Back to Journals » Journal of Experimental Pharmacology » Volume 12
Experimental Carbonic Anhydrase Inhibitors for the Treatment of Hypoxic Tumors
Authors Supuran CT
Received 25 October 2020
Accepted for publication 28 November 2020
Published 15 December 2020 Volume 2020:12 Pages 603—617
DOI https://doi.org/10.2147/JEP.S265620
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Bal Lokeshwar
Claudiu T Supuran
Neurofarba Department, Pharmaceutical and Nutraceutical Section, University of Florence, Florence 50019, Italy
Correspondence: Claudiu T Supuran
Neurofarba Department, Pharmaceutical and Nutraceutical Section, University of Florence, Via Ugo Schiff 6, Sesto Fiorentino, Florence 50019, Italy
Email [email protected]
Abstract: Carbonic anhydrase (CA, EC 4.2.1.1) isoforms IX and XII are overexpressed in many hypoxic tumors as a consequence of the hypoxia inducible factor (HIF) activation cascade, being present in limited amounts in normal tissues. These enzymes together with many others are involved in the pH regulation and metabolism of hypoxic cancer cells, and were validated as antitumor targets recently. A multitude of targeting strategies against these enzymes have been proposed and are reviewed in this article. The small molecule inhibitors, small molecule drug conjugates (SMDCs), antibody-drug conjugates (ADACs) or cytokine-drug conjugates but not the monoclonal antibodies against CA IX/XII will be discussed. Relevant synthetic chemistry efforts, coupled with a multitude of preclinical studies, demonstrated that CA IX/XII inhibition leads to the inhibition of growth of primary tumors and metastases and depletes cancer stem cell populations, all factors highly relevant in clinical settings. One small molecule inhibitor, sulfonamide SLC-0111, is the most advanced candidate, having completed Phase I and being now in Phase Ib/II clinical trials for the treatment of advanced hypoxic solid tumors.
Keywords: carbonic anhydrase, hypoxia, inhibitor, small molecule drug conjugates, anticancer drug, SLC-0111
Introduction
Tumor cells have many features which make them different from normal cells, among which are a more acidic pH outside the cell (pHe in the range of 6.5–6.8), connected with a slightly alkaline cytosolic pH (pHi values around 7.2–7.5), as well as lower than normal levels of O2 (hypoxia): these and other irregularities are known as the Warburg effect,1 from the name of the German physiologist who discovered them and who was awarded a Nobel prize in 1931, for this and similar breakthrough discoveries related to metabolism. In 2019, another Nobel prize for medicine was awarded to three scientists who explained the Warburg effect at the molecular level: Kaelin, Ratcliffe and Semenza, who discovered the transcription factors involved in tumor oxygen levels sensing, i.e., the hypoxia inducible factors 1 and 2 (HIF-1/2).2–5 HIF-1/2 are also involved in the regulation of genes implicated in the metabolism/homeostasis of cancer cells, such as the glucose transporters (GLUT1-4), pH regulation (e.g., carbonic anhydrases, CAs; Na+/H+ exchangers; vacuolar ATPase, etc.), transport of anions, such as the monocarboxylate transporters (MCTs), the sodium bicarbonate co-transporters, as well as angiogenesis (vascular endothelial growth factor VEGF).6–18 These abnormalities of tumor microenvironment (lower levels of oxygen, disturbed pH balance, upregulated glucose metabolisms, etc.) were considered as potential opportunities to develop anti-tumor drugs, which might specifically target tumor cells without affecting normal ones, more than one decade ago.18,19 In fact, the Warburg effect started to be exploited for diagnostic purposes several decades ago, in the 1980s, through the use of 18F-fluoro-deoxyglucose (FDG) for positron emission tomography (PET) imaging, as this glucose analog is a substrate for several GLUT isoforms and has a rapid uptake in cancer cells, thus making possible precise tumor imaging by means of computer tomography (CT) scans.20–23 However, the development of novel antitumor drugs based on this approach was more difficult than originally expected, although already in 2006 Pouysségur,18 and later Neri and Supuran,19 proposed various potential drug targets, such as the Na+/HCO3− co-transporters, Na+/H+ exchangers, anion exchangers (e.g., chloride for bicarbonate exchangers), monocarboxylate transporters (MCTs), vacuolar ATPase as well as several CA isoforms. At present only therapies which target VEGF, such as the monoclonal antibody (Mab) bevacizumab and similar biological drugs (known as anti-angiogenesis therapies), have been highly successful and constitute an important component of antitumor therapies for a variety of cancers.24–27 Other proteins whose genes are under the control of HIF-1/2 proved to be less “druggable” than originally thought.18,19,28 There is just one exception, which is constituted by the CA isoforms overexpressed in tumors as a consequence of the HIF-1/2 activation cascade, CA IX and XII, which will be discussed in this article.
Validation of CA IX/XII as Antitumor Drug Targets
The CAs (EC 4.2.1.1) are a superfamily of metalloenzymes widespread in all life kingdoms, that catalyze the conversion of CO2 to bicarbonate.29–33 As the hydration of CO2 generates a proton, whereas the reversed reaction, i.e., bicarbonate dehydration consumes one, these enzymes are involved primarily in pH regulation in many cells, tissues and organisms, but also in several metabolic processes.29–33 The field of CAs29–33 and their inhibitors34–40 with pharmacological applications has been reviewed extensively and will be not discussed in detail here. It should however be mentioned that CA inhibitors (CAIs) of the sulfonamide/sulfamate type have been known for decades and are still in clinical use as diuretics, anti-glaucoma, anti-epileptic and anti-obesity drugs.29,33–39 More recent applications of these as well as newer pharmacological agents belonging to the CAIs, demonstrated their potential in the management of neuropathic pain,40 cerebral ischemia,41 rheumatoid arthritis,42,43 or as anti-infective agents (for the treatment of bacterial, fungal and protozoan infections).44–50 However, the use of CAIs as antitumor agents51–57 constituted the subject of very intense research over the last two decades, with significant progress being achieved, and these derivatives will be reviewed here.
Two of the 15 known human (h) CA isoforms, hCA IX58 and XII59 are predominantly found in tumor cells and show a rather limited diffusion in normal cells. Both isoforms are multi-domain trans-membrane proteins with an extracellular CA domain, and were demonstrated to participate in the rather complex machinery of pH regulation,57–63 which as mentioned above, is dysregulated in cancer cells due to the activation of HIF-1/2. It should also be noted that these two enzymes are just a part of the complex network of proteins/processes which regulate pH and metabolism in tumor cells, most of which were mentioned in the Introduction.18,19 However, proof-of-concept studies from Pastorekova’s60,61 and Harris’62,63 laboratories demonstrated that hCA IX (but presumably hCA XII has a similar role) participates significantly in the extracellular acidification of tumor cells, with the concomitant alkalization of the cytosol.
Essential for the validation were the fluorescent sulfonamides 1 and 2 incorporating fluorescein thioureido moieties,60,61 which unlike the classical, clinically used sulfonamide acetazolamide 3 (Figure 1) showed a slightly selective inhibition of the transmembrane isoforms hCA IX/XII over the cytosolic enzymes hCA I and II, and were also membrane-impermeant due to the presence of the carboxylate moieties.61 The use of these inhibitors demonstrated on one hand the significant effect of catalytically active hCA IX in the extracellular acidification but also the fact that the inhibitor effectively binds to the enzyme only in hypoxia and not in normoxia, which was considered as a very promising finding. Indeed, inhibition of hCA IX with such compounds reverted the acidification of extracellular pHe in cell cultures, with an almost normalization of the parameter which from 6.5 (pHe of the tumor cells) returned to a more normal physiological value of 7.2–7.3 (after the use of inhibitors such as 1 and 2).60,61 Furthermore, the fact that the fluorescent inhibitors were observed bound only in hypoxia, constituted the first evidence that targeting hCA IX/XII may also have applications for imaging, not only for the treatment of hypoxic tumors. Indeed, intense research was thereafter initiated in several laboratories for finding more effective, drug-like anti-tumor CAIs.
