Back to Journals » Infection and Drug Resistance » Volume 15
Excessively Prolonged Early Antibiotic Duration in Very-Low-Birth-Weight Infants: A Multicenter Prospective Cohort Study in a Developing Country
Authors Hou S, Wang X, Wang F, Li Z, Wang H, Li J, Wang J, He H, Deng L, Feng Y, Fan X, Li W, Lu Q, Ma Y, Zhao G, Reddy S, Wu Y, Yu Y
Received 15 November 2021
Accepted for publication 16 March 2022
Published 19 April 2022 Volume 2022:15 Pages 1921—1931
DOI https://doi.org/10.2147/IDR.S349478
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Shanshan Hou,1,2 Xiaokang Wang,3 Fang Wang,4 Zhongliang Li,5 Hui Wang,6 Jiahui Li,7 Jing Wang,8 Haiying He,9 Liping Deng,10 Yushu Feng,11 Xiufang Fan,12 Wen Li,13 Qinghua Lu,14 Yanying Ma,15 Guoying Zhao,16 Simmy Reddy,17 Yanqiu Wu,2,* Yonghui Yu1,3,*
1Department of Neonatology, Shandong Provincial Hospital, Cheeloo College of Medicine, Shandong University, Jinan, 250021, People’s Republic of China; 2Department of Pediatric, The Affiliated Yantai Yuhuangding Hospital of Qingdao University, Yantai, 264000, People’s Republic of China; 3Department of Neonatology, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, Shandong, 250021, People’s Republic of China; 4Department of Neonatology, Liaocheng People’s Hospital, Liaocheng, 252000, People’s Republic of China; 5Department of Neonatology, W.F. Maternal and Child Health Hospital, Weifang, 261011, People’s Republic of China; 6Department of Neonatology, Hebei PetroChina Central Hospital, Langfang, 065000, People’s Republic of China; 7Department of Neonatology, The First Affiliated Hospital of Shandong First Medical University, Jinan, 250014, People’s Republic of China; 8Department of Neonatology, Women and Children’s Healthcare Hospital of Linyi, Linyi, 276000, People’s Republic of China; 9Department of Neonatology, Baogang Third Hospital of Hongci Group, Baotou, 014010, People’s Republic of China; 10Department of Neonatology, Heze Municipal Hospital, Heze, 274031, People’s Republic of China; 11Department of Neonatology, Linyi People’s Hospital, Linyi, 276000, People’s Republic of China; 12Department of Neonatology, Jinan Maternity and Child Health Care Hospital, Jinan, 250001, People’s Republic of China; 13Department of Neonatology, Qilu Hospital of Shandong University, Jinan, 250012, People’s Republic of China; 14Department of Neonatology, Shandong Maternal and Child Health Hospital, Cheeloo College of Medicine, Shandong University, Jinan, 250014, People’s Republic of China; 15Department of Neonatology, Jinan Second Maternal and Child Health Care Hospital, Jinan, 271100, People’s Republic of China; 16Department of Neonatology, Binzhou Medical University Hospital, Binzhou, 256603, People’s Republic of China; 17Cheeloo College of Medicine, Shandong University, Jinan, 250021, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yonghui Yu, Department of Neonatology, Shandong Provincial Hospital Affiliated to Shandong First Medical University, No. 234, Jingwu, Road, Huai Yin District, Jinan, Shandong, 250021, People’s Republic of China, Tel +86-0531-66953201, Email [email protected]; [email protected] Yanqiu Wu, Department of Pediatric, the Affiliated Yantai Yuhuangding Hospital of Qingdao University, No. 20, Yuhuangding East Road, Zhi fu District, Yantai, 264000, People’s Republic of China, Tel +86-0535-6691999, Email [email protected]
Purpose: Significant antibiotic overuse due to prolonged antibiotic duration has not draw enough attention in developing countries with high antibiotic consumption. We aimed to describe the current status of prolonged early antibiotic duration in very-low-birth-weight (VLBW) infants in a large regional multicenter cohort in China.
Patients and Methods: Institution-based prospective cohort study was conducted in all VLBW infants admitted to 16 Grade A tertiary hospitals between January 1, 2019 and December 31, 2020. Early antibiotic use was defined as antibiotic initiation within the first 3 days of life. Prolonged early antibiotic course was defined as early antibiotic initiation for more than 7 days in infants with early-onset sepsis (EOS) or more than 3 days in infants with unlikely EOS. Antibiotic use was described as days of therapy (DOT) per 1000 patient days (PD).
