Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Excellent Repigmentation of Generalized Vitiligo with Oral Baricitinib Combined with NB-UVB Phototherapy
Authors Li X , Sun Y, Du J, Wang F , Ding X
Received 9 November 2022
Accepted for publication 27 January 2023
Published 11 March 2023 Volume 2023:16 Pages 635—638
DOI https://doi.org/10.2147/CCID.S396430
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Anne-Claire Fougerousse
Xinxin Li, Yifang Sun, Juan Du, Fang Wang, Xiaolan Ding
Department of Dermatology, Peking University People’s Hospital, Beijing, People’s Republic of China
Correspondence: Xiaolan Ding, Department of Dermatology, Peking University People’s Hospital, No. 11 Xizhimen South Street, Xicheng District, Beijing, 100044, People’s Republic of China, Tel +8613522658992, Fax +861088325474, Email [email protected]
Abstract: Vitiligo is an acquired autoimmune skin disorder, clinically characterized by distinct white macules and patches resulting from progressive melanocyte destruction. JAK-STAT pathway plays an important role in the loss of epidermal melanocytes. Baricitinib can block the JAK-STAT pathway and the downstream chemokines as a new JAK1/2 inhibitor. In this report, we describe the successful treatment of 2 patients with oral baricitinib combined with NB-UVB phototherapy, which provides a good alternative and supplementary to treat refractory vitiligo.
Keywords: IFN-γ signaling pathway, Janus kinase inhibitor, baricitinib, vitiligo
Introduction
Vitiligo is an acquired depigmentary skin disorder with variable response rate to current therapy.1 Narrowband ultraviolet B (NB-UVB) phototherapy is one of the first-line therapeutic options for treating generalized vitiligo and always be combined with other therapies to improve its efficacy, such as systemic corticosteroids, topical steroids, and topical tacrolimus. However, treatment resistance is often encountered.2 Janus kinase inhibitors (JAK) can block the Interferon-γ (IFN-γ) signaling pathway and relate chemokines which mediate melanocyte destruction and become an emerging treatment for vitiligo. As a newer JAK inhibitor, baricitinib can be a useful adjunct to phototherapy and other therapies.9 Here we report 2 cases of generalized vitiligo who achieved satisfactory repigmentation with oral baricitinib combined with narrow-band ultraviolet B (NB-UVB) phototherapy.
Case
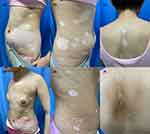
Patient 1 was a 17-year-old woman presented with white patches on her trunk and extremities for 3 years. These lesions had first presented on the chest 3 years ago after exposure to the sun during military training and had rapidly increased in size and quantity. She was diagnosed with vitiligo in a local hospital and was reluctant to conventional therapies, including topical steroids, tacrolimus, calcipotriol, systemic corticosteroids combined with NB-UVB phototherapy, oral compound glycyrrhizin tablets, and Chinese herbs. She had a history of hypothyroidism with 50µg levothyroxine daily as her only medication and no family history of vitiligo. Physical examination showed multiple, diffuse, depigmented patches with unclear boundaries and trichrome vitiligo involving her trunk and extremities. A halo nevi was also found on the right ribs (Figure 1a–c). Wood’s lamp examination accentuated these patches. Routine hematological and biochemical tests, virus serology and T-spot test were normal. The patient was given oral baricitinib 2mg twice daily, and 0.1% tacrolimus ointment twice daily together with NB-UVB phototherapy twice a week, the initial dose of phototherapy was 200 mJ/cm2 followed by 20% increments at each visit if tolerated. Repigmentation of the lesions was first observed after 1 month. Notably, 8 months after treatment initiation, the hypopigmented patches showed significant repigmentation with good tolerance (Figure 1d–f).
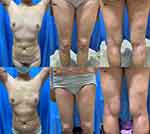
Patient 2 was a 56-year-old woman who presented with a 27-year history of vitiligo and a 1-year rapid progression. She also had a personal history of asthma and chronic rhinitis. The hypopigmented patches started on the trunk and then spread and enlarged slowly to facial area and extremities with the application of oral Chinese herbs, topical tacrolimus, NB-UVB and caloripuncture treatment. The lesions rapidly progressed due to insomnia and anxiety over the past 1 year. She had no family history of vitiligo. Physical examination showed multiple, hypopigmented-to-depigmented patches of skin on the facial area, trunk, and extremities (about 60% BSA, basic surface area) (Figure 2a–c). Wood’s lamp examination accentuated these patches. Routine hematological and biochemical tests, virus serology, and T-spot test were normal. For the rapid progression of the vitiligo, she was given diprospan injection every 4 weeks, oral ginkgo biloba 80mg twice daily together with topical 0.1% tacrolimus ointment, mometasone furoate cream, and full-body NB-UVB phototherapy with an initial dose of 200 mJ/cm2 followed by 20% increments at each subsequent treatment. After three months, the patient’s condition became stabilized. Subsequently, the systemic corticosteroids were substituted for oral baricitinib 2mg twice daily and the rest of the treatments were continued. Over 75% repigmentation of the lesions was observed after 6 months (Figure 2d–f). In addition, the drug was well-tolerated with no adverse effects in both patients.
