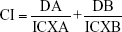Back to Journals » OncoTargets and Therapy » Volume 12
Evodiamine inactivates NF-κB and potentiates the antitumor effects of gemcitabine on tongue cancer both in vitro and in vivo
Authors Guo Q, Liu Y, Zhao J, Wang J, Li Y, Pang Y, Chen J, Wang J
Received 22 July 2018
Accepted for publication 22 November 2018
Published 27 December 2018 Volume 2019:12 Pages 257—267
DOI https://doi.org/10.2147/OTT.S181062
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Faris Farassati
Qi Guo,1 Yanmei Liu,1 Jiayuan Zhao,1 Jing Wang,1 Yue Li,1 Yunqing Pang,1 Jian Chen,2 Jing Wang1
1Department of Periodontology, School of Stomatology, Lanzhou University, Lanzhou, Gansu, China; 2Department of Pediatric Surgery, The First Hospital of Lanzhou University, Lanzhou, Gansu, China
Objective: The aim of this study was to investigate whether evodiamine (EVO) could potentiate the antitumor activity of gemcitabine (GEM) in tongue cancer cells and determine its potential underlying mechanisms.
Materials and methods: Human Tca8113 and CAL-27 tongue squamous carcinoma cell lines were treated with EVO and GEM in different sequences and doses, after which cell proliferation was measured. Drug interactions were analyzed using the Chou–Talalay method with CompuSyn software. Clonality, apoptosis, and migration were measured using the plate clone formation assay, annexin V/propidium iodide (PI) staining, Hoechst 33342 staining, and the wound-healing test. The activity of the nuclear factor kappa light-chain enhancer of activated B cell (NF-κB) p65 subunit and its downstream proteins was quantified by Western blotting. The effects of the drug combination in vivo were assessed using a CAL-27 heterotopic xenograft model.
Results: EVO and GEM had synergistic effects on CAL-27 and Tca8113 cell lines in time- and concentration-dependent manners. Combination of drugs inhibited cell proliferation and migration and reduced the expression of NF-κB p65, B cell lymphoma 2 (Bcl-2), and B cell lymphoma extra large (Bcl-xl) compared with the control and either drug alone. In vivo, combination treatment of the xenograft model with EVO and GEM led to a significant reduction in tumor volume growth and inhibited the activation of NF-κB p65 with no obvious adverse reactions.
Conclusion: The results of this study showed that EVO may inhibit cancer cells by suppressing NF-κB activity, and in combination with GEM, it may increase the chemosensitivity of tongue squamous cancer cells, thereby improving the treatment response.
Keywords: tongue cancer, evodiamine, gemcitabine, combination index, apoptosis, NF-κB
Introduction
Oral squamous cell carcinoma (OSCC) is the eighth most common cancer worldwide,1 and tongue cancer is estimated to account for more than 50% of oral cancer cases diagnosed each year globally.2 Research has shown that tongue squamous cell carcinoma is one of the most aggressive cancers of the oral cavity, leading to delays in early diagnosis and a positive prognosis of only 55%–65%.3 Chemoresistance and systemic adverse reactions from chemotherapeutic agents are the most intractable problems in tumor treatment, so better drugs are needed to target antitumor signaling molecules.