Results: Among 1684 eligible VLBW infants, 1544 (91.7%) infants were prescribed with prolonged early antibiotic course, including 618 infants with EOS and 926 infants with unlikely EOS. The median duration of early antibiotic course was 13 (IQR 8;20) days, with 78.0% of courses > 7 days and 43.6% of courses > 14 days. Total early antibiotic use was 408.3DOT/1000Pd, of which prolonged antibiotic courses accounted for 98.2% of all antibiotic use days. More than three antibiotics used, escalation antibiotic therapy, antibiotics for special use and the use of third generation cephalosporins and carbapenems were significantly common in prolonged courses compared to short courses in both infants with EOS and unlikely EOS group (P< 0.05).
Conclusion: A large proportion of VLBW infants had excessively prolonged early antibiotic durations in the regional multicenter in China. Timely discontinuation of antibiotics in VLBW infants according to standardized guidelines and limit on the use of third-generation cephalosporins and carbapenems may be key drivers in reducing the antibiotic overuse in developing countries like ours.
Keywords: very-low-birth-weight infants, early antibiotic use, prolonged antibiotic course, days of therapy
Introduction
Neonatal early-onset sepsis (EOS) is defined as a systemic infection occurring within the first 72h of life,1 causing significant morbidity and mortality worldwide, particularly among very-low-birth-weight(VLBW) infants.2,3 However, due to nonspecific clinical presentation, low predictive value of biomarkers and unreliable blood culture, it is difficult to rule out EOS immediately therefore clinicians often initiate early antibiotic treatment in VLBW infants after birth.4 Especially in developing countries with overcrowding, high infection and antimicrobial resistance burden, clinicians while more likely to prolong antibiotic use are often reluctant to stop it, often leading to prolonged duration of early antibiotic use in VLBW infants, even in infants with unlikely sepsis.5
It is well documented that excessively prolonged antibiotic duration is the major reason for overuse of antibiotics in preterm infants.6,7 Prolonged or unnecessarily early antibiotic use in VLBW infants can cause intestinal dysbiosis and has been associated with increased risk of serious neonatal mortality, morbidities and neurodevelopmental impairment.8 Besides, overuse of third generation cephalosporin and carbapenems can increase the risk of infection with multi-resistant bacteria and even ‘superbugs’, which is also an ongoing major global public threat.9
In recent years, antibiotic stewardship programs (ASPs) in neonates in high-income countries have made great effort in reducing antibiotic duration by standardizing antibiotic use for infection.10 Common guidelines for EOS suggest stopping antibiotic use in infants with unlikely sepsis within 36–72h hours after birth11,12 and limiting the antibiotic course of EOS to 5~7 days.11,13 This has lead to a significant decrease in the rate of prolonged antibiotic duration for VLBW infants over time.4 Only 26.5% of VLBW infants received early antibiotic use for more than 5 days in 297 hospitals across the United States from 2009 to 2015 and 55% of VLBW infants received early antibiotic use for more than 4 days in Canadian Neonatal Network from 2010 to 2014.14 As the most priority focused antibiotic resistance region in the World Health Organization (WHO), overuse of antibiotics is common in China, and is more serious in VLBW infants. But data on early antibiotic use in VLBW infants is scarcely recorded in China. The objective of this study was to comprehensively describe the prolonged early antibiotic duration for VLBW infants with EOS and unlikely EOS in a large multicenter cohort of regional China so as to provide bench-marking data to promote the neonatal-specific ASP in China and in countries with similar situations.
Materials and Methods
Study Design, Period and Area
This multicenter, prospective cohort study was conducted in all VLBW infants admitted to 16 Grade A tertiary hospitals between January 1, 2019 and December 31, 2020 from the Sino-northern Neonatal Network (SNN) database. Starting from 2018, we established a prospective cohort of Chinese Adverse Prognosis of Very Preterm infants’ cohort based on SNN database. SNN is a large, comprehensive administrative database of preterm inpatients with birth weight (BW) <1500g or gestational age (GA) <32 weeks from tertiary hospitals across 6 provinces and autonomous regions in northern China.15 Sixteen Grade A tertiary hospitals caring for more than 30 VLBW infants per year were included in this study. Of these hospitals, 14 were located in Shandong province (from which 7 major cities were involved), one tertiary hospital from Hebei province and the other from the Inner Mongolia Autonomous Region.
