity in the right orbital part of middle frontal gyrus in anxious depression. Front Psychiatry. 2020;10:994. PMID: 32038329; PMCID: PMC6987425. doi:10.3389/fpsyt.2019.00994
32. Zhang X, Zhu X, Wang X, et al. First-episode medication-naive major depressive disorder is associated with altered resting brain function in the affective network. PLoS One. 2014;9(1):e85241. PMID: 24416367; PMCID: PMC3887023. doi:10.1371/journal.pone.0085241
33. Kassuba T, Klinge C, Hölig C, et al. The left fusiform gyrus hosts trisensory representations of manipulable objects. Neuroimage. 2011;56(3):1566–1577. PMID: 21334444. doi:10.1016/j.neuroimage.2011.02.032
34. Petrusic I, Dakovic M, Kacar K, Zidverc-Trajkovic J. Migraine with aura: surface-based analysis of the cerebral cortex with magnetic resonance imaging. Korean J Radiol. 2018;19(4):767–776. PMID: 29962883; PMCID: PMC6005951. doi:10.3348/kjr.2018.19.4.767
35. Jung S, Kim JH, Kang NO, et al. Fusiform gyrus volume reduction associated with impaired facial expressed emotion recognition and emotional intensity recognition in patients with schizophrenia spectrum psychosis. Psychiatry Res Neuroimaging. 2021;307:111226. PMID: 33249305. doi:10.1016/j.pscychresns.2020.111226
36. Niu X, Gao X, Lv Q, et al. Increased spontaneous activity of the superior frontal gyrus with reduced functional connectivity to visual attention areas and cerebellum in male smokers. Front Hum Neurosci. 2023;17:1153976. PMID: 37007679; PMCID: PMC10063805. doi:10.3389/fnhum.2023.1153976
37. Zhe X, Tang M, Ai K, Lei X, Zhang X, Jin C. Decreased ALFF and functional connectivity of the thalamus in vestibular migraine patients. Brain Sci. 2023;13(2):183. PMID: 36831726; PMCID: PMC9954115. doi:10.3390/brainsci13020183
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.