 |
Figure 1 Fluorescent inhibitors 1, 2 used to validate hCA IX/XII as antitumor drug targets60–63 and the pan-inhibitor acetazolamide 3. |
Antitumor CAIs Developed in Zurich
The first CAIs which were reported to show antitumor effects in vivo, were compounds 4 and 5 discovered by Neri’s group in 2009 (Figure 2).64 Over the years, this group made significant contributions to the field, with many interesting approaches being proposed for targeting CA IX by scientists from this laboratory.65–72
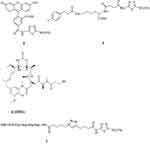 |
Figure 2 Acetazolamide-based CAIs 4, 5 and 7 reported by Neri’s group and the cytotoxin 6 (DM1).64,66 |
Derivatives 4 and 5 incorporated the acetazolamide scaffold as binding moiety to the enzyme zinc ion, and tails73 of the fluorescein carboxamide type in 4, similar to 1 and 2 reported earlier,60,61 as well as the albumin-binding moiety in 5.64 Both derivatives showed effective in vitro and in vivo binding to hCA IX, and 5 was also active in vivo, in a SK-RC-52 xenograft model of cancer.64
Neri’s group also reported the first DNA-encoded chemical libraries for obtaining tight binding CA IX inhibitors.65 This approach, apart from being highly innovative, also afforded sub-micromolar bis-sulfonamide CAIs, some of which have been prepared without the DNA-tag. They showed accumulation within the hypoxic tumor tissue and effective in vivo anti-tumor action.65 Subsequently, another pioneering approach has been proposed by the same group: small molecule drug conjugates (SMDCs) targeting CA IX and incorporating toxin payloads such as the cytotoxic, DNA-binding agents duocarmycins or the tubulin inhibitor mertansine (also known as DM1, compound 6 in Figure 2).66 Such SMDCs again incorporated the acetazolamide scaffold for targeting CA IX, to which a water-solubilizing tetrapeptide fragment has been attached by using click chemistry of the alkyne-azide cycloaddition type. The peptide fragment contained the Cys terminal amino acid residue, which reacts with the SH group of mertansine, forming a disulfide bond by which the drug conjugate has been obtained (derivative 7 in Figure 2). The SMDC 7 showed effective antitumor effects in subcutaneous SK-RC-52 (renal cell carcinoma tumors) xenograft models.66
The same approach has been thereafter explored and enriched for acetazolamide/benzenesulfonamide CAIs serving as selective delivery vehicles for radionuclides (99mTc),67 antibodies,68 dipeptide-linked renal cell carcinoma-targeting agents,68 cytokines (such as IL-2)70,72 and other small molecule toxins (e.g., auristatin methyl ester, cryptophycin)69,71 as drug conjugates. All these approaches were highly successful in leading to SMDCs, antibody-drug conjugates (ADACs) or cytokine-drug conjugates with an enhanced antitumor effect compared with the parent molecules from which they were obtained. The ETH–Neri group approach is definitely one of the most effective and innovative, in generating small molecules or conjugates with a very interesting pharmacological profile for targeting CA IX.
The Maastricht–Montpellier–Tampere–Florence Approach
Significant contributions to the discovery of interesting CA IX/XII inhibitors were made by Winum’s group in Montpellier, in collaboration with Lambin’s group in Maastricht, Parkkila’s group in Tampere and our group in Florence.74–79 Only the most relevant papers for the present review will be considered here. In fact many other contributions were achieved by these researchers in other fields connected to CAIs, which have been reviewed elsewhere.6,8,32–34
A very interesting idea from Winum’s laboratory was that of coupling the nitroazole chemotypes, present in many radio/chemosensitizing agents, with CAIs belonging to the sulfonamide, sulfamate or sulfamide type.74,75 Compounds prepared by this approach (derivatives 8–11, Figure 3) incorporated 2- or 5-nitroimidazoles and a range of aromatic scaffolds on which the zinc-binding groups (ZBGs) responsible for binding to CA were appended, particularly the primary sulfonamide, sulfamate and sulfamide moieties (Figure 3). The binding of some of these compounds (e.g., 10 and 11) to hCA II, an off-target isoform, was also investigated by means of X-ray crystallography, proving interesting interactions between the inhibitor scaffold and the enzyme active site.75
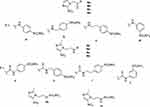 |
Figure 3 Nitroazole-containing CAIs of types 8–11.74–79 |
Effects on hypoxia-induced extracellular acidification in the presence of compounds such as 10 and 11 (which are low nanomolar hCA IX/XII inhibitors)75 was evaluated in vitro, in HT29 and HeLa cancer cells, whereas HT-29 tumor bearing mice were treated with the compounds alone or in combination with radiation.74–76 One compound, sulfamide 11, markedly enhanced sensitization towards radio- and chemotherapy, when administered alone or in combination with the anticancer agent doxorubicin, thus proving the usefulness of this approach.74,75 Furthermore, these compounds were shown to be non-toxic in vivo in several animal models77,78 whereas some newer congeners were also reported recently.79
University of Manchester–University of Florence Sulfamate CAIs
A series of aromatic sulfamates, highly effective as hCA IX/XII inhibitors, was reported by Williams’ group in Manchester in collaboration with our group.80,81 These derivatives, of types 12–15 (Figure 4), incorporate the sulfamate ZBG (present also in derivative 10 discussed above) and most of them possess aromatic-ureido (12) or thioureido (13) tails. The sulfamate fluorescent derivative 14, structurally similar to the early sulfonamide derivatives 1 and 2 which allowed the validation of CA IX as an antitumor drug target, was also reported in the same study. In this rather large series of congeneric ureas/thioureas 12 and 13, the compound which underwent a thorough investigation in vitro and in vivo was 15, also known as S4.80,81 This compound was a highly effective in vitro hCA IX (Ki of 7 nM) and hCA XII (Ki of 2 nM) inhibitor, and in an orthotopic MDA-MB-231 breast carcinoma tumor model in mice showed a significant reduction of the primary tumor and metastases growth at a dose of 10 mg/kg.80 Furthermore, S4 was also active in a small cell lung cancer (SCLC) animal model, in mice, both alone and in combination with cisplatin, through synergistic, hypoxia-specific targeting.81
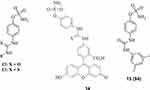 |
Figure 4 Sulfamates incorporating ureido (compounds 12, 14 and 15) and thioureido (13) moieties, with effective CA IX/XII inhibitory action and investigated for their antitumor effects. S4 was the most extensively investigated such derivative.80 |
Brisbane–Florence Glycomimetic CAIs
The collaboration between Poulsen’s group in Brisbane, Australia and the Florence group led to a large number of highly effective hCA IX/XII inhibitors belonging mainly to the sulfonamide, sulfamate and sulfamide classes.82–90
Many such compounds, among which an example of sulfonamides is presented in Figure 5, incorporate sugar moieties and they were termed as glycomimetics. The presence of sugars is beneficial both for the interaction with the enzyme, as many of these CAIs are highly effective inhibitors,82–90 but also for their physico-chemical and pharmacological properties, as the presence of the sugar moieties enhances water solubility and bioavailability. The drug design was attentively performed, as most of these compounds incorporate three fragments: the CA inhibitory fragment, belonging to the aromatic sulfonamide, aromatic/aliphatic sulfamate and sulfamide, linked through a triazole unit to the sugar fragment, which can be with free OH or acylated moieties. A range of sugars (mono-, di- and tri-saccharides), as well as their acetylated, propionylated or butylated esters were used for generating a remarkable number of CAIs. Furthermore, the chemical diversity was enhanced also by using various position of the sugar where the other two elements were appended (compare for example 16 and 22 in Figure 5). These compounds were prepared by so-called click-tailing, using the cycloaddition reaction between azides and alkynes, also known as “click chemistry”.82–90 In some cases, saccharin90 derivatives were also obtained with sugar clicked tails, based on the early finding from our and Klebe’s laboratory91 that saccharin is an effective CA IX inhibitor and binds to the metal ion from the CA active site as anion, through the secondary sulfonamide nitrogen atom. Some of these compounds showed effective CA IX/XII inhibition in vitro, and some of them were shown to interfere with cell proliferation and to induce cell apoptosis in T-cell lymphomas expressing CA XII.88
 |
Figure 5 Examples of glycomimetic CAIs incorporating benzenesulfonamide, sugar and 1,2,3-triazole moieties, of types 16–22.82–89 |
In another study, gallium-68 radiolabeled sulfonamides targeting CA IX were reported, which were investigated in a mouse xenograft HT29 tumor model.89 An accumulation of radioactivity within the tumor and a low uptake in blood, with clearing into the urine has been observed, which makes them of interest as imaging agents. It should be mentioned that this approach was in fact reported earlier by the Vancouver group.92–95