Study Population
Infants with BW<1500 g who were born in the participating hospitals were included in the study. The exclusion criteria were as follows: infants without early antibiotic use; infants who were admitted to the participating hospital for less than 7 days.
Data Collection and Quality Management
The perinatal information, and information related to the demographics of the infants that have been associated with neonatal infection and antibiotic use were all prospectively collected in SNN database, including GA, BW, Apgar score at 5 min of life, Neonatal Acute Physiology version II (SNAP-II) scores, perinatal risk factors, abnormal clinical presentation at the start of antibiotic therapy, laboratory tests, diagnoses, ventilator support mode, drug data (drug names, classes, the reason(s) for its initiation and dates), length of stay, morbidity and mortality. The aforementioned data sets were collected and transmitted by trained research staff utilizing a standardized manual of operations from each site to the coordinating center in Jinan, Shandong Province, China, and was audited by senior physicians.
Operational Definitions
Antibiotics for systemic use except for anti-fungal agents were included in this study. Days of therapy (DOT) was defined as accumulated days of systemic antibiotic therapy.16 DOT per 1000 Patient days (Pd) for early antibiotic use is calculated by dividing DOT by the total patient-days of follow-up and multiplying it by 1000. Early antibiotic use was defined as antibiotic initiation within the first 3 days of life.4 Prolonged early antibiotic course was defined as early antibiotic initiation for more than 7 days in infants with EOS or more than 3 days in infants with unlikely EOS.11,17 Antibiotics for special use were antibiotics including fourth generation cephalosporins, carbapenems and glycopeptides, based on their clinical effects and safety, which were predefined by the Chinese Ministry of Health.18 Perinatal risk factors for EOS were defined according to the American Academy Pediatrics (AAP) guidelines regarding management for suspected EOS: maternal cervical incompetence, preterm labor, GBS positivity, premature rupture of membranes more than 18h, chorioamnionitis, acute onset of unexplained non-reassuring fetal status.12 Multidrug-resistant (MDR) bacteria was defined as non-susceptibility to at least one agent in three or more antimicrobial categories.19
Our definition of neonatal sepsis was formulated with consideration to Chinese consensus of diagnosis.14,17 Neonatal EOS is defined as a systemic infection occurring within the first 72h of life by the following simultaneous presence: 1) clinical manifestations of newborn infection (according to≥1 items): respiratory distress, apnea; tachycardia or bradycardia; systemic hypotension or hypoperfusion; hypothermia or fever (T>38.5°C or <36°C); convulsion, hypotonia, irritability or lethargy; feeding intolerance or intestinal obstruction; 2) Abnormal non-specific infection index (according to ≥1 items): WBC<5×10^9/L, or WBC increased (>30×10^9/L); CRP≥10 mg/l; 3) antibiotics used or intended for use ≥5 days; If the blood or cerebrospinal fluid culture is positive, then it is diagnosed as proven EOS. Unlikely EOS is defined as an infant with perinatal risk factors or abnormal clinical manifestations but who has not clinically met the criteria for the definition mentioned above.11,17
Statistical Analysis
All the eligible infants were divided into infants with EOS group and infants with unlikely EOS group. Each group was than divided into short antibiotic course group and prolonged antibiotic course group according to the antibiotic duration. We compared the demographic characteristics and antibiotic metrics between infants with short antibiotic course and prolonged antibiotic course using Chi-square, Students’ t-test and non-parametric test depending on the case. Categorical variables were expressed as absolute and relative frequencies; continuous variables were expressed as mean (SD) or median and inter-quartile range (IQR) when appropriate. All statistical analyses were conducted using a software program (SPSS v.25.0 (SPSS Inc, Chicago, Illinois)), with statistical significance evaluated using 2-sided P values at the 5% testing level.
