Back to Journals » Clinical Ophthalmology » Volume 17
Ex-PRESS Implantation versus Trabeculectomy for Long-Term Maintenance in Patients with Open-Angle Glaucoma
Authors Tokumo K, Okada N , Onoe H, Komatsu K, Masuda S, Okumichi H , Hirooka K, Asaoka R, Kiuchi Y
Received 6 May 2023
Accepted for publication 10 August 2023
Published 28 August 2023 Volume 2023:17 Pages 2525—2537
DOI https://doi.org/10.2147/OPTH.S419765
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Kana Tokumo,1 Naoki Okada,1 Hiromitsu Onoe,1 Kaori Komatsu,1 Shun Masuda,1 Hideaki Okumichi,1 Kazuyuki Hirooka,1 Ryo Asaoka,2 Yoshiaki Kiuchi1
1Department of Ophthalmology and Visual Science, Hiroshima University Graduate School of Biomedical Sciences, Hiroshima, Japan; 2Seirei Hamamatsu General Hospital Department of Ophthalmology, Shizuoka, Japan
Correspondence: Kana Tokumo, Department of Ophthalmology and Visual Science, Hiroshima University Graduate School of Biomedical Sciences, 1-2-3 Kasumi, Minami-ku, Hiroshima, 734-8551, Japan, Tel +81-822575247, Fax +81-822575249, Email [email protected]
Purpose: To compare the efficacy of Ex-PRESS implantation (EXP) with that of trabeculectomy (TLE) with mitomycin C for maintaining low target intraocular pressure (IOP) in patients with open-angle glaucoma.
Patients and Methods: Patients were randomly assigned to receive EXP or TLE. Surgical success was defined according to three target mean IOP ranges (5 mmHg ≤ IOP ≤ 18 mmHg [criterion A], 5 mmHg ≤ IOP ≤ 15 mmHg [criterion B], and 5 mmHg ≤ IOP ≤ 12 mmHg [criterion C]) representing reductions of at least 20% below the baseline on two consecutive follow-up visits 3 months post-surgery, with or without antiglaucoma medication and without further glaucoma surgery. Participants were divided into three subgroups based on baseline mean deviation (MD) values: early (MD ≥ − 6 dB), moderate (− 6 dB > MD ≥ − 12 dB), and advanced (− 12 dB > MD). Survival rates were calculated by subgroup.
Results: A total of 73 patients, including 30 in the EXP group and 43 in the TLE group, were included in the study. No significant differences in baseline ocular or demographic characteristics were found between the two groups. No significant difference in IOP was noted every 6 months. After the 3-year follow-up, success rates were A) 60.0% and 60.2%, B) 45.7% and 58.1%, and C) 31.5% and 40.5% for the EXP and TLE groups, respectively. Moreover, there was no difference in success rate based on glaucoma level. Many glaucoma medications administered before surgery were associated with a higher failure rate in the TLE group but not in the EXP group.
Conclusion: Both procedures resulted in similar IOP reductions and success rates for a low target IOP. The number of preoperative glaucoma medications was a risk factor for TLE failure.
Keywords: filtration surgery, glaucoma, randomized controlled clinical trial, visual field
Introduction
Filtering surgery is widely employed in the treatment of glaucoma with uncontrollable intraocular pressure (IOP). Currently, the most popular filtering surgery is trabeculectomy (TLE), in which aqueous humor filtration is enhanced and the IOP is reduced by creating a scleral flap that leads to bleb formation in the conjunctiva. The success and complication rates for TLE are well established.1,2
The Ex-PRESS glaucoma filtration device (Alcon Laboratories, Fort Worth, TX, USA) was introduced as a modification to TLE. Although its surgical procedure and postoperative management are similar to those of TLE, Ex-PRESS (EXP) surgery does not require sclerectomy or iridectomy. Therefore, postoperative inflammation and hemorrhage may be reduced. Both surgical procedures can achieve comparable IOP reductions with few complications when the target pressure is set to 18 mmHg.3–5 However, the optimal target IOP for glaucoma treatment depends on the disease stage, IOP before treatment, age, and other risk factors. Generally, to maintain visual function, more severe glaucoma requires lower IOP post-surgery.6–8
The success of TLE depends on the preoperative IOP, history of previous surgery, number of preoperative medications, and race.9 A high incidence of normal-tension glaucoma (NTG) was reported in the Japanese population,10 and visual field impairment in patients with NTG often progresses even when IOP is within the normal range. Therefore, patients with NTG may benefit from a postoperative target pressure below that of patients with higher preoperative IOP.