Another research line opened by the Poulsen–Supuran collaboration was on the natural product sulfonamide Psammaplin C, 23 (Figure 6), which was discovered in 1991 in the marine sponge Pseudoceratina purpurea.96 The derivative comprises a bromotyrosine-oxime functionality as well as a functionalized aminoethyl-sulfonamide fragment, being one of the very few natural products to incorporate such a moiety. Psammaplin C was shown to be a low nanomolar hCA IX inhibitor (Ki of 12.3 nM) and a subnanomolar hCA XII inhibitor (Ki of 0.79 nM) and its X-ray crystal structure in complex with hCA II (Ki of 88 nM) was also resolved.96 In a subsequent study, it has been demonstrated that combining Psammaplin C (as well as some of its synthetic derivatives), with temozolomide, a clinically used chemotherapeutic agent, reversed multidrug resistance and significantly increased survival in an animal model of glioblastoma, a highly aggressive brain tumor.97
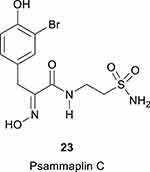 |
Figure 6 The natural product sulfonamide Psammaplin C, 23.96 |
Vancouver–Florence – The Winning Approach
The collaboration between Dedhar’s and Supuran’s groups started in 2010, and led shortly thereafter to the identification of a series of ureido-substituted benzenesulfonamides acting as CA IX/XII-selective in vitro CAIs, but also showing very promising in vivo antitumor/antimetastatic effects.98,99
Benzenesulfonamides incorporating carboxamido- and secondary sulfonamide linkers were in fact known for many years,29,32–34 and, although showing potent inhibitory action, no relevant selectivity for isoforms of interest was ever evidenced. Thus, it was rather surprising that the presence of ureido linkers, as in compounds 24 and 25 (Figure 7) as well as many of their congeners, led to many highly isoform-selective CAIs.98,100 This was explained when McKenna’s group reported the X-ray crystal structure of several of these derivatives bound to hCA II.98,100 It was observed that due to the flexibility conferred by the ureido linker, the tails of such sulfonamides were able to bind in very different regions of the active site, towards its entrance, which is the most variable part of the different CA isoforms. This in fact may explain the observed selectivity of many ureido sulfonamides to diverse CA isoforms. For example 25 (later known as SLC-0111, Figure 7) had a Ki of 4.5 nM for hCA XII, of 45 nM for hCA IX and > 400 nM for all other 10 catalytically active human isoforms.98–100 Initial in vivo experiments were done with the nitro-derivative 24 and thereafter with SLC-0111 25,98,99 as well as the coumarin CAIs 26 and 27,101 in orthotopic breast cancer models expressing CA IX (the 4T1 cell line) or without any CA IX expression (67NR cell line). A marked decrease of the primary tumor growth was observed only for the 4T1 line, irrespective of the CAI used (all these compounds 24–27 are low nanomolar CA IX/XII inhibitors and poorly inhibit the cytosolic off-target isoforms CA I and II). Furthermore, mice harboring the 4T1 tumors also spontaneously develop lung metastases. After treatment with the CAIs of type 24–27 a strong reduction in the formation of the lung metastases or even their total lack (at higher doses of inhibitor) was also observed proving that the inhibitors are effective in inhibiting the growth of the primary tumors and metastases.98–101
 |
Figure 7 Ureido-sulfonamides 24 and 25 and 7-glycosyl-substituted coumarins 26, 27.98–101 |
Regarding the coumarin CAIs, it should be mentioned that they were discovered in 2009 by a collaboration with Poulsen’s group in Brisbane,102 and they represented a completely new CAI chemotype with an unprecedented inhibition mechanism. The coumarins act as prodrug inhibitors, being hydrolyzed by the esterase CA activity at the lactone ring, and the formed hydroxyl-cinnamic acid thereafter binds at the entrance of the active site cavity, obstructing its entrance.102,103
Apart the dual effect on the primary tumors and metastases, subsequent work from Dedhar’s group104,105 with CAIs of the type mentioned above as well as genetic depletion of CA IX with small hairpin RNAs, showed a third beneficial effect of this class of compounds: depletion of the cancer stem cells population, which is considered a clinically significant phenomenon. In another paper from the same group106 it has also been shown that CA IX activates a matrix metalloproteinase isoform (MMP-14) for initiating the invasion which is essential for tumor cell migration from the primary tumor to other organs for the formation of metastases. Similar effects were thereafter observed with structurally related sulfonamides to SLC-0111, which possess the sulfonamide moiety in meta to the ureido functionality,107 proving that the antitumor/antimetastatic effects are a general feature of the potent, CA IX/XII – selective compounds.
Subsequent studies from the Vancouver group evidenced the synergistic effects of SLC-0111 in combination with other anticancer drugs in various animal models, such as pancreatic cancer (combination with gemcitabine);108 combination with immune check point inhibitors;109 combination with temozolomide for glioblastoma treatment,110 etc.
Other research groups apart from the Vancouver one were also active in employing SLC-0111, alone or in combination with other agents/procedures, observing interesting antitumor effects. They include the observation that interference with pH regulation in hepatocellular carcinomas, by using CA IX inhibitors, has a potent antitumor effect,111 which was also evident when CA IX inhibitors were administered together with radiation,112 proton pump inhibitors (lansoprazole, omeprazole),113 cisplatin,81 antimetabolites,114 apurinic/apyrimidinic endonuclease 1 (APE1) inhibitors,115 histone deacetylase inhibitors,116 etc. Another study showed the lack of endothelial toxicity of SLC-0111,117 whereas 24 (also known as U104) was shown to reduce prostate cancer118 and breast cancer119 cell growth by research groups which did not participate in the discovery of these drugs. Indeed, SLC-0111 was proved to be useful in diverse biomedical studies not related to carcinogenesis, when selective CAIs were needed, such as the demonstration that CA IX is involved in the pH regulation of pulmonary microvascular endothelial cells,120,121 or in proving that CA VB is involved in mitochondrial biogenesis and production of lactate by human Sertoli cells.122
SLC-0111 entered in Phase I clinical studies as an antitumor/antimetastatic agent in 2014 and the quite positive report of the study was recently published.123 In 2017 a Phase Ib/II study was initiated which is still in progress, for assessing the efficacy of SLC-0111 in combination with other therapeutic agents for the management of pancreatic cancer (ClinicalTrials.gov Identifier: NCT03450018).
Various Other Approaches for Designing CA IX/XII Inhibitors
The interesting results observed with SLC-0111 and its congeners as antitumor/antimetastatic agents, fostered a large number of research studies in the design of CAIs which used this compound as the lead molecule. For lack of space we will be unable to mention all of them, but some relevant examples will be presented.
As shown in Figure 8, a large number of compounds have been designed and often also investigated in anticancer studies for efficacy, using SLC-0111 as the lead compound.107,124–134
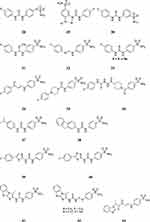 |
Figure 8 Sulfonamides 28–43 designed as anti-cancer derivatives using SLC-0111 as lead molecule. |
The main approaches for obtaining these compounds were: (i) modification of ZBG which in SLC-0111 is of the primary sulfonamide type, but it has been changed to sulfamate in compound 28;80 (ii) modification of the position of the sulfamoyl moiety, as in compounds 29 and 30, which have the ZBG in meta, not in para to the ureido functionality;107 (iii) modification of the ureido linker, as in compounds 30–36, which incorporate thioureido, selenoureido, N cyanoguanidino, triazene, enaminone, piperazino-carboxamide and piperidine-carboxamide functionalities;124–130 (iv) modification of the tail, as in compounds 37–41, in which the 4-fluorophenyl moiety has been changed to various other moieties, similar or much more different to the original compound;131–133 (v) more drastic changes of both the tail and the linker present in the lead, as in derivatives 42 and 43.134 It should be mentioned that polyaminocarboxylate-polycyclic moieties of the DOTA type have also been introduced in the SLC-0111 scaffold (in place of the fluorine atom) for obtaining compounds able to complexate PET emitting isotopes such as111 In- and 90Y-, with such labeled ureidosulfonamides being useful for SPECT imaging.135 For lack of space it is impossible to mention many other highly valuable studies of drug design of various other classes of CAIs.