Evodiamine (EVO), a Chinese herb of the evodia rutaecarpa family, may inhibit cell proliferation and induce apoptosis in cancer cell lines by downregulating B cell lymphoma 2 (Bcl-2), B cell lymphoma extra large (Bcl-xl), caspase-3, caspase-9, and vascular endothelial growth factor (VEGF), while upregulating Bcl-2-associated X protein (Bax).4–9 Our recent studies have indicated that EVO inhibits cell growth and promotes apoptosis in tongue cancer cells both in vitro and in vivo.10,11 Takada et al’s12 study showed that EVO abolished the constitutive and inducible activation of nuclear factor kappa light-chain enhancer of activated B cells (NF-κB) by inhibiting IκBα kinase activation, thereby suppressing NF-κB-mediated regulation of antiapoptotic and metastatic gene expression, upregulating apoptosis, and inhibiting invasion. Thus, EVO may be a natural NF-κB inhibitor. Data have shown that many chemotherapeutics and radiation can activate NF-κB, so it may be involved in radiation and chemotherapy resistance.13 Studies have suggested that NF-κB inhibitors and natural compounds with NF-κB inhibitory activity can induce tumor cell apoptosis.14,15 When EVO is combined with other chemotherapy drugs, it inhibits the expression of multidrug resistance (MDR) gene and P-glycoprotein (P-gp), reversing MDR and enhancing inhibition of chemotherapy drugs on tumor cells.16,17 Inhibitor of apoptosis (IAP) proteins and NF-κB contribute to the prevention of apoptosis, and there is a close interaction between their signaling pathways. It has been reported that EVO can simultaneously target both pathways18,19 and may augment chemotherapy by directly or indirectly inhibiting the PI3K/Akt pathway and targeting NF-κB, thereby sensitizing pancreatic cancer cells to treatment.20 However, the use of EVO for the treatment of tongue cancer has not been explored. Thus, we determined whether EVO could increase the antitumor effects of chemotherapeutics to treat tongue cancer via inhibition of NF-κB. To this end, we used gemcitabine (GEM), which is the first-line treatment of head and neck cancer,21 and EVO. GEM can be combined with cisplatin,22 paclitaxel,23,24 and oxaliplatin,25,26 but no standard combination has been recommended. Long-term and heavy use of GEM can induce chemoresistance and ultimately weaken treatment effects. GEM kills cancer cells while activating other protective mechanisms, which may correlate with the upregulation of NF-κB signaling.27,28 Zhang et al’s29 research showed that pancreatic cancer stem cells developed chemoresistance toward GEM through the NAPDH oxidase/ROS (Nox/ROS)/NF-κB/STAT 3 signaling pathway (STAT3).
To study the activity of the EVO/GEM drug combination, we used the Chou–Talalay method, which is based on the median-effect principle. We combined EVO with GEM and treated tongue cancer cell lines to study their synergism and then studied their effects on cell proliferation, apoptosis, and migration. We also studied the potential mechanisms underlying the effects of NF-κB signaling on the apoptosis of tongue cancer cells.
Materials and methods
Reagents, cell lines, and animals
EVO (Natural Institutes for Food and Drug Control, Beijing, China) was dissolved in complete medium containing 0.2% dimethyl sulfoxide (DMSO; Sigma-Aldrich Co., St Louis, MO, USA) as a 100-mM stock solution and stored at −20°C. GEM (Eli Lilly and Company, Indianapolis, IN, USA) was reconstituted as a 200-μg/mL stock solution in sterile normal saline (NS) and stored at −20°C. Antibodies against NF-κB p65 were obtained from Cell Signaling Technology (CST, Danvers, MA, USA). Anti-Bcl-2, anti-Bcl-xl, and anti-β-actin antibodies were obtained from Immune Way (Plano, TX, USA). Human Cal-27 (American Type Culture Collection [ATCC], Manassas, VA, USA) and Tca-8113 (Medical University of the Air Force, Xian, China; approved by the medical ethics committee of Lanzhou University) tongue squamous carcinoma cell lines were cultured in DMEM, supplemented with 10% FBS, and maintained in a humidified atmosphere with 5% CO2 at 37°C. Male nude BALB/c mice aged 4–5 weeks old (18–22 g) were purchased from Beijing Vital River Laboratory Animal Technology Company (Beijing, China). Mice were housed in specific pathogen-free laboratory of Gansu University of Chinese Medicine (Lanzhou, China).
MTT assay
Cells were plated at a density of 4×103 cells/well in 96-well culture plates and cultured overnight. After treatment, 20 μL MTT (Sigma-Aldrich Co.) solution was added to each well and incubated for 4 hours. Medium was discarded, MTT formazan was dissolved in 150 μL DMSO, and the absorbance was measured at 570 nm with a microplate reader.
Drug interaction analysis
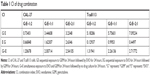
Drug interactions between EVO and GEM were assessed using a combination index (CI), where CI <1, CI =1, and CI >1 indicated synergistic, additive, and antagonistic effects, respectively. The CI value was calculated as follows:
|
where ICXA and ICXB are 50% inhibitory concentrations of cell growth (IC50) of EVO and GEM, respectively, and DA and DB are drug concentrations in combination treatments that inhibit cell growth by 50%. IC50 and CI were calculated with CompuSyn software.
Apoptosis assay
After the designated treatment, cells were washed, harvested, and counted. Then, 1×105 cells were resuspended in 100 μL PBS, after which 10 μL annexin V and 5 μL propidium iodide (PI) were added, and cells were incubated in the dark for 15 minutes at room temperature according to the manufacturer’s instructions (Bioseal Biotech, Guangzhou, China). Apoptosis (%) was assayed using flow cytometry. Apoptotic morphology was confirmed by fluorescence microscopy after Hoechst33342 staining (Beyotime, Beijing, China).