This study compared the efficacy and safety of EXP to TLE to achieve target postoperative IOPs of ≤18, ≤15, and ≤12 mmHg, each representing reductions of at least 20% below the baseline, among Japanese patients with open-angle glaucoma (OAG, including exfoliation glaucoma and primary OAG).
Materials and Methods
The institutional review board of Hiroshima University approved the study protocol before recruitment. This study was registered with the University Hospital Medical Information Network Clinical Trials Registry of Japan (Identifier University Hospital Medical Information Network 000008981; date of access and registration, September 25, 2012). All patients provided written informed consent before participation, and the study was conducted in accordance with the tenets of the Declaration of Helsinki.
Randomization and Treatment
The study was conducted at the Department of Ophthalmology, Hiroshima University Hospital. Patients were enrolled between November 2012 and March 2015. They were followed up for 1 year, 3 years if possible. Patients were randomized to receive TLE or EXP using a computer-based random number generator. Random allocations were prepared by staff not associated with the study and they assigned participants to interventions. Clinicians enrolled the participants in this study. After patient enrollment was completed, only then the surgery to be performed was confirmed. Both patients and clinicians were aware of the therapy; therefore, this was an open-label, randomized study.
Patients and Inclusion Criteria
This single-center clinical trial enrolled patients with OAG aged >20 years with or without cataracts and scheduled for filtration surgery. Patients who previously underwent glaucoma surgery were excluded. However, those who underwent cataract surgery more than 6 months before the planned TLE or EXP were included. When both eyes met the inclusion criteria, only the first eye was analyzed. Patients with preoperative IOP <15 mmHg were excluded after randomization, and their data were analyzed in another study.
Outcome Measures
Goldmann applanation tonometry was used to measure the IOP. The preoperative IOP and best-corrected visual acuity (VA) were recorded during the examination immediately before surgery. The IOP and VA were documented the day after surgery and at every follow-up visit. Postoperative IOP and VA values measured every 6 months for 3 years were included in the efficacy (surgical success) analysis. The surgical procedures and postoperative management were comparable for each case.
Anesthesia included subconjunctival 2% lidocaine injection. A fornix-based conjunctival flap was used, and a half-thickness scleral flap was created. Small pieces of gelatin sponge soaked with 0.4 mg/mL mitomycin C (MMC) were applied to the exposed tissue, including the posterior surface of Tenon’s capsule and conjunctiva, adjacent episcleral tissue, and scleral flap for 5 min. After 5 min, all sponges were removed, and the wound was irrigated with 100 mL of a balanced salt solution. For the TLE group, a block incision was made in the region of the trabecular meshwork, followed by peripheral iridectomy. For the EXP group, a small scleral tunnel was created as a shunt in the region of the trabecular meshwork using a 25-G needle. In all cases in the EXP and TLE groups, the scleral flap was sutured using five 10–0 Nylon sutures, and the conjunctiva were closed using 10–0 Nylon sutures.
Patients were treated with laser suture lysis if the IOP was higher than the target IOP. The target IOP for most patients was set at 8–10 mmHg 2 weeks postoperatively.8 All patients received a similar topical medical regimen, including postoperatively 1.5% levofloxacin and 0.1% topical betamethasone three times per day. Patients receiving combined cataract surgery were also administered nepafenac three times a day. Topical atropine sulfate (1%) was used as needed. All doses were tapered at the surgeon’s discretion.
Parameters for Surgical Success
The primary outcome was surgical success based on the target postoperative IOP range at 6-month intervals with or without antiglaucoma medications according to the guidelines of the World Glaucoma Association.11 The three target IOP ranges were (A) 5 mmHg ≤ IOP ≤ 18 mmHg, (B) 5 mmHg ≤ IOP ≤ 15 mmHg, and (C) 5 mmHg ≤ IOP ≤ 12 mmHg. Participants were divided into three subgroups based on the baseline mean deviation (MD) values: early (MD ≥ −6 dB), moderate (−6 dB > MD ≥ −12 dB), and advanced (−12 dB> MD). Failure was defined as an IOP above the indicated range on two consecutive follow-up visits 3 months after surgery and a reduction of <20% below baseline or the need for further glaucoma surgery. The need for laser suture lysis or bleb needling was not considered a surgical failure because these procedures are part of routine postoperative management for filtration surgeries.
