Back to Journals » Neuropsychiatric Disease and Treatment » Volume 15
Exercise training increases spatial memory via reducing contralateral hippocampal NMDAR subunits expression in intracerebral hemorrhage rats
Authors Zheng S , Zhang F , Liu Q, Jian R, Yang M
Received 4 March 2019
Accepted for publication 24 June 2019
Published 9 July 2019 Volume 2019:15 Pages 1921—1928
DOI https://doi.org/10.2147/NDT.S207564
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yuping Ning
Shulin Zheng,1 Feixue Zhang,1 Qiusheng Liu,2 Rui Jian,1 Min Yang1
1Department of Rehabilitation Medicine, Affiliated Hospital of Southwest Medical University, Luzhou, Sichuan 646000, People’s Republic of China; 2Department of Cardiovascular Medicine, Luzhou Traditional Chinese Medicine Hospital, Luzhou, Sichuan 646000, People’s Republic of China
Objective: The aim of this study was to explore the effect of exercise training on spatial memory in rats with intracerebral hemorrhage (ICH) and to analyze its related neurobiological mechanisms.
Methods: A total of 26 Sprague-Dawley rats were randomly divided into 3 groups: exercise (EX) group undergoing exercise training after ICH, model (MD) group and sham-operated (SM) group. The ICH rats model were induced by infusion of type I collagenase into caudate nucleus of rats. Morris water maze (MWM) test was performed at the same time in three groups to evaluate spatial memory in rats. All rats were sacrificed for evaluation of expression of N-methyl-d-aspartate receptor 1 (NR1) and N-methyl-d-aspartate receptor 2B (NR2B) in the CA3 region of the hippocampus by Western blot.
Results: MWM test results showed that the spatial memory of MD group was significantly decreased compared to that of SM operation group (P<0.05), while exercise training significantly improved the spatial memory of rats with cerebral hemorrhage (P<0.05). Western blot analysis showed that exercise training significantly decreased the expression of NR1 and NR2B in CA3 region of the contralateral hippocampus (P<0.05), but there was no significant difference between MD and SM groups (P>0.05).
Conclusion: Exercise training improves the spatial memory in the rats with ICH via down-regulating NR1 and NR2B expression in CA3 region of the contralateral hippocampus.
Keywords: exercise training, intracerebral hemorrhage, NR1, NR2B, hippocampus
Introduction
Intracerebral hemorrhage (ICH) is a common cerebrovascular disease with high mortality and disability rate, and a large part of the survivors have functional impairment. The research shows that 50% of the survivors have cognitive or neurobehavioral dysfunction, and the loss of social work and living ability caused by cognitive impairment will cause a great burden on social economy.1 Unfortunately, we still lack effective treatment methods.
In addition to relying on surgical treatment and routine drug therapy, current studies have shown that exercise training can effectively improve cognitive impairment after stroke.2–4 However, whether and how exercise training improves the cognitive function after ICH is still unexplored. It has been found that ICH often accompanied by changes in hippocampal structure and function, including necrosis of nerve cells, destruction of synaptic plasticity, increased expression of inflammatory factors, large release of glutamate, and overactivation of N-methyl-d-aspartate receptor (NMDAR).5,6
NMDAR is a class of ionotropic glutamate receptor, which is considered to be a complex heteromer consisting of two NR1 and two NR2 subunits (NR2A, NR2B, NR2C, NR2D). NMDAR has been proved to be an important factor for synapse function and behavior cognition via different mechanisms. First, NMDAR is involved in the long-term potentiation, an activity-dependent amplification in the efficiency of synaptic transmission thought to underlie certain kinds of memory.7,8 Second, NMDAR has also been reported in participating in the excitation and inhibition balance (E-I balance). Previous studies have shown that imbalance in E-I balance has been linked to several disorders, such as schizophrenia, Alzheimer’s disease, and Huntington’s disease.9 It has been shown that the E-I balance is broken after stroke. Large glutamate release after stroke will lead to the continuous activation of NMDAR, resulting in influx of calcium ions, which causes neuronal death and cognitive impairment. This is called glutamic acid excitatory toxicity.10,11 NR1 and NR2B are the most widely expressed receptors of NMDAR receptors. In adult hippocampus, neurons mainly express NMDAR containing NR1 and NR2B subunits.12 Reducing NR1 and NR2B is beneficial to the recovery of neurological function in stroke rats.5,13
It is well known that the brain after stroke relies on the contralateral hemisphere part to improve functional improvement as a compensation.14–16 Does exercise training promote contralateral hippocampus function in intracerebral hemorrhage rats? Does it affect the NMDAR expression in the contralateral hippocampus? At present, it is still unknown.