Results
Sociodemographic Characteristics of Patients
In the two-year duration of this study, 2082 VLBW infants were admitted in the level 3 NICUs of 16 Grade A tertiary hospitals. After excluding 398 infants who met the exclusion criteria, the remaining 1684 eligible VLBW infants were enrolled in our study, with 700 (41.6%) VLBW infants with EOS and 984 (58.4%) infants with unlikely EOS. In the EOS group, 618 (89.4%) infants received >7 days of early antibiotics, while in the unlikely EOS group, 926 (93.6%) infants received >3 days of early antibiotics. Overall, 1544 (91.7%) eligible infants were prescribed with prolonged early antibiotic course (Figure 1). The mean GA ±SD of all eligible infants was 29.6±2.0 weeks and the median BW was 1200 (IQR 1022;1340) g. In comparing socio-demographic characteristics between short and prolonged courses in each group, we found that there was no significant difference in antepartum risk factors, clinical characteristics, laboratory abnormalities and outcomes (P>0.05). (Table 1).
 |
Table 1 Descriptive Characteristics Comparison of Short and Prolonged Early Antibiotic Course |
 |
Figure 1 Flowchart of the study and distribution of DOT/1000Pd for early antibiotic use. Abbreviations: NICU, neonatal intensive care units; EOS, early onset sepsis; DOT, days of therapy. |
Overall Antibiotic Use
The durations of early antibiotic use for all eligible infants are shown in Figure 2, and the median duration of early antibiotic initiation was 13 (IQR 8;20) days. Only 4.3% of courses were ≤3 days while 78.0% of courses were >7 days and 43.6% of courses were >14 days (Figure 2). The total DOT/1000Pd of early antibiotic use in our cohort was 408.3. Prolonged early antibiotic courses accounted for 98.2% of all early antibiotic use days, including 190.0DOT/1000Pd (46.5%) of prolonged courses coming from infants with EOS and 211.2DOT/1000Pd (51.7%) of prolonged courses coming from infants with unlikely EOS (Table 2). When calculating the antibiotic use in each group, the maximum DOT/1000Pd (480.4) was in VLBW infants with EOS with prolonged courses, while the minimum DOT/1000Pd (68.1) was in VLBW infants with unlikely EOS with short courses (Figure 1).
 |
Table 2 Antibiotic Metrics Comparison of Short and Prolonged Early Antibiotic Courses |
 |
Figure 2 Distribution of duration of early antibiotic courses in VLBW infants. Abbreviations: EOS; early onset sepsis. d, days. |
A total of 21 different antibiotic agents were prescribed during the study period. The top 12 frequently used antibiotic agents are listed in Supplementary Table 1. The most 3 commonly used antibiotics were Piperacillin/tazobactam (37.5%), Cefoperazone/sulbactam (25.0%) and Meropenem (24.0%). The median duration of almost all antibiotic agents was more than 7 days except for Amoxicillin/Clavulanate Acid. After normalizing our results by follow-up time, the most commonly used antibiotic classes by percentage of total DOT were third generation cephalosporins (31.2%), followed by penicillinase inhibitor (26.4%), carbapenems (20.1%), penicillins (15.4%) and glycopeptide (3.7%) (Figure 3).
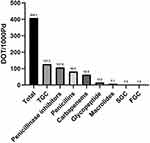 |
Figure 3 Early antibiotic classes used by DOT/1000PD. Abbreviations: TGC, third generation cephalosporins; SGC, second generation cephalosporins; FGC, fourth generation cephalosporins. |
Comparison of Early Antibiotic Use in Short and Prolonged Courses Among VLBW Infants with EOS
The median duration of early antibiotic initiation in VLBW infants with EOS was 15 (IQR 10;24) days. Only 11.0% of courses were ≤7 days while 53.0% of courses were >14 days (Figure 2). Within the EOS group, 39 patients were diagnosed with proven EOS, 12 with purulent meningitis and the median durations of early antibiotic use in infants with proven EOS and purulent meningitis were 18 (IQR 10;28) and 16 (IQR 15;29) days, respectively. The DOT/patient and duration of antibiotic course were both significantly longer in prolonged courses compared to short courses. Similarly, more than three antibiotics used, escalation antibiotic therapy and antibiotics for special use were all significantly increased in prolonged courses (P<0.05). The escalated antibiotic therapy was mainly to upgrade to meropenem (92.1%), and it is significantly more common in the prolonged course, the remaining escalation antibiotic therapy was to upgrade to third generation cephalosporins (8.9%). In the analysis of antibiotic agents, the third generation cephalosporins, penicillins and carbapenems were more likely used in prolonged courses than in the short courses, but penicillins were all combined with third generation cephalosporin or carbapenems.