Statistical Analysis
All statistical analyses were performed using JMP version 15 (Cary, NC, USA). Results are presented as mean ± standard deviation (SD) unless otherwise indicated. Mean values of normally distributed continuous variables were compared between groups by Student’s t-test and proportion (eg, patients with visual stability or surgical success) by the chi-square test. IOP values were compared using the Mann–Whitney nonparametric test with Bonferroni correction for repeated measures. Patients requiring additional surgery were censored from the analysis after reoperation. Kaplan–Meier survival curves were used to analyze success rates over time. The survival data were analyzed using regression analysis based on the Cox proportional hazards model. All statistical tests were two-sided, and P-values <0.05 were considered statistically significant. The sample size calculation determined that 30 eyes in each group were required to detect a 2.5-mmHg difference in the IOP with an SD of 2.75 mmHg and power of 80%. However, to account for patient dropout, 73 eyes were included.
Results
This study enrolled a total of 105 patients with OAG (105 eyes). Two patients who had glaucoma surgery were excluded. After randomization, 30 patients whose preoperative IOP was <15 mmHg were excluded. In total, 30 eyes were treated with EX-PRESS implantation (EXP group) and 43 eyes with TLE (TLE group). The number of patients who could be followed is shown in Figure 1. Patients who had mild-to-severe cataract underwent combined cataract surgery. All patients were Japanese. Patient demographics and basic characteristics are summarized in Table 1. The mean follow-up periods were 25.9 ± 12.9 and 23.8 ± 13.1 months in the EXP and TLE groups, respectively. No differences were found in baseline IOP, sex ratio, age, and number of glaucoma medications between the EXP and TLE groups. In the EXP group, 22 patients received EXP surgery alone, 8 patients received combined EXP and cataract surgery, 29 patients received TLE surgery alone, and 14 patients received combined TLE and cataract surgery.
 |
Table 1 Baseline Demographics and Ocular Characteristics of the EXP Group (30 Eyes) and TLE Group (43 Eyes) |
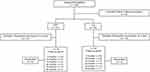 |
Figure 1 Flowchart of the follow-up. |
IOP Reduction
The mean IOP at each postoperative visit is presented in Table 2 and graphed in Figure 2. Both surgical procedures led to a significant sustained reduction in IOP. In the EXP group, the IOP decreased from 20.4 ± 4.9 to 13.2 ± 1.9 mmHg at the 3-year follow-up visit (P = 0.001, paired t-test). In the TLE group, the IOP decreased from 21.9 ± 7.9 to 13.5 ± 5.0 mmHg at the 3-year follow-up visit (P = 0.0012, paired t-test). Both surgical procedures resulted in a significant IOP reduction after surgery compared with the preoperative IOP. During observation, no differences in IOP were found between the two groups.
 |
Table 2 Mean Intraocular Pressure and Medication Use |
Kaplan–Meier survival analysis was used to compare success rates between the two treatment groups. Three definitions of success were created, and the survival curves were compared. At the 1-year follow-up, the success rates of EXP and TLE were 72.0% and 70.8%, 65.3% and 68.3%, and 55.1% and 58.5% for criteria A, B, and C, respectively. At the 3-year follow-up, the success rates of EXP and TLE were 60.0% and 60.2%, 45.7% and 58.1%, and 31.5%and 40.5% for criteria A, B, and C, respectively (Figure 3). No significant differences in treatment efficacy were found between the strata (A, P = 0.93; B, P = 0.53; C, P = 0.58, Log rank test).
We also analyzed success rates according to subgroups of glaucoma severity. Glaucoma was classified by the severity of the visual field: 11 patients were classified as early (4 form the EXP group, 7 from the TLE group), 16 patients as moderate (6 from the EXP group, 10 from the TLE group), and 46 patients as advanced (20 from the EXP group, 26 from the TLE group). No significant differences in treatment efficacy were found between the strata (Figure 4). Table 3 shows the P-values indicating significant differences in survival rates by severity of visual field.