Understanding whether and how exercise training improves cognitive impairment in rats with ICH is helpful for us to further understand the neurobiological mechanism and to develop effective rehabilitation training programs. Considering that the hippocampal CA3 region is related to spatial memory,17 we explored the role of exercise training onto spatial memory as well as the expression of NR1 and NR2B in the contralateral hippocampal CA3 region of rats with cerebral hemorrhage.
Materials and methods
Animals
Animal Experimental Center of Southwest Medical University provided 26 male Sprague-Dawley rats, weighing (300±50) g and aged 8 weeks. The agreement on research animals has been approved by the Medical Ethics Committee of Southwest Medical University. Five rats in each cage were placed in a temperature control room (20–30°C). Light/dark cycles lasted for 12/12 hrs to obtain food and water at will. Rats were fed for 1 week to adapt to their habitat conditions. Before grouping, adaptive swimming training was arranged for the rats. The rats were trained for swimming for 10 mins on the first day, 20 mins on the second day and 30 mins on the third day. On the fourth day, the rats were randomly divided into three groups: sham-operated (SM) group, model (MD) group, exercise (EX) group. The rats were randomly allocated to three groups of 5 rats each. All the experimental procedures were carried out in accordance with the Guidelines for the Feeding and Use of Laboratory Animals issued by the Ministry of Science and Technology of China.
Intracerebral hemorrhage model
Rats were anesthetized with 10% chloral hydrate (350 mg/kg) by intraperitoneal anesthesia after 6 hrs of fasting and abstinence before the operation. After successful anesthesia, the top of the rat’s skull was sheared and skinned, then the prone position was fixed on the stereotaxic brain locator. The method was to fix the interauricular line first, then the incisor was fixed on the hook, so that the plane of the hook was 2.4 mm lower than that of the interauricular line. Thereafter, iodophor disinfected the top of the head skin, laid a hole scarf, and cut a sagittal incision about 1 cm between the binocular line and the binocular line to the subcutaneous area, and 30% of the incisor hydrogen peroxide corrodes the epicranium, exposes the anterior fontanel and coronal suture, locates the right caudate nucleus (0.2 mm in front of the anterior fontanel, 3 mm in the right side of the midline) and drills a round hole about 1 mm in diameter to reach the dural surface. Type I collagenase 2 µL was extracted with 5 µL microinjector, then heparin sodium 1 µL was extracted. The microinjector was fixed on the stereotaxic brain locator. The microinjector was inserted vertically along the drill hole for 6 mm. The collagenase I and heparin sodium were injected into the caudate nucleus of rats at a speed of 0.3 µL/min and a total injection time of 10 mins. After the liquid injection, the needle was retained for 10 mins, then the needle was withdrawn slowly for 10 mins. After withdrawing the needle, the drill hole was sealed with medical bone wax to prevent the liquid from spilling along the needle passage. The wound was sterilized with Iodophor and sutured again. The rats were placed in a separate cage. After awakening, the rats were assessed by masked observer through modified Neurological Severity Score (mNSS) (Table 1).18 The score (>8.5 points) was the success of the model. The control group rats were treated with 3 µL saline instead of collagenase and heparin sodium.
 |
Table 1 Modified Neurological Severity Score (mNSS) in rats |
Exercise training
On the third day after the success of the model, rats in EX group underwater exercise training. Each rat was put into a cylinder (diameter: 0.6 m, height: 1 m), water temperature was 29–31°C, water surface was 0.4 m away from the edge of the container, 30 mins a day, once a day, 5 days a week, 3 weeks in a row, once an hour. The other two groups were free to move.