In the infants with proven EOS, the most common Gram-positive isolated pathogens were coagulase-negative staphylococcus, with 13 confirmed episodes, and a 20% resistance rate to oxacillin. In the Gram-negative group, the most common bacteria were E. coli and Klebsiella pneumoniae, with a total of 23 cases and a 43.5% third generation cephalosporins resistance rate, approximately 30% (3/23) of those isolates were MDR but all sensitivity to Piperacillin/Tazobactam. 42.3% (11/26) of total Gram-negative group pathogen were resistant to third generation cephalosporins (Table 3).
 |
Table 3 Antibiotic Resistance Patterns of Isolated Pathogens |
Comparison of Early Antibiotic Use in Short and Prolonged Courses Among VLBW Infants with Unlikely EOS
The median duration of early antibiotic initiation in VLBW infants with unlikely EOS was 11 (IQR 7;18) days. Only 6.0% of courses were ≤ 3 days while 70.0% of courses were >7 days with 37.0% of courses being >14 days (Figure 2). Similar to EOS group, DOT/patient and duration of antibiotic course were both significantly longer in prolonged courses as compared to short courses (P<0.05). More than three antibiotics used, combination antibiotic therapy, escalation antibiotic therapy and antibiotics for special use were all significantly increased in prolonged courses (P<0.05). All escalation antibiotic therapy was found in prolonged courses and also tended to upgrade to meropenem (86.7%), the remaining escalation antibiotic therapy upgrades were to third generation cephalosporins (7.8%) and to Piperacillin/tazobactam (5.5%), respectively. In the analysis of antibiotic agents, the third generation cephalosporins, penicillins and carbapenems tended to be used in prolonged courses more often than in short courses, but penicillins were combined with the third generation cephalosporins or carbapenems except for mezlocillin.
Discussion
Antibiotic overuse is a common and serious issue in developing countries like China, while in preterm infants excessively prolonged antibiotic duration is verifiably the major reason for that problem. Thus, our study is, to the best of our knowledge, the first study to exhaustively describe the prolonged early antibiotic duration for VLBW infants in a large-sample prospective cohort of regional China. We identified that more than 75% of enrolled VLBW infants were prescribed with antibiotic therapy for longer than 7 days after birth and over 98% of total early antibiotic use days were for prolonged courses. Besides, the escalation antibiotic therapy to third generation cephalosporins or carbapenems increased significantly in prolonged courses although there were no significant difference in antepartum risk factors, clinical characteristics and laboratory abnormalities between short courses and prolonged courses in infants with EOS or unlikely EOS, which is dramatically different than that in high-income countries.20 However, approximately half of Gram-negative bacteria pathogen of proven EOS were resistant to third generation cephalosporins. This descriptive study reflects a real-life situation and emphasizes the severity of antibiotic overuse and the urgent need for ASP in neonate.
Overall, only 4.3% of early antibiotic courses were ≤3 days but 88.0% of courses were >5 days with 78.0% of courses being >7 days and 43.6% of courses being >14 days. Our findings were significantly longer than those in a similar cohort study in a multicenter in the USA, which were about 40.0% for early antibiotic courses ≤3 days and about 26.5% of courses being >5 days.4 When we divided the VLBW infants into EOS group and unlikely EOS group according to infection diagnosis, we found 93.6% infants in unlikely EOS group were prescribed with early antibiotic for more than 3 days and 89.4% infants in EOS group were given prescription for more than 7 days, which were only 68% of infants with unlikely EOS for more than 2 days and 63% of infants with EOS for more than 7 days in Cantey’s survey in Dallas, USA.21 In addition, the total antibiotic use for early antibiotic courses was 408.3DOT/1000Pd, which was dramatically higher than that in the high-income countries, which were 233.8DOT/1000Pd in France and 250.0 DOT/1000Pd in Canada.14,22 It is also striking that prolonged antibiotic courses accounted for 98.2% of all early antibiotic use days in the further analysis. High proportion and antibiotic consumption of prolonged antibiotic courses could not be fully explained by higher infection rates, because the incidence of confirmed infections was not significantly higher in our centers compared with high-income countries.23 Besides, there was no significant difference in antepartum risk factors, severity of illness and laboratory tests between short courses and prolonged courses in our study, which was different from similar analysis in high-income countries,20 indicating that the prescribers’ subjective decision may be the major cause of prolonged antibiotic duration rather than objective factors.