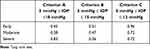 |
Table 3 P-values aIndicating Significant Differences in Survival Rates Based on Severity of Visual Field |
Glaucoma Medication Use
The mean number of glaucoma medications required at each postoperative visit is presented in Table 2. Both groups required significantly fewer glaucoma medications after surgery, and no significant differences were noted between the groups. The EXP group required more postoperative eye drops than the TLE group. The difference increased gradually with time.
VAs
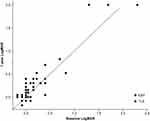
Decimal VA was converted to the logarithm of a minimum angle of resolution equivalents (logMAR) for statistical analysis. All patients who underwent glaucoma surgery combined with cataract surgery showed VA improvement; thus, these patients were excluded from the VA analysis. Figure 5 shows the VA results 1 year after glaucoma surgery. LogMAR VA immediately after the procedure did not differ significantly between the groups (EXP group, 0.30 ± 0.60; TLE group, 0.14 ± 0.33; P = 0.20, Mann–Whitney U-test) at 1 year after surgery (EXP group, 0.37 ± 0.61; TLE group, 0.20 ± 0.43, P = 0. 24, Mann–Whitney U-test) or 3 years after surgery (EXP group, 0.44 ± 0.55; TLE group, 0.33 ± 0.52; P = 0.34, Mann–Whitney U-test). Six patients in each group lost more than two lines at the last visit, whereas one patient in the TLE group developed cataract, which resulted in VA reduction. The others lost their vision because of glaucoma progression.
Postoperative Complications
Table 4 shows the early and late postoperative complications of the two groups. At 1 month postoperatively, 13 patients in the EXP group and 10 patients in the TLE group developed early complications. These included hyphema, choroidal detachment, shallow anterior chamber, hypotony maculopathy, and conjunctiva leakage. Both groups had similar complications. No statistically significant difference was found in the total prevalence of early complications between the groups (P = 0.07, Student’s t-test). Only the TLE group showed late postoperative complications, i.e., one case of choroidal detachment and one of conjunctiva leakage. However, no statistically significant difference was found in the prevalence of late complications between the groups (P = 0.27, Student’s t-test).
 |
Table 4 Early Postoperative Complications and Late Postoperative Complications |
Risk Factors for Surgical Failure by Multivariate Cox Proportional Hazards Model
The baseline characteristics, including a history of cataract surgery, glaucoma diagnosis, preoperative IOP, and number of glaucoma medications, were evaluated as prognostic factors for surgical failure (Table 5). In the TLE group, the number of preoperative medications was a risk factor for failure to satisfy criteria A and B. In the EXP group, glaucoma surgery with cataract surgery reduced the risk of failure for criterion C. Factors not significantly associated with target IOP success rates included preoperative IOP, lens status, and diagnosis.
 |
Table 5 Multivariate Cox Proportional Hazard Ratios for Risk Factors for Failure |
Discussion
Most previous studies have defined successful TLE or EXP as a postoperative IOP of ≤18 mmHg. However, the Advanced Glaucoma Intervention Study reported that visual field impairment progressed unless the IOP was maintained at <12.3 mmHg,8 and patients with NTG often have IOP values ≤18 mmHg. Therefore, in this study, we set the target IOP at several lower levels (criteria B and C).
Filtering surgery, including TLE, is a common and standard modality for long-standing glaucoma. Based on previous studies, while EXP was expected to reduce IOP as effectively as TLE,3,4,12 an equivalent efficacy has not been demonstrated for lower target IOP ranges. As the baseline IOP of some patients was in the normal range under maximum medical therapy, some studies have used percentage reduction from the baseline as an outcome metric in addition to absolute IOP levels. However, this criterion does not apply to all situations. In Japan, NTG accounts for the majority of glaucoma cases.10 A low preoperative IOP value as in NTG makes it difficult to achieve a 20% reduction from baseline after filtering surgery without complications.13,14 Therefore, only patients with IOP ≥ 15 mmHg were included in this study.