Essential for these studies were also the X-ray crystallography data obtained by many groups, but in which McKenna’s was undoubtedly the most relevant, with a huge number of compounds belonging to a variety of classes crystallized in complex with hCA II, hCA IX (or one of its mimics) as well as other isoforms.98,100,136–144 This led to a thorough understanding of the favorable interactions between the inhibitor scaffold and the enzyme and paved the way towards more efficient and isoform-selective inhibitors.
A Recent Controversy on Antitumor CAIs
Recently, in a controversial paper, Jonsson and Liljas145 queried the validity of CA IX/XII as antitumor drug targets as well as the inhibition data generated in my laboratory, with a direct attack on the most advanced drug candidate available to date, SLC-0111. They state that “it is doubtful that SLC-0111 has the required Ki to advance to clinical trials” and that the “commercial interests of pharmaceutical companies and patients may be hurt”.145 As shown in this review, and elsewhere, as a reply to this attack,53,146 a multitude of studies form various laboratories all over the world, through an effort of more than 20 years, allowed the validation of the two enzymes as drug targets. Furthermore, very diverse techniques such as stopped-flow kinetics,53 native mass spectrometry measurements,147–149 and X-ray crystallography strongly support the Ki values obtained in our laboratory using the stopped-flow kinetic method for a range of different types of CAIs and demonstrate that this provides sufficient accuracy levels for drug discovery and development applications. It should also be noted that more than 10,000 different CAIs were synthesized and assayed in our laboratory and a number of >15,000 were assayed through collaborations with >100 academic research groups and 15 pharmaceutical companies worldwide during the three decades of activity in CA research. I have not mentioned in detail here, but we have reported more than 80% of the new chemotypes acting as CAIs and discovered at least three innovative CA inhibition mechanisms, i.e., anchoring to zinc coordinated water, occlusion of the active site entrance and binding out of the active site.146,150 Liljas is a crystallographer who only reported the X-ray crystal structure of just one isoform (hCA II) in complex with three sulfonamides and five inorganic anions, and never worked in anticancer drug design.145 We thus consider the statements from the above-mentioned paper as totally erroneous and motivated by non-scientific issues.
Conclusions
CA IX/XII are activated through the HIF-1/2 cascade in hypoxic tumors and were validated as antitumor/antimetastatic targets in recent years. CA IX and XII play crucial roles in regulating the extracellular pH in tumor cells, and were shown to be abundantly expressed in many types of advanced solid metastatic tumors present in a variety of organs (kidneys, lung, breast, colon, liver, prostate, melanoma, etc). As a result of careful studies involving chemical synthesis, discovery of new chemotypes and CA inhibition mechanisms, stopped-flow kinetic measurements, X-ray crystallography, mass spectrometry and other techniques, involving the screening of tens of thousands of potential inhibitors, many key lead compounds targeting CA IX and XII emerged, including the sulfonamide CA inhibitor SLC-0111, which is presently in Phase Ib/II clinical trials. A variety of pre-clinical cancer models were developed, all demonstrating that these two enzymes are promising cancer therapeutic targets in advanced, hypoxic solid tumors, due to the fact that they possess at least three beneficial effects: they reduce the growth of the primary tumor, inhibit the formation of metastases, and deplete the number of cancer stem cells. We anticipate that the use of selective CA IX/XII inhibitors, such as SLC-0111 or many of the interesting compounds developed in other laboratories, will be beneficial in combination with chemo-, radiation- and immuno- therapies for eliminating resistant cancer cell populations and for a durable suppression of tumor cell growth and metastasis.
Disclosure
Claudiu T Supuran reports Welichem Biotech Inc. is developing the drug that I have discovered, SLC-0111, during the conduct of the study; in addition, Claudiu T Supuran has a patent WO2012/021963 issued to Wellichem. The author reports no other potential conflicts of interest for this work.
References
1. Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309–314. doi:10.1126/science.123.3191.309
2. Semenza GL. Pharmacologic targeting of hypoxia-inducible factors. Annu Rev Pharmacol Toxicol. 2019;59:379–403.
3. Pugh CW, Ratcliffe PJ. New horizons in hypoxia signaling pathways. Exp Cell Res. 2017;356:116–121.
4. Kaelin WG. The VHL tumor suppressor gene: insights into oxygen sensing and cancer. Trans Am Clin Climatol Assoc. 2017;128:298–307.
5. Schito L, Semenza GL. Hypoxia-inducible factors: master regulators of cancer progression. Trends Cancer. 2016;2:758–770.
6. Ebbesen P, Pettersen EO, Gorr TA, et al. Taking advantage of tumor cell adaptations to hypoxia for developing new tumor markers and treatment strategies. J Enzyme Inhib Med Chem. 2009;24(Suppl 1):1–39.
7. Schwartz L, Supuran CT, Alfarouk KO. The Warburg effect and the hallmarks of cancer. Anticancer Agents Med Chem. 2017;17:164–170.
8. Pettersen EO, Ebbesen P, Gieling RG, et al. Targeting tumour hypoxia to prevent cancer metastasis. From biology, biosensing and technology to drug development: the METOXIA consortium. J Enzyme Inhib Med Chem. 2015;30(5):689–721.
9. Wykoff CC, Beasley NJ, Watson PH, et al. Hypoxia-inducible expression of tumor-associated carbonic anhydrases. Cancer Res. 2000;60:7075–7083.
10. Wykoff CC, Beasley N, Watson PH, et al. Expression of the hypoxia-inducible and tumor-associated carbonic anhydrases in ductal carcinoma in situ of the breast. Am J Pathol. 2001;158(3):1011–1019. doi:10.1016/S0002-9440(10)64048-5
11. Beasley NJ, Wykoff CC, Watson PH, et al. Carbonic anhydrase IX, an endogenous hypoxia marker, expression in head and neck squamous cell carcinoma and its relationship to hypoxia, necrosis, and microvessel density. Cancer Res. 2001;61:5262–5267.
12. Loncaster JA, Harris AL, Davidson SE, et al. Carbonic anhydrase (CA IX) expression, a potential new intrinsic marker of hypoxia: correlations with tumor oxygen measurements and prognosis in locally advanced carcinoma of the cervix. Cancer Res. 2001;61:6394–6399.
13. Giatromanolaki A, Koukourakis MI, Sivridis E, et al. Expression of hypoxia-inducible carbonic anhydrase-9 relates to angiogenic pathways and independently to poor outcome in non-small cell lung cancer. Cancer Res. 2001;61:7992–7998.
14. Turner KJ, Crew JP, Wykoff CC, et al. The hypoxia-inducible genes VEGF and CA9 are differentially regulated in superficial vs invasive bladder cancer. Br J Cancer. 2002;86:1276–1282.
15. Mandriota SJ, Turner KJ, Davies DR, et al. HIF activation identifies early lesions in VHL kidneys: evidence for site-specific tumor suppressor function in the nephron. Cancer Cell. 2002;1:459–468.
16. Stessels F, Van den Eynden G, Van der Auwera I, et al. Breast adenocarcinoma liver metastases, in contrast to colorectal cancer liver metastases, display a non-angiogenic growth pattern that preserves the stroma and lacks hypoxia. Br J Cancer. 2004;90:1429–1436.
17. Chia SK, Wykoff CC, Watson PH, et al. Prognostic significance of a novel hypoxia-regulated marker, carbonic anhydrase IX, in invasive breast carcinoma. J Clin Oncol. 2001;19:3660–3668.
18. Pouysségur J, Dayan F, Mazure NM. Hypoxia signalling in cancer and approaches to enforce tumour regression. Nature. 2006;441:437–443.