Guidelines in the UK have always recommended performing a blood culture before giving the first dose of antibiotic and measuring C-reactive protein (CRP) at least twice within 24h after birth to assist diagnosis of infection. It suggested to stop antibiotic use within 36–72h hours after birth in infants with unlikely EOS and monitor the levels and trends of CRP every 24h to help to judge the antibiotic duration in infants with EOS.11 Common guidelines for EOS suggest limiting the antibiotic course of EOS to 5~7 days.11,13 The latest literature recommended the use of two bottles for aerobic and anaerobic cultures with a volume of at least 0.5 mL in each one of them to increase the credibility of the results. Unfortunately, we did not register the exact amounts of the blood culture samples. Only 55% of infants had at least two CRP within 3 days of life, and the infection indicators were always measured only once a week after 3 days of life in most infants, which were much lower than the recommended sample count. In addition, about 75% infants with prolonged courses were not presenting with abnormal indicators at the same time. As a result, the lower credibility of blood culture and the limited infection indicators may be the potential causes for prolonged treatment. Moreover, insufficient consciousness of adverse outcomes in antibiotic overuse and tense doctor-patient relationships made the physicians in China more likely to continue antibiotics until infants have no abnormal clinical presentation. However, persistent cardio-respiratory instability is common among VLBW infants and should not alone an indication for prolonged empirical antibiotic use.12 Clinical practice guidelines have the potential to improve decision making in antibiotic prescription, particularly important in areas with limited laboratory and specialist capacity.24 Therefore, it is urgently needed to develop guidelines for management of EOS based on local characteristics, meanwhile promoting an organizational culture on appropriate antibiotic use, strengthening education and supervising the compliance of guidelines may be the primary target drivers to ASP in neonates.
In our study, more than three antibiotics used and escalation antibiotic therapy were significantly common in prolonged course in both infants with EOS or unlikely EOS group, and most escalation antibiotic therapy was escalated to third generation cephalosporins or carbapenems. As a result, the third generation cephalosporins and carbapenems use rate were significantly higher in prolonged course. Ampicillin and gentamicin (93%) are the most frequent used antibiotics for infection in high-income countries,21 but prescription of gentamicin is forbidden for children younger than 8 years in national antibacterial guidelines due to possible risk of hearing loss. So, third generation cephalosporins are commonly recommended as the first-line antibiotics for neonatal EOS. However, in the current study, E. coli and Klebsiella pneumoniae accounted for the majority of pathogen in proven EOS with an almost 50% third generation cephalosporins resistance rate, while the total MDR rate was only 7.7% (3/39). Therefore, empiric antibiotics with third generation cephalosporins or meropenem for EOS may be one major reason for irrational antibiotic use. Additionally, long-term use of third generation cephalosporins in neonate may disrupt the establishment of microbiome in early life and lead to a higher incidence of antibiotic resistance, NEC, invasive candidiasis, even death. In particular, overuse of meropenem can lead to growing of MDR bacteria, which is a significant global health problem.9 In 2017, the WHO has classified antibiotics into three groups (Access, Watch, and Reserve, AWaRe) according to use priority and agent spectrum. Cefoperazone/sulbactam belongs to the “not recommended” group, while other broad-spectrum antibiotics in our study all belong to “watch group” that should be restricted to use according to the WHO guidelines.25 Thus, limiting exposure to third generation cephalosporins and carbapenems should be prioritized as key targets of stewardship programs because of their higher resistance potential.
The strength of our study lies in the large number of participating VLBW infants included, as well as the fact that we comprehensively reported the description of antibiotic use, including combination use, switching patterns and antimicrobial sensitivity data, which were scarcely presented in the previous study. Furthermore, due to defined daily dose (DDD) usually used as adult doses which limits its applicability to pediatric populations, we chose DOT as the antibiotic consumption metric, which is main benchmark for most ASP nowadays, and can best represent the degree of antibiotic exposure and thus the impact on the infant’s microbiome than length of therapy (LOT) or DDD in previous study. However, our study has several limitations. First, incidence of proven sepsis in this study is relatively lower than reported in previous reports.24 We speculate that cultures may be falsely negative because of inappropriate sampling practices such as obtaining insufficient blood volume and performing cultures after antibiotics have been started.26 Therefore, we have focused on standardizing blood culture collection in future practice. Besides, we did not analyze variations of antibiotic use among NICUs. We are planning to implement a survey on antibiotic use strategies in multicenter NICUs to identify additional possible reasons for prolonged antibiotic exposure while exploring optimized practices in the center with the lowest antibiotic consumption to develop more effective clinical guideline.