Several previous prospective studies have compared the success rate of TLE with that of EXP with and without the use of glaucoma medications. Gonzalez-Rodriguez et al4 defined surgical success as 5 ≤ IOP ≤18 (criterion A in our study) and a 20% reduction from baseline and reported success rates of 59% and 76% for EXP and TLE groups at 24 months and 52% and 61% at 36 months, respectively. In accordance with our results using criterion A, these rates did not differ between procedures. We could not find any studies comparing differences in survival based on severity of visual field. We also compared the difference in survival rates by severity of disease and found no difference between the two groups at any disease severity.
Most studies have not found a difference in the number of glaucoma medications required by patients following EXP or TLE,4,5,15–17 whereas a few have reported that the EXP group required fewer glaucoma medications,3,18 and one study reported that patients required more medications following EXP implantation.19 In the present study, no significant difference was found in the number of glaucoma medications required before or after surgery. However, the EXP group required more glaucoma medications than the TLE group after surgery, and the group difference in the number of eye drops required increased gradually during follow-up. Thus, the EXP group will eventually require a significantly greater number or significantly higher doses of glaucoma medications.
In this study, we found a slight difference in complication rates following EXP or TLE. A patient in the TLE group developed cataract, which resulted in VA loss during the observation period, whereas no cases of cataract progression were reported in the EXP group. Similarly, Beltran-Agullo et al reported that cataract progression was more frequent following TLE than EXP20 because EXP does not require peripheral iridectomy and thus carries a lower risk of hypotony. Aqueous flare elevation may occur during or following filtration surgery because of the disruption of the blood–aqueous barrier and flare severity increases with inflammatory activity in the anterior segment.21 A case study reported a substantial flare on day 1 after EXP, which subsided on day 3,22 whereas another study reported severe flares at 4 weeks after TLE.23 The more sustained flare after TLE may also result from iridectomy. Further, the reduced inflammation following EXP may prevent cataract progression. Moreover, Arimura et al16 reported a significantly greater total number of complications following TLE than following EXP. However, other studies have reported comparable incidences of most early complications, except for hyphema.24 In the present study, no significant differences in early and late postoperative complications were found between treatment groups. No late complications due to hypotony, such as hypotony maculopathy and choroidal detachment, were observed in the EXP group. However, a patient in the TLE group exhibited choroidal detachment related to over-filtration and low IOP. This may be because EXP surgery creates a constant aqueous outflow route. From this, we expect that EXP is more suitable for patients at risk for complications related to low IOP. For example, young patients with long axial length tend to develop hypotony maculopathy after TLE,25 which could lead to VA reduction. Compared with TLE, EXP surgery requires the cost of implants. Therefore, it is well indicated for patients at risk of complications related to low IOP; however, TLE may be more cost-effective for other patients.
A Cox regression model identified the number of glaucoma medications before surgery as a risk factor for surgical failure in the TLE group, consistent with the findings of previous studies,26,27 suggesting that preservatives of glaucoma medication increase the risk of TLE failure.28 Postoperative subconjunctival fibrosis after ophthalmic surgery increased the number of conjunctival fibroblasts and inflammatory cells,29 and preservatives will further increase the number of conjunctival fibroblasts and macrophages while reducing the number of conjunctival goblet cells.28 These alterations lead to the predisposition to heal the fistula drainage, resulting in uncontrolled IOP.26,28 Since the role of the conjunctiva is the same in TLE and EXP, glaucoma medication may reduce the surgical success of EXP through similar mechanisms. However, in this study, the number of glaucoma medications was not a risk factor for EXP failure. EXP does not require iridectomy and leads to less bleeding and inflammatory cell infiltration.
This study has some limitations. First, there is group imbalance, and the number of patients was relatively small because patients with preoperative IOP <15 mmHg were excluded after the randomization. Second, we followed patients for 3 years in this study; thus, the number of dropouts increased with long-term follow-up. As glaucoma requires lifelong treatment, longer-term clinical follow-up is required.
Conclusion
In conclusion, no differences in surgical success, IOP, VA, and failure rates were found between the EXP and TLE groups. In addition, the results indicated that the number of glaucoma medications was a risk factor for surgical failure with TLE but not with EXP.
Abbreviations
EXP, Ex-PRESS; IOP, Intraocular pressure; MD, Mean deviation; NTG, Normal-tension glaucoma; OAG, Open-angle glaucoma; SD, Standard deviation; VA, Visual acuity.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author, Kana Tokumo, upon reasonable request.