Morris water maze test (MWM)
Three groups of rats were assessed by MWM test. The MWM (S1000; Chengdu Taimeng Technology Co, Ltd, Chengdu, China) consisted of a stainless steel circular water tank (120 cm in diameter; 55 cm in height), which was equipped with a platform (10 cm in diameter; 21 cm in height), divided into four quadrants. The platform was located in the third quadrant and submerged at 2 cm below the water surface. The pool is opaque and is made of black edible-pigmented liquid. The experiment is divided into place navigation task and spatial probe task. Before the experiment, all rats swam freely in the water maze for 15 mins. In the place navigation task, the rats were placed at each release marker in different quadrants at a fixed time in a certain order every day for 4 days. The latency period was determined when the rats found the target platform within 120 s and stayed on the platform for 10 s. If the rats did not find the target platform within 120 s, the latency period would be the escape latency. Finding the platform will guide it to the platform and stay on the platform for 10 s, recording the escape latency time of 120 s. After 24 hrs of the place navigation task, the spatial memory ability of rats was evaluated by spatial probe task. The hidden platform was removed and the rats were placed from the previous platform. The percentage of time and distance of the target quadrant within 120 s was recorded systematically. The swimming speed was also recorded in the whole test.19
Western blot analysis
After MWM, the rat brain was decapitated and the CA3 area of the opposite hippocampus was quickly separated on ice and under a stereomicroscope, placed in a pre-cooled EP tube, and then stored in a refrigerator at −80°C. The operation needed to be completed in 2 mins. After cutting the tissue into fine fragments, the cracking solution (protease and phosphatase inhibitor were added into the cracking solution) was added. The homogenizer was homogenized until complete cracking. After pyrolysis, the sample was weighed and centrifuged for 15 mins at 4°C, then the supernatant was taken, the protein was quantified and stored in −80°C refrigerator. Protein concentration was measured by bicinchoninic acid assay protein analysis kit (Bioswamp, Wuhan, Hubei, China). The equivalent sample (10 µg) was denatured and separated by SDS-PAGE. After separation, we used wet rotation. Specifically, in wet rotation, sandwiches were arranged as follows: filter paper/glue/membrane/filter paper, soaked with electro-rotary buffer, placed in the clamp used for membrane rotation, the black face of the clamp was on the black face of the groove, the white face of the clamp was on the red face of the groove, and the film was on the positive electrode. When electricity was transferred, heat was generated and ice blocks were placed on one side of the tank to cool down. 90 V transmembrane was used for 50 mins. Polyvinylidene fluoride (PVDF) membrane (Millipore, Billerica, MA, USA) was immersed in methanol for 5 mins before conversion, and incubated in cold electro-conversion buffer for 2 mins. After transmembrane, the blocker antibody was incubated: 5% skimmed milk powder was blocked at room temperature for 2 hrs, and primary antibody (antibody name: NMDAR1, antibody species: rabbit, antibody brand: Abcam number: Ab109182; antibody name: NMDAR2B, antibody species: rabbit, antibody brand: Abcam number: Ab71178; Abcam, Cambridge, UK) was cultured overnight at 4°C. After incubating, the membrane was washed with PBST for 3 times, 5 mins each time. Then, according to the dosage, the second antibody labeled with horseradish peroxidase diluted at 1:10,000 was incubated with the membrane at room temperature for 1 hr. And, washed 3 times with PBST, 5 mins each time. Then, the film was placed in the darkroom; the ECL luminescent liquid A and B was equally mixed according to the dosage and was added into the front of the film to have ful contact. Then, the film was detected by a fully automatic chemiluminescence analyzer, and the gray values of the relevant strips were read by TANON GIS software (Shanghai, China).