19. Neri D, Supuran CT. Interfering with pH regulation in tumours as a therapeutic strategy. Nat Rev Drug Discov. 2011;10:767–777.
20. Beaney RP. Positron emission tomography in the study of human tumors. Semin Nucl Med. 1984;14(4):324–341.
21. Nanni C, Zamagni E. Fluorodeoxyglucose-PET/Computed Tomography as a Predictor of Prognosis in Multiple Myeloma. PET Clin. 2019;14(3):383–389.
22. Rubello D, Marzola MC, Colletti PM. The Prognostic Value of 18F-FDG PET/CT in Monitoring Chemotherapy in Ovarian Cancer Both at Initial Diagnosis and at Recurrent Disease. Clin Nucl Med. 2018;43(10):735–738.
23. Kozaka K, Kobayashi S, Takamura H, et al. Differences in 18F-FDG Uptake and Expression of Glucose Transporter Between 2 Distinct Subtypes of Mass-Forming Intrahepatic Cholangiocarcinomas. Clin Nucl Med. 2020;45(6):e267–e273.
24. Glade-Bender J, Kandel JJ, Yamashiro DJ. VEGF blocking therapy in the treatment of cancer. Expert Opin Biol Ther. 2003;3(2):263–276.
25. Singh N, Badrun D, Ghatage P. State of the art and up-and-coming angiogenesis inhibitors for ovarian cancer. Expert Opin Pharmacother. 2020;21(13):1579–1590.
26. De Luca E, Marino D, Di Maio M. Ramucirumab, a second-line option for patients with hepatocellular carcinoma: a review of the evidence. Cancer Manag Res. 2020;12:3721–3729.
27. Papachristos A, Sivolapenko GB, Pharmacogenomics P. Circulating proteins as biomarkers for bevacizumab treatment optimization in patients with cancer: a review. J Pers Med. 2020;10(3):E79.
28. Chafe SC, McDonald PC, Dedhar S. pH regulators of the tumor microenvironment. A general overview. In: Supuran CT, Carradori S, editors. Ph-Interfering Agents as Chemosensitizers in Cancer Therapy. London, UK: Elsevier; 2021:13–33.
29. Supuran CT. Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov. 2008;7:168–181.
30. Supuran CT. Structure and function of carbonic anhydrases. Biochem J. 2016;473:2023–2032.
31. Nocentini A, Supuran CT. Advances in the structural annotation of human carbonic anhydrases and impact on future drug discovery. Expert Opin Drug Discov. 2019;14:1175–1197.
32. Supuran CT. Advances in structure-based drug discovery of carbonic anhydrase inhibitors. Expert Opin Drug Discov. 2017;12:61–88.
33. Supuran CT. Exploring the multiple binding modes of inhibitors to carbonic anhydrases for novel drug discovery. Expert Opin Drug Discov. 2020;15(6):671–686.
34. Supuran CT. How many carbonic anhydrase inhibition mechanisms exist? J Enzyme Inhib Med Chem. 2016;31:345–360.
35. Supuran CT. Carbonic anhydrase inhibitors and their potential in a range of therapeutic areas. Expert Opin Ther Pat. 2018;28:709–712.
36. Scozzafava A, Supuran CT, Carta F. Antiobesity carbonic anhydrase inhibitors: a literature and patent review. Expert Opin Ther Pat. 2013;23:725–735.
37. Supuran CT. Applications of carbonic anhydrases inhibitors in renal and central nervous system diseases. Expert Opin Ther Pat. 2018;28:713–721.
38. Supuran CT, Altamimi ASA, Carta F. Carbonic anhydrase inhibition and the management of glaucoma: a literature and patent review 2013–2019. Expert Opin Ther Pat. 2019;29:781–792.
39. Supuran CT. The management of glaucoma and macular degeneration. Expert Opin Ther Pat. 2019;29:745–747.
40. Supuran CT. Carbonic anhydrase inhibition and the management of neuropathic pain. Expert Rev Neurother. 2016;16:961–968.
41. Di Cesare Mannelli L, Micheli L, Carta F, et al. Carbonic anhydrase inhibition for the management of cerebral ischemia: in vivo evaluation of sulfonamide and coumarin inhibitors. Enzyme Inhib Med Chem. 2016;31:894–899.
42. Margheri F, Ceruso M, Carta F, et al. Overexpression of the transmembrane carbonic anhydrase isoforms IX and XII in the inflamed synovium. J Enzyme Inhib Med Chem. 2016;31(sup4):60–63.
43. Bua S. Design and Synthesis of Novel Nonsteroidal Anti-Inflammatory Drugs and Carbonic Anhydrase Inhibitors Hybrids (NSAIDs-CAIs) for the Treatment of Rheumatoid Arthritis. J Med Chem. 2017;60:1159–1170.
44. Kaur J, Cao X, Abutaleb NS, et al. Optimization of Acetazolamide-Based Scaffold as Potent Inhibitors of Vancomycin-Resistant Enterococcus. J Med Chem. 2020;63(17):9540–9562.
45. Supuran CT, Capasso C. Antibacterial carbonic anhydrase inhibitors: an update on the recent literature. Expert Opin Ther Pat. 2020;1–20. doi:10.1080/13543776.2020.1811853
46. Vermelho AB, Rodrigues GC, Supuran CT. Why hasn’t there been more progress in new Chagas disease drug discovery? Expert Opin Drug Discov. 2020;15(2):145–158.
47. Supuran CT, Capasso C. Biomedical applications of prokaryotic carbonic anhydrases. Expert Opin Ther Pat. 2018;28(10):745–754.
48. Capasso C, Supuran CT. Bacterial, fungal and protozoan carbonic anhydrases as drug targets. Expert Opin Ther Targets. 2015;19(12):1689–1704.
49. Supuran CT, The CC. η-class carbonic anhydrases as drug targets for antimalarial agents. Expert Opin Ther Targets. 2015;19(4):551–563.
50. Capasso C, Supuran CT. Anti-infective carbonic anhydrase inhibitors: a patent and literature review. Expert Opin Ther Pat. 2013;23(6):693–704.
51. Supuran CT. Carbonic anhydrase inhibitors as emerging agents for the treatment and imaging of hypoxic tumors. Expert Opin Investig Drugs. 2018;27(12):963–970.
52. Berrino E, Supuran CT. Novel approaches for designing drugs that interfere with pH regulation. Expert Opin Drug Discov. 2019;14(3):231–248.
53. Angeli A, Carta F, Nocentini A, et al. Carbonic Anhydrase Inhibitors Targeting Metabolism and Tumor Microenvironment. Metabolites. 2020;10(10):E412.
54. Supuran CT, Anhydrase C. Inhibition and the Management of Hypoxic Tumors. Metabolites. 2017;7(3):48.
55. Iessi E, Logozzi M, Mizzoni D, Di Raimo R, Supuran CT, Fais S. Rethinking the Combination of Proton Exchanger Inhibitors in Cancer Therapy. Metabolites. 2017;8(1):2.
56. McDonald PC, Swayampakula M, Dedhar S. Coordinated Regulation of Metabolic Transporters and Migration/Invasion by Carbonic Anhydrase IX. Metabolites. 2018;8(1):20.
57. Benej M, Svastova E, Banova R, et al. Intracellular pH to Maintain Metabolic Reprogramming and Proliferation in Hypoxia. Front Oncol. 2020;2(10):1462.
58. Pastorek J, Pastoreková S, Callebaut I, et al. Cloning and characterization of MN, a human tumor-associated protein with a domain homologous to carbonic anhydrase and a putative helix-loop-helix DNA binding segment. Oncogene. 1994;9:2877–2888.
59. Türeci O, Sahin U, Vollmar E, et al. Human carbonic anhydrase XII: cDNA cloning, expression, and chromosomal localization of a carbonic anhydrase gene that is overexpressed in some renal cell cancers. Proc Natl Acad Sci U S A. 1998;95:7608–7613.
60. Svastová E, Hulíková A, Rafajová M, et al. Hypoxia activates the capacity of tumor-associated carbonic anhydrase IX to acidify extracellular pH. FEBS Lett. 2004;577:439–445.