Ethics Approval and Informed Consent
The Institutional Review Board of Hiroshima University Hospital approved the study protocol before the recruitment began. This study was registered with the University Hospital Medical Information Network Clinical Trials Registry of Japan (Identifier University Hospital Medical Information Network 000008981; date of access and registration, September 25, 2012). The study followed the tenets of the Declaration of Helsinki.
Consent for Publication
Written informed consent was obtained from all patients before the surgical procedure.
Acknowledgments
The authors would like to thank Enago for the English language review.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all of these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The authors did not receive support from any organization for the submitted work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Borisuth NS, Phillips B, Krupin T. The risk profile of glaucoma filtration surgery. Curr Opin Ophthalmol. 1999;10(2):112–116. doi:10.1097/00055735-199904000-00006
2. Sugimoto Y, Mochizuki H, Ohkubo S, Higashide T, Sugiyama K, Kiuchi Y. Intraocular pressure outcomes and risk factors for failure in the collaborative bleb-related infection incidence and treatment study. Ophthalmology. 2015;122(11):2223–2233. doi:10.1016/j.ophtha.2015.06.038
3. de Jong L, Lafuma A, Aguadé AS, Berdeaux G. Five-year extension of a clinical trial comparing the EX-PRESS glaucoma filtration device and trabeculectomy in primary open-angle glaucoma. Clin Ophthalmol. 2011;5:527–533. doi:10.2147/OPTH.S18565
4. Gonzalez-Rodriguez JM, Trope GE, Drori-Wagschal L, Jinapriya D, Buys YM. Comparison of trabeculectomy versus Ex-PRESS: 3-year follow-up. Br J Ophthalmol. 2016;100(9):1269–1273. doi:10.1136/bjophthalmol-2015-307161
5. Wagschal LD, Trope GE, Jinapriya D, Jin YP, Buys YM. Prospective randomized study comparing Ex-PRESS to trabeculectomy: 1-year Results. J Glaucoma. 2015;24(8):624–629. doi:10.1097/IJG.0000000000000029
6. de Moraes CG, Liebmann JM, Medeiros FA, Weinreb RN. Management of advanced glaucoma: characterization and monitoring. Surv Ophthalmol. 2016;61(5):597–615. doi:10.1016/j.survophthal.2016.03.006
7. Peters D, Bengtsson B, Heijl A. Factors associated with lifetime risk of open-angle glaucoma blindness. Acta Ophthalmol. 2014;92(5):421–425. doi:10.1111/aos.12203
8. The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. Am J Ophthalmol. 2000;130(4):429–440. doi:10.1016/S0002-9394(00)00538-9
9. Husain R, Clarke JCK, Seah SKL, Khaw PT. A review of trabeculectomy in East Asian people--the influence of race. Eye. 2005;19(3):243–252. doi:10.1038/sj.eye.6701477
10. Iwase A, Suzuki Y, Araie M, et al. The prevalence of primary open-angle glaucoma in Japanese: the Tajimi Study. Ophthalmology. 2004;111(9):1641–1648. doi:10.1016/j.ophtha.2004.03.029
11. Shaarawy T, Sherwood M, Grehn F. Guidelines on Design and Reporting of Glaucoma Surgical Trials. Amsterdam: Kugler Publications; 2009.