Discussion
Vitiligo is an acquired autoimmune skin disorder, clinically characterized by depigmented macules and patches resulting from progressive loss of epidermal melanocytes.1 Vitiligo has significant psychosocial impacts on patients and is challenging to manage.3 Repigmentation rates and efficacy of current treatment options have been variable. Recent studies have revealed that IFN-γ and the related chemokine (C-X-C motif) ligand (CXCL) 9 and 10 appear as key drivers of vitiligo pathogenesis and are mediated by JAK/signal transducer and activator of transcription (STAT) pathway in local keratinocytes.4,5 The JAK family comprises JAK1, JAK2, JAK3, and tyrosine kinase 2 (TYK2).5 Jak inhibitors can block this pathway, thereby blocking the effects of IFN-γ and the downstream chemokines.
There have been an increasing number of studies suggesting promising results for tofacitinib (JAK1/3 inhibitor) and ruxolitinib (JAK1/2 inhibitor) in vitiligo treatment. Baricitinib, a newer JAK1/2 inhibitor, has recently been approved for the treatment of rheumatoid arthritis. Mumford et al6 first reported a case of vitiligo repigmentation after taking oral baricitinib for 8 months in 2020. The patient acquired poor improvement using tofacitinib 5mg twice daily for 5 months. Then she switched to baricitinib 4mg daily and got almost complete repigmentation after 8 months. In a retrospective study, Liu et al7 reported that skin exposure to ultraviolet light appears to be required to achieve repigmentation using JAK inhibitors as evidenced by increased JAK1 levels in vitiligo patients that were downregulated after NBUVB treatment.8 Recently, Dong et al10 found that in vitro baricitinib could promote tyrosinase activity, melanin content and tyrosinase, tyrosinase-related protein-1 gene expression of melanocytes damaged model, which will be contributed to repigmentation of vitiligo.
Conclusion
In the present cases, combination treatment with oral baricitinib and NB-UVB phototherapy showed satisfactory repigmentation and good tolerance. These patients will continue to be followed up and randomized control trials (RCTs) in large populations are still needed to verify the efficacy and safety of JAK inhibitors in vitiligo treatment.
Abbreviations
JAK, Janus kinase inhibitors; STAT, signal transducer and activator of transcription; IFN-γ, Interferon-γ; NB-UVB, narrow-band ultraviolet B; TYK2, tyrosine kinase 2; CXCL, chemokine (C-X-C motif) ligand; RCTs, randomized control trials.
Consent Statement
Written informed consents were provided by both patients to have the case details and any accompanying images published. Institutional approval was not required to publish the case details.
Funding
This work was supported by the Peking University People’s Hospital Research And Development Funds [RDX2020-09].
Disclosure
The authors report no conflicts of interest in this work.
References
1. Rahimi H, Zeinali R, Tehranchinia Z. Photodynamic therapy of vitiligo: a pilot study. Photodiagnosis Photodyn Ther. 2021;36:102439. doi:10.1016/j.pdpdt.2021.102439
2. Yazdani Abyaneh M, Griffith RD, Falto-Aizpurua L, Nouri K. Narrowband ultraviolet B phototherapy in combination with other therapies for vitiligo: mechanisms and efficacies. J Eur Acad Dermatol Venereol. 2014;28(12):1610–1622. doi:10.1111/jdv.12619
3. Moya EC, Bruinsma RL, Kelly KA, Feldman SR. How suitable are JAK inhibitors in treating the inflammatory component in patients with alopecia areata and vitiligo? Expert Rev Clin Immunol. 2022;18(3):189–191. doi:10.1080/1744666X.2022.2036607
4. Frisoli ML, Essien K, Harris JE. Vitiligo: mechanisms of pathogenesis and treatment. Annu Rev Immunol. 2020;38:621–648. doi:10.1146/annurev-immunol-100919-023531
5. Relke N, Gooderham M. The use of janus kinase inhibitors in vitiligo: a review of the literature. Review. J Cutan Med Surg. 2019;23(3):298–306. doi:10.1177/1203475419833609
6. Mumford BP, Gibson A, Chong AH. Repigmentation of vitiligo with oral baricitinib. Australas J Dermatol. 2020;61(4):374–376. doi:10.1111/ajd.13348
7. Liu LY, Strassner JP, Refat MA, Harris JE, King BA. Repigmentation in vitiligo using the Janus kinase inhibitor tofacitinib may require concomitant light exposure. J Am Acad Dermatol. 2017;77(4):675–682 e1. doi:10.1016/j.jaad.2017.05.043
8. Nada HR, El Sharkawy DA, Elmasry MF, Rashed LA, Mamdouh S. Expression of Janus Kinase 1 in vitiligo & psoriasis before and after narrow band UVB: a case–control study. Arch Dermatol Res. 2018;310(1):39–46. doi:10.1007/s00403-017-1792-6
9. Urso B. Jak-inhibitors and UV-B: potential combined therapy for vitiligo. Dermatol Ther. 2017;30(5):e12531. doi:10.1111/dth.12531
10. Dong J, Huang X, Ma LP, et al. Baricitinib is effective in treating progressing vitiligo in vivo and in vitro. Dose Response. 2022;20(2):15593258221105370. doi:10.1177/15593258221105370
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.