61. Cecchi A, Hulikova A, Pastorek J, et al. Carbonic anhydrase inhibitors. Design of fluorescent sulfonamides as probes of tumor-associated carbonic anhydrase IX that inhibit isozyme IX-mediated acidification of hypoxic tumors. J Med Chem. 2005;48:4834–4841.
62. Swietach P, Wigfield S, Cobden P, et al. Tumor-associated carbonic anhydrase 9 spatially coordinates intracellular pH in three-dimensional multicellular growths. J Biol Chem. 2008;283:20473–20483.
63. Swietach P, Patiar S, Supuran CT, et al. The role of carbonic anhydrase 9 in regulating extracellular and intracellular pH in three-dimensional tumor cell growths. J Biol Chem. 2009;284:20299–20310.
64. Ahlskog JK, Dumelin CE, Trüssel S, Mårlind J, Neri D. In vivo targeting of tumor-associated carbonic anhydrases using acetazolamide derivatives. Bioorg Med Chem Lett. 2009;19(16):4851–4856.
65. Buller F, Steiner M, Frey K, et al. Selection of Carbonic Anhydrase IX Inhibitors from One Million DNA-Encoded Compounds. ACS Chem Biol. 2001;6(4):336–344.
66. Krall N, Pretto F, Decurtins W, Bernardes GJ, Supuran CT, Neri D. A small-molecule drug conjugate for the treatment of carbonic anhydrase IX expressing tumors. Angew Chem Int Ed Engl. 2014;53(16):4231–4235.
67. Krall N, Pretto F, Mattarella M, Müller C, Neri D. A 99mTc-Labeled Ligand of Carbonic Anhydrase IX Selectively Targets Renal Cell Carcinoma In Vivo. J Nucl Med. 2016;57(6):943–949.
68. Cazzamalli S, Dal Corso A, Neri D. Acetazolamide Serves as Selective Delivery Vehicle for Dipeptide-Linked Drugs to Renal Cell Carcinoma. Mol Cancer Ther. 2016;15(12):2926–2935.
69. Cazzamalli S, Dal Corso A, Widmayer F, Neri D. Chemically Defined Antibody- and Small Molecule-Drug Conjugates for in Vivo Tumor Targeting Applications: A Comparative Analysis. J Am Chem Soc. 2018;140(5):1617–1621.
70. Cazzamalli S, Ziffels B, Widmayer F, et al. Enhanced Therapeutic Activity of Non-Internalizing Small-Molecule-Drug Conjugates Targeting Carbonic Anhydrase IX in Combination with Targeted Interleukin-2. Clin Cancer Res. 2018;24(15):3656–3667.
71. Cazzamalli S, Figueras E, Pethő L, et al. In Vivo Antitumor Activity of a Novel Acetazolamide-Cryptophycin Conjugate for the Treatment of Renal Cell Carcinomas. ACS Omega. 2018;3(11):14726–14731.
72. Gouyou B, Millul J, Villa A, Cazzamalli S, Neri D, Sortase-Mediated Site-Specific MM. Modification of Interleukin-2 for the Generation of a Tumor-Targeting Acetazolamide-Cytokine Conjugate. ACS Omega. 2020;5(40):26077–26083.
73. Scozzafava A, Menabuoni L, Mincione F, et al. Carbonic anhydrase inhibitors. Synthesis of water-soluble, topically effective, intraocular pressure-lowering aromatic/heterocyclic sulfonamides containing cationic or anionic moieties: is the tail more important than the ring? J Med Chem. 1999;42:2641–2650.
74. Dubois L, Peeters SG, van Kuijk SJ, et al. Targeting carbonic anhydrase IX by nitroimidazole based sulfamides enhances the therapeutic effect of tumor irradiation: a new concept of dual targeting drugs. Radiother Oncol. 2013;108(3):523–528.
75. Rami M, Dubois L, Parvathaneni NK, et al. Hypoxia-targeting carbonic anhydrase IX inhibitors by a new series of nitroimidazole-sulfonamides/sulfamides/sulfamates. J Med Chem. 2013;56(21):8512–8520.
76. van Kuijk SJA, Parvathaneni NK, Niemans R, et al. New approach of delivering cytotoxic drugs towards CAIX expressing cells: A concept of dual-target drugs. Eur J Med Chem. 2017;15(127):691–702.
77. Aspatwar A, Becker HM, Parvathaneni NK, et al. Nitroimidazole-based inhibitors DTP338 and DTP348 are safe for zebrafish embryos and efficiently inhibit the activity of human CA IX in Xenopus oocytes. J Enzyme Inhib Med Chem. 2018;33(1):1064–1073.
78. Aspatwar A, Parvathaneni NK, Barker H, et al. Design, synthesis, in vitro inhibition and toxicological evaluation of human carbonic anhydrases I, II and IX inhibitors in 5-nitroimidazole series. J Enzyme Inhib Med Chem. 2020;35(1):109–117.
79. Anduran E, Aspatwar A, Parvathaneni NK, et al. Hypoxia-Activated Prodrug Derivatives of Carbonic Anhydrase Inhibitors in Benzenesulfonamide Series: synthesis and Biological Evaluation. Molecules. 2020;25(10):2347.
80. Gieling RG, Babur M, Mamnani L, et al. Antimetastatic effect of sulfamate carbonic anhydrase IX inhibitors in breast carcinoma xenografts. J Med Chem. 2012;55(11):5591–5600.
81. Bryant JL, Gieling RG, Meredith SL, et al. Novel carbonic anhydrase IX-targeted therapy enhances the anti-tumour effects of cisplatin in small cell lung cancer. Int J Cancer. 2018;142(1):191–201.
82. Wilkinson BL, Bornaghi LF, Houston TA, Innocenti A, Supuran CT, Poulsen SA. A novel class of carbonic anhydrase inhibitors: glycoconjugate benzene sulfonamides prepared by “click-tailing”. J Med Chem. 2006;49(22):6539–6548.
83. Wilkinson BL, Bornaghi LF, Houston TA, et al. Carbonic anhydrase inhibitors: inhibition of isozymes I, II, and IX with triazole-linked O-glycosides of benzene sulfonamides. J Med Chem. 2007;50(7):1651–1657.
84. Wilkinson BL, Innocenti A, Vullo D, Supuran CT, Poulsen SA. Inhibition of carbonic anhydrases with glycosyltriazole benzene sulfonamides. J Med Chem. 2008;51(6):1945–1953.
85. Lopez M, Paul B, Hofmann A, et al. S-glycosyl primary sulfonamides–a new structural class for selective inhibition of cancer-associated carbonic anhydrases. J Med Chem. 2009;52(20):6421–6432.
86. Lopez M, Bornaghi LF, Innocenti A, et al. Sulfonamide linked neoglycoconjugates–a new class of inhibitors for cancer-associated carbonic anhydrases. J Med Chem. 2010;53(7):2913–2926.
87. Morris JC, Chiche J, Grellier C, et al. Targeting hypoxic tumor cell viability with carbohydrate-based carbonic anhydrase IX and XII inhibitors. J Med Chem. 2011;54(19):6905–6918.
88. Lounnas N, Rosilio C, Nebout M, et al. Pharmacological inhibition of carbonic anhydrase XII interferes with cell proliferation and induces cell apoptosis in T-cell lymphomas. Cancer Lett. 2013;333(1):76–88.
89. Tanpure RP, Ren B, Peat TS, Bornaghi LF, Vullo D, Supuran CT. Carbonic anhydrase inhibitors with dual-tail moieties to match the hydrophobic and hydrophilic halves of the carbonic anhydrase active site. J Med Chem. 2015;58(3):1494–1501.
90. Moeker J, Peat TS, Bornaghi LF, Vullo D, Supuran CT, Poulsen SA. Cyclic secondary sulfonamides: unusually good inhibitors of cancer-related carbonic anhydrase enzymes. J Med Chem. 2014;57(8):3522–3531.
91. Köhler K, Hillebrecht A, Schulze Wischeler J, et al. Saccharin inhibits carbonic anhydrases: possible explanation for its unpleasant metallic aftertaste. Angew Chem Int Ed Engl. 2007;46(40):7697–7699.
92. Sneddon D, Niemans R, Bauwens M, et al. Synthesis and in Vivo Biological Evaluation of (68)Ga-Labeled Carbonic Anhydrase IX Targeting Small Molecules for Positron Emission Tomography. J Med Chem. 2016;59(13):6431–6443.