Conclusion
A large proportion of VLBW infants had excessively prolonged early antibiotic duration at regional multicenter of China, whose antibiotic consumption accounted for the majority of total early antibiotic use. In addition, the use of third generation cephalosporins and carbapenems increased significantly in prolonged courses although approximately half of Gram-negative bacteria pathogen of proven EOS were resistant to third generation cephalosporins and no significant difference was found in illness severity in infants with EOS or unlikely EOS. Physicians and policymakers should focus their efforts to standardize clinical guidelines for timely antibiotic discontinuation and limit use of third generation cephalosporins and carbapenems to decrease the burden of antibiotic use, and further reduce the rising bacterial resistance in developing countries like ours.
Ethical Considerations
The studies involving human participants were reviewed and approved by Ethics Committee of Shandong Provincial Hospital affiliated to Shandong First Medical University and Shandong University (LCYJ: NO.2019-132). Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin. This study was conducted in accordance with the Declaration of Helsinki.
Acknowledgments
The authors would like to thank those who were involved in this research.
Author Contributions
All authors made substantial contributions to designed the study, trained and supervised the data collectors, the data collection, interpreted the results, and took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
This study was supported by Project of “Impact of Pulmonary Surfactant on Premature Infants with Neonatal Respiratory Distress Syndrome”. The funder of our study is the corresponding author of this study, professor Yonghui Yu.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Stoll BJ, Gordon T, Korones SB, et al. Early-onset sepsis in very low birth weight neonates: a report from the National Institute of Child Health and Human Development Neonatal Research Network. J Pediatric. 1996;129:72–80. doi:10.1016/S0022-3476(96)70192-0
2. Hornik CP, Fort P, Clark RH, et al. Early and late onset sepsis in very-low-birth-weight infants from a large group of neonatal intensive care units. Early Hum Dev. 2012;88(Suppl.2):S69–74. doi:10.1016/S0378-3782(12)70019-1
3. Simonsen KA, Anderson-Berry AL, Delair SF, et al. Early-onset neonatal sepsis. Clin Microbiol Rev. 2014;27(1):21–47. doi:10.1128/CMR.00031-13
4. Flannery DD, Ross RK, Mukhopadhyay S, et al. Temporal Trends and Center Variation in Early Antibiotic Use Among Premature Infants. JAMA network open. 2018;1:164. doi:10.1001/jamanetworkopen.2018.0164
5. Rueda MS. Antibiotic Overuse in Premature Low-Birth-Weight Infants in a Developing Country. Pediatr Infect Dis. 2019;38(3):302–307. doi:10.1097/INF.0000000000002055
6. Patel SJ, Oshodi A, Prasad P, et al. Antibiotic use in neonatal intensive care units and adherence with centers for disease control and prevention 12 step campaign to prevent antimicrobial resistance. Pediatr Infect Dis J. 2009;28(12):1047–1051.
7. Cantey JB, Pyle AK, Wozniak PS, et al. Early antibiotic exposure and adverse outcomes in preterm, very low birth weight infants. J Pediatr. 2018;203:62–67.
8. Kuppala VS, Meinzen-Derr J, Morrow AL, et al. Prolonged initial empirical antibiotic treatment is associated with adverse outcomes in premature infants. J Pediatr. 2011;159(5):720–725. doi:10.1016/j.jpeds.2011.05.033
9. Zakir A, Regasa Dadi B, Aklilu A. Investigation of Extended-Spectrum β-Lactamase and Carbapenemase Producing Gram-Negative Bacilli in Rectal Swabs Collected from Neonates and Their Associated Factors in Neonatal Intensive Care Units of Southern Ethiopia. Infect Drug Resist. 2021;14:3907–3917. doi:10.2147/IDR.S333603
10. Willis Z, Annabelle D. Strategies to improve antibiotic use in the neonatal ICU. Curr Opin Pediatr. 2019;31(1):127–134. doi:10.1097/MOP.0000000000000716
11. NICE. Neonatal Infection: Antibiotics for Prevention and Treatment. PMID: 34133110. London: National Institute for Health and Care Excellence (NICE);2021.