Statistics analysis
The data were analyzed by SPSS 18.0 software (SPSS Inc., Chicago, IL, USA). The results were expressed as mean ± SD. First, repeated measures ANOVA or one-way ANOVA were used first, then least significant difference (LSD)-t test was used. P<0.05 was considered to have statistical significance.
Results
Exercise training improves memory function in the intracerebral hemorrhage rats
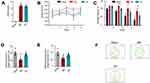
We first used the mNSS to assess the functional outcome in the rodent ICH model. We found that there was no statistical significance between the exercise group and the model group (P=0.566). Meanwhile, in the sham-operation group, the mNSS was 0 (Figure 1A). Next, the EX group underwent the exercise training. We used the water maze experiment to evaluate the memory function in the different groups. The water maze experiment lasted for 5 days. There was no significant difference in the average daily swimming speed among the three groups at each time point (P>0.05), and there was no significant difference in the average daily swimming speed among the three groups (P>0.05) (Figure 1B). The escape latency of the three groups decreased with time (Figure 1C). On the first day, there was no significant difference between the three groups. From the second day, the escape latency of the MD group was longer than that of the SM group (P<0.05), and that of the EX group was shorter than that of the MD group (P<0.05). On the fifth day of the spatial probe task, the percentage of time and distance in the target quadrant in the MD group decreased significantly compared with the SM group (P<0.05), while the percentage of time and distance in the target quadrant in the EX group increased significantly compared with the MD group (P<0.05) (Figure 1D–F). These results suggest that exercise training significantly improved the memory in the ICH rats.
Exercise training reduces NR1 and NR2B expression in the contralateral hippocampal CA3 area of intracerebral hemorrhage rats
We used the Western blot to evaluate the expression level of NR1 and NR2B in the contralateral hippocampal CA3 area of different groups. Compared with MD group and SM group, the expression of NR1 and NR2B in contralateral hippocampal CA3 area in the EX group was significantly decreased (P<0.05). The expression of NR1 and NR2B in CA3 area of hippocampus in model group was lower than that in sham-operation group, but there was no statistical significance (P>0.05) (Figure 2A–C).
Discussion
It is well known that exercise training is closely related to hippocampal-dependent learning and memory.20 Many previous experimental studies have found that exercise training can improve cognitive impairment by promoting the release of neurotrophic factors in hippocampus, reducing synaptic damage, promoting synaptic growth, and synaptic plasticity in cerebral infarction model animals.2–4 However, whether exercise training can also improve the cognitive function after ICH is still unknown. In the results of this study, we found that the spatial memory of rats in MD group was significantly lower than that of SM group, while the spatial memory of EM groups was significantly improved compared to that of MD group. These results showed that exercise training could effectively improve the spatial memory of ICH rats.