Clonogenic assay
Cells were plated at a density of 2×103 cells/well in cell culture dishes. After treatment, cells were cultured for another 10 days and then fixed and stained with Giemsa (Abcam, Cambridge, UK). Colonies (≥50 cells) were counted to calculate percent growth inhibition.
Migration assay
Cells were grown to 90%–100% confluence in six-well tissue culture plates. After drug treatment, a wound was made by scraping the middle of the cell monolayer with a 200 μL pipette tip. Then, floating cells were removed by washing with PBS, and fresh complete DMEM was added to each well. After 24, 48, and 72 hours of cell migration, wound areas were observed under an inverted microscope. ImageJ software was used to analyze the images.
Western blot
After treatment, cells were washed with ice-cold PBS and harvested in buffer (Beyotime) containing protease inhibitors (Beyotime). Extracted proteins were resolved with SDS-PAGE and electrophoretically transferred onto polyvinylidene difluoride membranes (Solarbio Science and Technology, Beijing, China). Proteins were probed with specific antibodies as previously described.
In vivo xenografts
Animal experiments were performed in accordance with the National Regulations on the Management of Experimental Animals (Beijing, China) and approved by the experimental animal ethics committee of School of Stomatology, Lanzhou University (Lanzhou, China). CAL-27 cells were washed with PBS and harvested in serum-free medium. Then, ~2×106 cells were injected subcutaneously into the right armpit regions of nude mice. When the length of the transplanted tumor reached 5 mm, nude mice were randomized into four groups (n=6/group) to receive intraperitoneal injections as follows: control (NS), EVO (10 mg/kg, dissolved in vehicle), GEM (50 mg/kg, dissolved in vehicle), or EVO and GEM twice per week for 5 weeks. Tumor volumes and body weight were measured twice per week. Tumor volume was measured along the longest orthogonal axes and calculated as volume = (length × width2/2), where width is the shortest measurement. All mice were sacrificed at 35 days after the first day of treatment.
Transmission electron microscopy (TEM) observations of morphological changes
Morphological changes were observed under TEM. Cells were fixed in 2.5% glutaraldehyde/1% osmium tetroxide for 1.5 hours. After embedding and aggregating, specimens were sliced and stained with uranyl acetate and lead citrate.
Statistical analyses
All experiments were repeated at least three times, and data are expressed as mean ± SD. Differences among groups of cells or mice were analyzed with the chi-squared test or ANOVA, followed by the Student’s t-test using SPSS v16.0 software (SPSS Inc., Chicago, IL, USA). P-values of <0.05 were considered statistically significant.
Results
Synergistic inhibition of proliferation with EVO and GEM in vitro
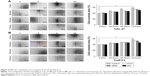
CAL-27 and Tca8113 cells were treated with chosen dosages of GEM (0, 0.01, 0.1, 1, 10, and 100 μg/mL), EVO (0, 1, 2, 4, 8, 16, and 32 μM), or their combinations. Figure 1 shows that EVO and GEM inhibited the proliferation of tongue squamous cells in dose- and time-dependent manners; the IC50 data are given. CI data showed that different treatment sequences and dose ratios induced different drug interactions. Table 1 summarizes that sequential exposure to EVO for 24 hours followed by GEM for 24 hours at a 2:1 ratio of EVO:GEM produced synergistic effects in both cell lines, although the effects were more pronounced in Tca8113 cells.
Effect of EVO and GEM on apoptosis in vitro
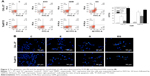
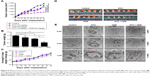
CAL-27 and Tca8113 cells were treated with GEM (1.8 and 2.8 μg/mL, respectively), 5 μM EVO, or their combination for 48 hours. EVO and GEM increased apoptosis in CAL-27 cells (P<0.01). It was shown that pretreatment with EVO followed by GEM treatment induced apoptosis in a greater proportion when compared with treatment with either drug alone (Figure 2A, P<0.05). Figure 2B shows that the nuclei of the controls were regular and oval. Part of the nucleus dissolved, was irregularly shaped, and stained bright blue after treatment with a single drug. The number of cells significantly decreased after combination treatment, and nuclei had significant shrinkage, fragmentation, dissolution, and irregularities.