93. Pan J, Lau J, Mesak F, et al. Synthesis and evaluation of 18F-labeled carbonic anhydrase IX inhibitors for imaging with positron emission tomography. J Enzyme Inhib Med Chem. 2014;29(2):249–255.
94. Lau J, Zhang Z, Jenni S, et al. Imaging of Carbonic Anhydrase IX Expression of HT-29 Tumor Xenograft Mice with (68)Ga-Labeled Benzenesulfonamides. Mol Pharm. 2016;13(3):1137–1146.
95. Zhang Z, Lau J, Zhang C, et al. Design, synthesis and evaluation of 18F-labeled cationic carbonic anhydrase IX inhibitors for PET imaging. J Enzyme Inhib Med Chem. 2017;32(1):722–730.
96. Mujumdar P, Teruya K, Tonissen KF, Vullo D, Supuran CT, Peat TS. An Unusual Natural Product Primary Sulfonamide: synthesis, Carbonic Anhydrase Inhibition, and Protein X-ray Structures of Psammaplin C. J Med Chem. 2016;59(11):5462–5470.
97. Mujumdar P, Kopecka J, Bua S, Supuran CT, Riganti C. Poulsen SA.Carbonic Anhydrase XII Inhibitors Overcome Temozolomide Resistance in Glioblastoma. J Med Chem. 2019;62(8):4174–4192.
98. Pacchiano F, Carta F, McDonald PC, et al. Ureido-substituted benzenesulfonamides potently inhibit carbonic anhydrase IX and show antimetastatic activity in a model of breast cancer metastasis. J Med Chem. 2011;54(6):1896–1902.
99. Lou Y, McDonald PC, Oloumi A, et al. Targeting tumor hypoxia: suppression of breast tumor growth and metastasis by novel carbonic anhydrase IX inhibitors. Cancer Res. 2011;71(9):3364–3376.
100. Pacchiano F, Aggarwal M, Avvaru BS, et al. Selective hydrophobic pocket binding observed within the carbonic anhydrase II active site accommodate different 4-substituted-ureido-benzenesulfonamides and correlate to inhibitor potency. Chem Commun (Camb). 2010;46(44):8371–8373.
101. Touisni N, Maresca A, McDonald PC, et al. Glycosyl coumarin carbonic anhydrase IX and XII inhibitors strongly attenuate the growth of primary breast tumors. J Med Chem. 2011;54(24):8271–8277. doi:10.1021/jm200983e
102. Maresca A, Temperini C, Vu H, et al. Non-Zinc Mediated Inhibition of Carbonic Anhydrases: coumarins Are a New Class of Suicide Inhibitors #. J Am Chem Soc. 2009;131(8):3057–3062. doi:10.1021/ja809683v
103. Maresca A, Temperini C, Pochet L, Masereel B, Scozzafava A, Supuran CT. Deciphering the mechanism of carbonic anhydrase inhibition with coumarins and thiocoumarins. J Med Chem. 2010;53(1):335–344. doi:10.1021/jm901287j
104. McDonald PC, Winum J-Y, Supuran CT, Dedhar S. Recent developments in targeting carbonic anhydrase IX for cancer therapeutics. Oncotarget. 2012;3(1):84–97. doi:10.18632/oncotarget.422
105. Lock FE, McDonald PC, Lou Y, et al. Targeting carbonic anhydrase IX depletes breast cancer stem cells within the hypoxic niche. Oncogene. 2013;32(44):5210–5219. doi:10.1038/onc.2012.550
106. Swayampakula M, McDonald PC, Vallejo M, et al. The interactome of metabolic enzyme carbonic anhydrase IX reveals novel roles in tumor cell migration and invadopodia/MMP14-mediated invasion. Oncogene. 2017;36(45):6244–6261.
107. Bozdag M, Carta F, Ceruso M, et al. Discovery of 4-Hydroxy-3-(3-(phenylureido)benzenesulfonamides as SLC-0111 Analogues for the Treatment of Hypoxic Tumors Overexpressing Carbonic Anhydrase IX. J Med Chem. 2018;61(14):6328–6338. doi:10.1021/acs.jmedchem.8b00770
108. McDonald PC, Chafe SC, Brown WS, et al. Regulation of pH by Carbonic Anhydrase 9 Mediates Survival of Pancreatic Cancer Cells With Activated KRAS in Response to Hypoxia. Gastroenterology. 2019;157(3):823–837.
109. Chafe SC, McDonald PC, Saberi S, et al. Targeting Hypoxia-Induced Carbonic Anhydrase IX Enhances Immune-Checkpoint Blockade Locally and Systemically. Cancer Immunol Res. 2019;7(7):1064–1078. doi:10.1158/2326-6066.CIR-18-0657
110. Boyd NH, Walker K, Fried J, et al. Addition of carbonic anhydrase 9 inhibitor SLC-0111 to temozolomide treatment delays glioblastoma growth in vivo. JCI Insight. 2017;2(24):92928. doi:10.1172/jci.insight.92928
111. Kuchuk O, Tuccitto A, Citterio D, et al. pH regulators to target the tumor immune microenvironment in human hepatocellular carcinoma. Oncoimmunology. 2018;7(7):e1445452. doi:10.1080/2162402X.2018.1445452
112. Ward C, Meehan J, Gray M, et al. Carbonic Anhydrase IX (CAIX), Cancer, and Radiation Responsiveness. Metabolites. 2018;8(1):E13. doi:10.3390/metabo8010013
113. Federici C, Lugini L, Marino ML, et al. Lansoprazole and carbonic anhydrase IX inhibitors sinergize against human melanoma cells. J Enzyme Inhib Med Chem. 2016;31(sup1):119–125. doi:10.1080/14756366.2016.1177525
114. Andreucci E, Peppicelli S, Carta F, et al. Carbonic anhydrase IX inhibition affects viability of cancer cells adapted to extracellular acidosis. J Mol Med (Berl). 2017;95(12):1341–1353. doi:10.1007/s00109-017-1590-9
115. Logsdon DP, Grimard M, Luo M, et al. Regulation of HIF1 under Hypoxia by APE1/Ref-1 Impacts CA9 Expression: dual Targeting in Patient-Derived 3D Pancreatic Cancer Models. Mol Cancer Ther. 2016;15(11):2722–2732. doi:10.1158/1535-7163.MCT-16-0253
116. Peppicelli S, Andreucci E, Ruzzolini J, et al. The Carbonic Anhydrase IX inhibitor SLC-0111 as emerging agent against the mesenchymal stem cell-derived pro-survival effects on melanoma cells. J Enzyme Inhib Med Chem. 2020;35(1):1185–1193. doi:10.1080/14756366.2020.1764549
117. Genah S, Angeli A, Supuran CT, Morbidelli L. Effect of Carbonic Anhydrase IX inhibitors on human endothelial cell survival. Pharmacol Res. 2020;159:104964. doi:10.1016/j.phrs.2020.104964
118. Riemann A, Güttler A, Haupt V, et al. Inhibition of Carbonic Anhydrase IX by Ureidosulfonamide Inhibitor U104 Reduces Prostate Cancer Cell Growth, But Does Not Modulate Daunorubicin or Cisplatin Cytotoxicity. Oncol Res. 2018;26(2):191–200. doi:10.3727/096504017X14965111926391
119. Güttler A, Theuerkorn K, Riemann A, et al. Cellular and radiobiological effects of carbonic anhydrase IX in human breast cancer cells. Oncol Rep. 2019;41(4):2585–2594.
120. Lee JY, Alexeyev M, Kozhukhar N, Pastukh V, White R, Stevens T. Carbonic anhydrase IX is a critical determinant of pulmonary microvascular endothelial cell pH regulation and angiogenesis during acidosis. Am J Physiol Lung Cell Mol Physiol. 2018;315(1):L41–L51.
121. Lee JY, Onanyan M, Garrison I, et al. Extrinsic acidosis suppresses glycolysis and migration while increasing network formation in pulmonary microvascular endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2019;317(2):L188–L201.
122. Bernardino RL, Dias TR, Moreira BP, et al. Carbonic anhydrases are involved in mitochondrial biogenesis and control the production of lactate by human Sertoli cells. FEBS J. 2019;286(7):1393–1406.