The survival of nerve cells requires normal physiological activity. Glutamate is a common excitatory transmitter in the brain. At present, NMDAR and alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor (AMPAR) are considered to be the two main types of ionic glutamate receptor. In the resting state, the channel holes of NMDAR are usually blocked by Mg2+. When glutamate is released from the presynaptic site, activated AMPAR leads to partial depolarization of the postsynaptic membrane, which is sufficient to remove Mg2+ blockade from NMDARs. Once NMDARs are activated, a large amount of influx of Ca2+ through NMDARs causes cell death.8,21,22 In the physiological conditions, glutamine synthetase can convert glutamic acid into non-neuroactive compound glutamine. However, under pathological condition, like ischemia and hypoxia, the expression of glutamic acid synthetase decreases rapidly, which leads to the persistence of excitatory toxicity caused by release of glutamic acid.6,21,23 The neuronal damage caused by this excitotoxicity also directly leads to cognitive impairment in rats with ICH.5
The two subunits of NMDAR, NR2A and NR2B, are mainly located at synapses and extrasynapses, respectively. NR2A participates in cell survival, while the activation of NR2B leads to excitotoxicity and neuronal apoptosis.22 NR2B is considered to play a neuropathological role after stroke. Using NR2B antagonists and inhibiting, NR2B expression can regulate excitotoxicity induced by glutamate, protect neurons after cerebral infarction, reduce infarction area and promote the recovery of nerve function.24–26 In cognitive-related research, Xu et al19 used N2B antagonist in the experiment and found that N2B antagonist could effectively inhibit nerve injury and improve spatial memory of rats with cerebral infarction. The results showed that inhibiting NR2B expression was beneficial to cognitive recovery. Liu et al5 also found that the decrease of spatial learning and memory ability after cerebral hemorrhage modeling was related to the increased expression of NR2B in the hippocampus, and inhibiting the expression of NR2B was beneficial to cognitive recovery. In this study, we also found that exercise training improves the spatial memory of rats with cerebral hemorrhage and down-regulates the expression of NR2B in the hippocampus.
NR1 subunit is essential for the formation of functional NMDARs. It has been found that secondary inflammation after ICH is associated with the expression and phosphorylation of NR1.27 NR1 autoantibody can reduce stroke area in stroke patients with complete blood–brain barrier, which may have protective effect on nerve cells.28 Numerous experimental studies have proved that the use of NR1 vaccine and drugs to reduce the expression of NR1 can also protect neurons after stroke, make the structure of neurons and dendrites more complete, and reduce excitotoxicity.13,29–32 In this study, we also found that the down-regulation of NR1 expression mediates the cognitive improvement of exercise training.
Early studies have shown that the brain can be compensated by the contralateral hemisphere after cerebral infarction.14–16 And, Jiang et al33 also found that exercise training can promote the hypermetabolism of motor cortex in the contralateral cerebral hemisphere of cerebral infarction through PET-CT study. In the study of Shimada et al,2 after cerebral infarction, they showed that the cognitive improvement of exercise training was related to the histological changes of bilateral hippocampus. In this study, we found that the expression of NR2B and NR1 regulated by the exercise training happened in the contralateral hippocampal CA3 area.
Activation of NMDAR in the cerebral hemisphere after ICH caused damage to nerve cells and cognitive impairment. After exercise training, the expression of NR2B and NR1 in both cerebral hemisphere and contralateral hippocampal CA3 area was down-regulated, and the excitotoxicity was inhibited. The expression of NR2B and NR1 in the MD group was lower than that in the SM group, but there was no statistical significance. Therefore, we considered that exercise training could promote the compensation of contralateral hippocampus.
There are several limitations in this study. Firstly, because of the small sample size, we have not dynamically observed the changes of NR1 and NR2B in the study; secondly, there have been no morphological related research; thirdly, whether the cognitive improvement effect of exercise training in ICH rats is sustained or not, needs further study.
Conclusion
These results suggest that exercise training can improve the spatial memory of rats with cerebral hemorrhage via down-regulating the expression of NR2B and NR1 in the contralateral hippocampus.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Springer MV, Schmidt JM, Wartenberg KE, et al. Predictors of global cognitive impairment 1 year after subarachnoid hemorrhage. Neurosurgery. 2009;65(6):1043–1051. doi:10.1227/01.NEU.0000359317.15269.20
2. Shimada H, Hamakawa M, Ishida A, et al. Low-speed treadmill running exercise improves memory function after transient middle cerebral artery occlusion in rats. Behav Brain Res. 2013;243:21–27. doi:10.1016/j.bbr.2012.12.018
3. Lin Y, Dong J, Yan T, et al. Involuntary, forced and voluntary exercises are equally capable of inducing hippocampal plasticity and the recovery of cognitive function after stroke. Neurol Res. 2015;37(10):893–901. doi:10.1179/1743132815Y.0000000074
4. Yang L, Zhang J, Deng Y, et al. The effects of early exercise on motor, sense, and memory recovery in rats with stroke. Am J Phys Med Rehabil. 2017;96(3):e36–e43. doi:10.1097/PHM.0000000000000670
5. Liu Z, Li R, Jiang C, et al. The neuroprotective effect of lithium chloride on cognitive impairment through glycogen synthase kinase-3β inhibition in intracerebral hemorrhage rats. Eur J Pharmacol. 2018;840:50–59. doi:10.1016/j.ejphar.2018.10.019
6. Sharp F, Liu DZ, Zhan X, et al. Intracerebral hemorrhage injury mechanisms: glutamate neurotoxicity, thrombin, and Src. Acta Neurochir Suppl. 2008;105:43–46.