12. Gedde SJ, Herndon LW, Brandt JD, Budenz DL, Feuer WJ, Schiffman JC. Surgical complications in the tube versus trabeculectomy study during the first year of follow-up. Am J Ophthalmol. 2007;143(1):23–31. doi:10.1016/j.ajo.2006.07.022
13. Yuasa Y, Sugimoto Y, Hirooka K, et al. Effectiveness of trabeculectomy with Mitomycin C for glaucomatous eyes with low intraocular pressure on treatment eye drops. Acta Ophthalmol. 2020;98(1):e81–e87. doi:10.1111/aos.14195
14. Schultz SK, Iverson SM, Shi W, Greenfield DS. Safety and efficacy of achieving single-digit intraocular pressure targets with filtration surgery in eyes with progressive normal-tension glaucoma. J Glaucoma. 2016;25(2):217–222. doi:10.1097/IJG.0000000000000145
15. Moisseiev E, Zunz E, Tzur R, Kurtz S, Shemesh G. Standard trabeculectomy and ex-press miniature glaucoma shunt: a comparative study and literature review. J Glaucoma. 2015;24(6):410–416. doi:10.1097/IJG.0000000000000047
16. Arimura S, Miyake S, Iwasaki K, et al. Randomised clinical trial for postoperative complications after EX-PRESS implantation versus trabeculectomy with 2-year follow-up. Sci Rep. 2018;8(1):16168. doi:10.1038/s41598-018-34627-w
17. Netland PA, Sarkisian SR, Moster MR, et al. Randomized, prospective, comparative trial of EX-PRESS glaucoma filtration device versus trabeculectomy (XVT study). Am J Ophthalmol. 2014;157(2):433–440.e3. doi:10.1016/j.ajo.2013.09.014
18. Dahan E, Ben Simon GJ, Lafuma A. Comparison of trabeculectomy and Ex-PRESS implantation in fellow eyes of the same patient: a prospective, randomised study. Eye. 2012;26(5):703–710. doi:10.1038/eye.2012.13
19. Hashimoto Y, Michihata N, Matsui H, Fushimi K, Yasunaga H, Aihara M. Reoperation rates after Ex-PRESS versus trabeculectomy for primary open-angle or normal-tension glaucoma: a national database study in Japan. Eye. 2020;34(6):1069–1076. doi:10.1038/s41433-019-0641-6
20. Beltran-Agullo L, Trope GE, Jin Y, Wagschal LD, Jinapriya D, Buys YM. Comparison of visual recovery following ex-press versus trabeculectomy: results of a prospective randomized controlled trial. J Glaucoma. 2015;24(3):181–186. doi:10.1097/IJG.0b013e31829e1b68
21. Sawa M. Laser flare-cell photometer: principle and significance in clinical and basic ophthalmology. Jpn J Ophthalmol. 2017;61(1):21–42. doi:10.1007/s10384-016-0488-3
22. Ishida K, Moroto N, Murata K, Yamamoto T. Effect of glaucoma implant surgery on intraocular pressure reduction, flare count, anterior chamber depth, and corneal endothelium in primary open-angle glaucoma. Jpn J Ophthalmol. 2017;61(4):334–346. doi:10.1007/s10384-017-0512-2
23. Inatani M, Ogata-Iwao M, Takihara Y, et al. A prospective study of postoperative aqueous flare in trabeculectomy alone versus phacotrabeculectomy. Nippon Ganka Gakkai Zasshi. 2012;116(9):856–861.
24. Wang W, Zhang X. Meta-analysis of randomized controlled trials comparing EX-PRESS implantation with trabeculectomy for open-angle glaucoma. PLoS One. 2014;9(6):e100578. doi:10.1371/journal.pone.0100578
25. Sakamoto M, Matsumoto Y, Mori S, et al. Excessive scleral shrinkage, rather than choroidal thickening, is a major contributor to the development of hypotony maculopathy after trabeculectomy. PLoS One. 2018;13(1):e0191862. doi:10.1371/journal.pone.0191862
26. Issa de Fendi L, Cena de Oliveira T, Bigheti Pereira C, Pereira Bigheti C, Viani GA. Additive effect of risk factors for trabeculectomy failure in glaucoma patients: a risk-group from a cohort study. J Glaucoma. 2016;25(10):e879–e883. doi:10.1097/IJG.0000000000000490
27. Landers J, Martin K, Sarkies N, Bourne R, Watson P. A twenty-year follow-up study of trabeculectomy: risk factors and outcomes. Ophthalmology. 2012;119(4):694–702. doi:10.1016/j.ophtha.2011.09.043
28. Sherwood MB, Grierson I, Millar L, Hitchings RA. Long-term morphologic effects of antiglaucoma drugs on the conjunctiva and Tenon’s capsule in glaucomatous patients. Ophthalmology. 1989;96(3):327–335. doi:10.1016/S0161-6420(89)32888-0
29. Broadway DC, Grierson I, Hitchings RA. Local effects of previous conjunctival incisional surgery and the subsequent outcome of filtration surgery. Am J Ophthalmol. 1998;125(6):805–818. doi:10.1016/S0002-9394(98)00045-2
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.