12. Puopolo KM, Benitz WE, Zaoutis TE. Committee On Fetus and Newborn, Committee On Infectious Diseases. Management of Neonates Born at ≤34 6/7 Weeks’ Gestation with Suspected or Proven Early-Onset Bacterial Sepsis. Pediatrics. 2018;142(6):65. doi:10.1542/peds.2018-2896
13. Cantey JB, Wozniak PS, Pruszynski JE, et al. Reducing unnecessary antibiotic use in the neonatal intensive care unit (SCOUT): a prospective interrupted time-series study. Lancet Infect Dis. 2016;1:1178–1184. doi:10.1016/S1473-3099(16
14. Ting JY, Synnes A, Roberts A, et al. Canadian Neonatal Network Investigators: association between antibiotic use and neonatal mortality and morbidities in very low-birth-weight infants without culture-proven sepsis or necrotizing enterocolitis. JAMA Pediatr. 2016;170:1181–1187.
15. Shi R, Zhang M, Chen Y, et al. Dynamic Change of Thyroid Hormones with Postmenstrual Age in Very Preterm Infants Born with Gestational Age. Front Endocrinol (Lausanne). 2021;2:11. doi:10.3389/fendo.2020.585956
16. Flannery DD, Horbar JD. Metrics of neonatal antibiotic use. Semin Perinatol. 2020;44(8):151329. doi:10.1016/j.semperi.2020.151329
17. Chinese Pediatric Society. Chinese consensus of diagnosis and treatment of neonatal sepsis. Chin J Peditr. 2019;57(4):252–257. doi:10.3760/cma.j.issn.0578-1310.
18. Wozniak B. Implementation of the Smart Use of Antibiotics Program to Reduce Unnecessary Antibiotic Use in a Neonatal ICU: a Prospective Interrupted Time-Series Study in a Developing Country. Crit Care Med. 2019;47(1):e1–e7. doi:10.1097/CCM.0000000000003463
19. Liu J, Fang Z, Yu Y, et al. Pathogens Distribution and Antimicrobial Resistance in Bloodstream Infections in Twenty-Five Neonatal Intensive Care Units in China, 2017-2019. Antimicrob Resist Infect Control. 2021;10(1):121. doi:10.1186/s13756-021-00989-6
20. Corinne S, Gregory S, Nishant S, et al. Factors influencing antibiotic duration in culture‐negative neonatal early‐onset sepsis. Pharmacotherapy. 2021;41(2):148–161. doi:10.1002/phar.2507
21. Joseph C, Wozniak B. Prospective Surveillance of Antibiotic Use in the Neonatal Intensive Care Unit: results from the SCOUT Study. Pediatr Infect Dis J. 2015;34(3):267–272. doi:10.1097/INF.0000000000000542.
22. Martin-Mons S, Lorrain S, Iacobelli S, et al. Antibiotics Prescription Over Three Years in a French Benchmarking Network of 23 Level 3 Neonatal Wards. Front Pharmacol. 2021;11:585018. doi:10.3389/fphar.2020.585018
23. Schrag SJ, Farley MM, Petit S, et al. Epidemiology of invasive early-onset neonatal sepsis, 2005 to 2014. Pediatrics. 2016;138(6):e20162013. doi:10.1542/peds.2016-2013
24. Maina M, Mcknight J, Tosas-Auguet O, et al. Using treatment guidelines to improve antibiotic use: insights from an antibiotic point prevalence survey in Kenya. Br Med J Global Health. 2021;6(1):e003836. doi:10.1136/bmjgh-2020-003836
25. Jimenez-Juarez RN. Use of the WHO Access, Watch, and Reserve classification to define patterns of hospital antibiotic use (AWaRe): an analysis of paediatric survey data from 56 countries. Lancet Glob Health. 2019;7(7):e861–e8712019. doi:10.1016/S2214-109X(19)30071-3
26. Connell TG, Rele M, Cowley D, et al. How reliable is a negative blood culture result? Volume of blood submitted for culture in routine practice in a children’s hospital. Pediatrics. 2007;119(5):891–896. doi:10.1542/peds.2006-0440
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