7. Jolly S, Bazargani N, Quiroga AC, et al. G protein-coupled receptor 37-like 1 modulates astrocyte glutamate transporters and neuronal NMDA receptors and is neuroprotective in ischemia. J Cell Physiol. 2018;66(1):47–61.
8. Hardingham GE, Bading H. The Yin and Yang of NMDA receptor signalling. Trends Neurosci. 2003;26(2):81–89. doi:10.1016/S0166-2236(02)00040-1
9. Paoletti P, Bellone C, Zhou Q. NMDA receptor subunit diversity: impact on receptor properties, synaptic plasticity and disease. Nat Rev Neurosci. 2013;14(6):383–400. doi:10.1038/nrn3504
10. Mayor D, Tymianski M. Neurotransmitters in the mediation of cerebral ischemic injury. Neuropharmacology. 2018;134(Pt B):178–188.
11. Jaenisch N, Liebmann L, Guenther M, et al. Reduced tonic inhibition after stroke promotes motor performance and epileptic seizures. Sci Rep. 2016;6:26173. doi:10.1038/srep26173
12. Gogas KR. Glutamate-based therapeutic approaches: NR2B receptor antagonists. Curr Opin Pharmacol. 2006;6(1):68–74. doi:10.1016/j.coph.2006.06.002
13. Huang M, Cheng G, Tan H, et al. Capsaicin protects cortical neurons against ischemia/reperfusion injury via down-regulating NMDA receptors. Exp Neurol. 2017;295:66–76. doi:10.1016/j.expneurol.2017.05.001
14. Murphy TH, Corbett D. Plasticity during stroke recovery: from synapse to behaviour. Nat. Rev Neurosci. 2009;10(12):861. doi:10.1038/nrn2735
15. Takatsuru Y, Fukumoto D, Yoshitomo M, et al. Neuronal circuit remodeling in the contralateral cortical hemisphere during functional recovery from cerebral infarction. J Neurosci. 2009;29(32):10081–10086. doi:10.1523/JNEUROSCI.1638-09.2009
16. Rehme AK, Fink GR, von Cramon DY, et al. The role of the contralesional motor cortex for motor recovery in the early days after stroke assessed with longitudinal FMRI. Cereb Cortex. 2011;21(4):756–768. doi:10.1093/cercor/bhq140
17. Penner MR, Mizumori SJ. Age-associated changes in the hippocampal-ventral striatum-ventral tegmental loop that impact learning, prediction, and context discrimination. Front Aging Neurosci. 2012;4:22. doi:10.3389/fnagi.2012.00022
18. Liu T, Zhou J, Cui H, et al. Quantitative proteomic analysis of intracerebral hemorrhage in rats with a focus on brain energy metabolism. Brain Behav. 2018;8(11):e01130.