123. McDonald PC, Chia S, Bedard PL, et al. A Phase 1 Study of SLC-0111, a Novel Inhibitor of Carbonic Anhydrase IX, in Patients With Advanced Solid Tumors. Am J Clin Oncol. 2020;43(7):484–490.
124. Akocak S, Lolak N, Bua S, Turel I, Supuran CT. Synthesis and biological evaluation of novel N, N’-diaryl cyanoguanidines acting as potent and selective carbonic anhydrase II inhibitors. Bioorg Chem. 2018;77:245–251. doi:10.1016/j.bioorg.2018.01.022;
125. Lolak N, Akocak S, Bua S, Koca M, Supuran CT. Design and synthesis of novel 1,3-diaryltriazene-substituted sulfonamides as potent and selective carbonic anhydrase II inhibitors. Bioorg Chem. 2018;77:542–547. doi:10.1016/j.bioorg.2018.02.015
126. Lomelino CL, Mahon BP, Carta F, Supuran CT, Kinetic MR. and X-ray crystallographic investigations on carbonic anhydrase isoforms I, II, IX and XII of a thioureido analog of SLC-0111 Bioorg. Med Chem. 2016;24:976–981. doi:10.1016/j.bmc.2016.01.019;
127. Angeli A, Tanini D, Peat TS, et al. Discovery of new selenoureido analogues of 4-(4-fluorophenylureido) benzenesulfonamide as carbonic anhydrase inhibitors ACS Med. Chem Lett. 2017;8:963–968. doi:10.1021/acsmedchemlett.7b00280
128. Eldehna WM, Abo-Ashour MF, Berrino E, et al. SLC-0111 enaminone analogs, 3/4-(3-aryl-3-oxopropenyl) aminobenzenesulfonamides, as novel selective subnanomolar inhibitors of the tumor-associated carbonic anhydrase isoform IX. Bioorg Chem. 2019;83:549–558. doi:10.1016/j.bioorg.2018.11.014
129. Congiu C, Onnis V, Deplano A, Balboni G, Dedeoglu N, Supuran CT. Synthesis of sulfonamides incorporating piperazinyl-ureido moieties and their carbonic anhydrase I, II, IX and XII inhibitory activity. Bioorg Med Chem Lett. 2015;25(18):3850–3853.
130. Moi D, Nocentini A, Deplano A, et al. Appliance of the piperidinyl-hydrazidoureido linker to benzenesulfonamide compounds: synthesis, in vitro and in silico evaluation of potent carbonic anhydrase II, IX and XII inhibitors. Bioorg Chem. 2020;98:103728.
131. Abo-Ashour MF, Eldehn WM, Nocentini A, et al. Novel synthesized SLC-0111 thiazole and thiadiazole analogues: determination of their carbonic anhydrase inhibitory activity and molecular modeling studies. Bioorg Chem. 2019;87:794–802. doi:10.1016/j.bioorg.2019.04.002.
132. Alkhaldi AAM, Al-Sanea MM, Nocentini A, et al. 3-Methylthiazolo[3,2-a]benzimidazole-benzenesulfonamide conjugates as novel carbonic anhydrase inhibitors endowed with anticancer activity: design, synthesis, biological and molecular modeling studies. Eur J Med Chem. 2020;207:112745.
133. Eldehna WM, Fares M, Ceruso M, et al. Amido/ureidosubstituted benzenesulfonamides-isatin conjugates as low nanomolar/subnanomolar inhibitors of the tumor-associated carbonic anhydrase isoform XII. Eur J Med Chem. 2016;110:259–266.
134. Eldehna WM, Abo-Ashour MF, Nocentini A, et al. Enhancement of the tail hydrophobic interactions within the carbonic anhydrase IX active site via structural extension: design and synthesis of novel N-substituted isatins-SLC-0111 hybrids as carbonic anhydrase inhibitors and antitumor agents. Eur J Med Chem. 2019;162:147e160.
135. Iikuni S, Ono M, Watanabe H, Shimizu Y, Sano K, Saji H. Cancer radiotheranostics targeting carbonic anhydrase-IX with 111In- and 90Y-labeled ureidosulfonamide scaffold for SPECT imaging and radionuclide-based therapy. Theranostics. 2018;8(11):2992–3006.
136. Andring JT, Fouch M, Akocak S, et al. Structural Basis of Nanomolar Inhibition of Tumor-Associated Carbonic Anhydrase IX: X-Ray Crystallographic and Inhibition Study of Lipophilic Inhibitors with Acetazolamide Backbone. J Med Chem. 2020. doi:10.1021/acs.jmedchem.0c01390
137. Bonardi A, Nocentini A, Bua S, et al. Sulfonamide inhibitors of human carbonic anhydrases designed through a three-tails approach: improving ligand/isoform matching and selectivity of action. J Med Chem. 2020;63(13):7422–7444.
138. Petreni A, Bonardi A, Lomelino C, Osman SM. Inclusion of a 5-fluorouracil moiety in nitrogenous bases derivatives as human carbonic anhydrase IX and XII inhibitors produced a targeted action against MDA-MB-231 and T47D breast cancer cells. Eur J Med Chem. 2020;190:112112.
139. Bua S, Lomelino C, Murray AB, Osman SM. “A sweet combination”: developing saccharin and acesulfame k structures for selectively targeting the tumor-associated carbonic anhydrases IX and XII. J Med Chem. 2020;63(1):321–333.
140. Nocentini A, Trallori E, Singh S, Lomelino CL, Bartolucci G. 4-Hydroxy-3-nitro-5-ureido-benzenesulfonamides Selectively Target the Tumor-Associated Carbonic Anhydrase Isoforms IX and XII Showing Hypoxia-Enhanced Antiproliferative Profiles. J Med Chem. 2018;61(23):10860–10874.
141. Lomelino CL, Murray AB, Supuran CT, Sweet Binders: MR. Carbonic Anhydrase IX in Complex with Sucralose. ACS Med Chem Lett. 2018;9(7):657–661.
142. Murray AB, Lomelino CL, Supuran CT, McKenna R. “Seriously Sweet”: acesulfame K Exhibits Selective Inhibition Using Alternative Binding Modes in Carbonic Anhydrase Isoforms. J Med Chem. 2018;61(3):1176–1181.
143. Mboge MY, Mahon BP, Lamas N, et al. Structure activity study of carbonic anhydrase IX: selective inhibition with ureido-substituted benzenesulfonamides. Eur J Med Chem. 2017;132:184–191.
144. Moeker J, Mahon BP, Bornaghi LF, et al. Structural insights into carbonic anhydrase IX isoform specificity of carbohydrate-based sulfamates. J Med Chem. 2014;57(20):8635–8645.
145. Jonsson BH, Liljas A. Perspectives on the Classical Enzyme Carbonic Anhydrase and the Search for Inhibitors. Biophys J. 2020;119:1275–1280.
146. Mishra CB, Tiwari M, Supuran CT. Progress in the development of human carbonic anhydrase inhibitors and their pharmacological applications: where are we today? Med Res Rev. 2020;40:2485–2565.
147. Nguyen GTH, Tran TN, Podgorski MN, Bell SG, Supuran CT, Donald WA. Nanoscale Ion Emitters in Native Mass Spectrometry for Measuring Ligand-Protein Binding Affinities. ACS Cent Sci. 2019;5(2):308–318.
148. Nguyen GTH, Nocentini A, Angeli A, Gratteri P, Supuran CT, Donald WA. Perfluoroalkyl Substances of Significant Environmental Concern Can Strongly Inhibit Human Carbonic Anhydrase Isozymes. Anal Chem. 2020;92(6):4614–4622.
149. Nguyen GTH, Leung WY, Tran TN, Wang H, Murray V, Donald WA. Mechanism for the Binding of Netropsin to Hairpin DNA Revealed Using Nanoscale Ion Emitters in Native Mass Spectrometry. Anal Chem. 2020;92(1):1130–1137.
150. D’Ambrosio K, Carradori S, Monti SM, et al. Out of the active site binding pocket for carbonic anhydrase inhibitors. Chem Commun. 2015;51:302–305.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