19. Xu CS, Liu AC, Chen J, et al. Overactivation of NR2B-containing NMDA receptors through entorhinal-hippocampal connection initiates accumulation of hyperphosphorylated tau in rat hippocampus after transient middle cerebral artery occlusion. J Neurochem. 2015;134(3):566–577. doi:10.1111/jnc.13134
20. Hashimoto M, Araki Y, Takashima Y, et al. Hippocampal atrophy and memory dysfunction associated with physical inactivity in community-dwelling elderly subjects: the Sefuri study. Brain Behav. 2017;7(2):e00620. doi:10.1002/brb3.620
21. Weilinger NL, Lohman AW, Rakai BD, et al. Metabotropic NMDA receptor signaling couples Src family kinases to pannexin-1 during excitotoxicity. Nat Neurosci. 2016;19(3):432–442.
22. Wu QJ, Tymianski M. Targeting NMDA receptors in stroke: new hope in neuroprotection. Mol Brain. 2018;11(1):15. doi:10.1186/s13041-018-0357-8
23. Lee A, Lingwood BE, Bjorkman ST, et al. Rapid loss of glutamine synthetase from astrocytes in response to hypoxia: implications for excitotoxicity. J Chem Neuroanat. 2010;39(3):211–220. doi:10.1016/j.jchemneu.2009.12.002
24. Hu J, Pang WS, Han J, et al. Gualou Guizhi decoction reverses brain damage with cerebral ischemic stroke, multi-component directed multi-target to screen calcium-overload inhibitors using combination of molecular docking and protein-protein docking. J Enzyme Inhib Med Chem. 2018;33(1):115–125. doi:10.1080/14756366.2017.1396457
25. Jie P, Lu Z, Hong Z, et al. Activation of transient receptor potential vanilloid 4 is involved in neuronal injury in middle cerebral artery occlusion in mice. Mol Neurobiol. 2016;53(1):8–17. doi:10.1007/s12035-014-8992-2
26. Fan M, Xu H, Wang L, et al. Tissue plasminogen activator neurotoxicity is neutralized by recombinant ADAMTS 13. Sci Rep. 2016;6:25971. doi:10.1038/srep25971
27. Weng X, Tan Y, Chu X, et al. N-methyl-D-aspartic acid receptor 1 (NMDAR1) aggravates secondary inflammatory damage induced by hemin-NLRP3 pathway after intracerebral hemorrhage. Chin J Traumatol. 2015;18(5):254–258. doi:10.1016/j.cjtee.2015.11.010
28. Zerche M, Weissenborn K, Ott C, et al. Preexisting serum autoantibodies against the NMDAR subunit NR1 modulate evolution of lesion size in acute ischemic stroke. Stroke. 2015;46(5):1180–1186. doi:10.1161/STROKEAHA.114.008323
29. During MJ, Symes CW, Lawlor PA, et al. An oral vaccine against NMDAR1 with efficacy in experimental stroke and epilepsy. Science. 2000;287(5457):1453–1460. doi:10.1126/science.287.5457.1453
30. Yun X, Maximov VD, Yu J, et al. Nanoparticles for targeted delivery of antioxidant enzymes to the brain after cerebral ischemia and reperfusion injury. J Cereb Blood Flow Metab. 2013;33(4):583–592. doi:10.1038/jcbfm.2012.209
31. Gu J, Chen J, Yang N, et al. Combination of Ligusticum chuanxiong and Radix Paeoniae ameliorate focal cerebral ischemic in MCAO rats via endoplasmic reticulum stress-dependent apoptotic signaling pathway. Nat Neurosci. 2016;187:313–324.
32. Balsara R, Dang A, Donahue DL, et al. Conantokin-G attenuates detrimental effects of NMDAR hyperactivity in an ischemic rat model of stroke. PLoS One. 2015;10(3):e0122840. doi:10.1371/journal.pone.0122840
33. Jiang XF, Zhang T, Sy C, et al. Dynamic metabolic changes after permanent cerebral ischemia in rats with/without post-stroke exercise: a positron emission tomography (PET) study. Neurol Res. 2014;36(5):475–482. doi:10.1179/1743132814Y.0000000350
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.