Effect of EVO and GEM on cloning and migration in vitro
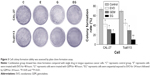
CAL-27 and Tca8113 cells were treated with GEM (0.35 and 0.55 μg/mL, respectively), 1 μM EVO, or their combination for 48 hours. Figure 3 shows the clones of CAL-27 cells in each treatment group and that combination treatment inhibited clone formation more than single drug treatment. This effect was more pronounced in Tca8113 cells (P<0.01). The results of the wound healing assay showed that cell coverage in each groups increased over time, with coverage in the control group increasing the fastest which scratches almost completely healing by 72 hours (Figure 4, P<0.05). Cell coverage between controls and treatments was statistically different at 72 hours in both cell lines (P<0.05).
EVO enhanced GEM-induced apoptosis by regulating NF-κB and Bcl-2 family protein
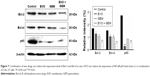
We investigated whether the inhibitory effects of EVO on tongue cancer cell survival were mediated via the activity of NF-κB-related proteins. Protein extracts of untreated, EVO-treated (5 μM), GEM-treated (2 μg/mL), and dual-treated cells were analyzed by Western blotting to assess the expression of p65 and downstream apoptosis-related proteins (Figure 5). CAL-27 and Tca8113 cells had NF-κB signal activation, but GEM treatment did not significantly activate NF-κB signaling. EVO inhibited NF-κB signaling and GEM-induced NF-κB activity in CAL-27 cells (P<0.05). The effects were modest in Tca8113 cells. EVO and GEM downregulated Bcl-2 and Bcl-xl in both cell lines (P<0.05).
Antitumor effect of EVO plus GEM on the growth of implanted tongue tumor in vivo
We evaluated the in vivo antitumor activity of EVO in a xenograft tongue cancer model. Tumor growth was significantly inhibited in mice treated with EVO (P<0.05) or GEM (P<0.05) compared with vehicle, and tumor growth after EVO + GEM treatment was slower than with any single agent (P<0.05). Mouse weight slowly increased in each group, and there was no significant difference among groups. Protein extracts from the specimens of all groups were also analyzed by Western blotting to assess NF-κB p65, Bcl-2, and Bcl-xl expression, and the data were in accordance with the in vitro data. TEM showed that tumor cell nuclei in the controls were intact, clear, and homogeneous. Cell volume was reduced, and chromatin was concentrated, showing early apoptosis in the single drug treatment groups. Nuclear heterochromatin fragmentation and concentration at the nuclear membrane were observed in the combined treatment group, which showed delayed apoptotic characteristics.
Discussion
EVO significantly decreased the growth of human tongue cancer cells, sensitized tongue cancer cells to chemotherapy, and inhibited NF-κB signaling. In vitro experiments revealed that growth inhibitory effects of EVO in combination with GEM were time dependent. Sequential exposure to EVO for 24 hours followed by GEM for 24 hours at a ratio of 2:1 produced synergistic effects in both cell lines. Other regimens resulted in additive or antagonistic effects, suggesting that the drug combination was almost equally or as weakly cytotoxic as each drug alone, in accordance with the literature,27,28,30,31 suggesting that sequential administration of small molecules that suppress NF-κB activation followed by GEM may be preferable, and simultaneous administration may be inappropriate. The Chou–Talalay method can quantify the synergistic, additive, and antagonistic effects of two or more drugs and help with optimal combination selection as it reveals single and combination drug dose–response curves.
The mechanisms underlying the antagonistic or synergistic interaction with sequential exposure are unclear. The antagonistic effects of EVO and GEM suggest that they cancel out their individual cytotoxic effects. EVO and GEM are cell cycle specific. EVO inhibits cells at the G2/M phase,27,32 and GEM prevents cells from entering the S phase, arresting the cell cycle at the G1 phase.28 Thus, drug effects may be associated with cell proliferation kinetics. In addition, EVO and GEM at a dose ratio of 2:1 offer better synergistic effects, perhaps due to the rapid growth of tongue squamous cells, the longer G2/M phase, and better EVO inhibition.
MDR may arise from ATP-binding cassette (ABC) transporter irregularities including P-gp, MDR1, and breast cancer resistance protein, as cell membranes of chemotherapy-insensitive tumors tend to overexpress ABC transporters.33 These transporters can increase drug efflux to reduce the therapeutic concentration. In addition, blockade of pro-survival signaling pathways can increase tumor cell sensitivity to chemotherapy. Enhanced antiapoptotic activity may be a mechanism of MDR. In addition, autophagy-induced chemotherapy resistance, tumor stem cell-regulated MDR, micro-RNA-regulated MDR, hypoxia-induced MDR, DNA repair mechanism-enhanced MDR, and MDR epigenetic regulation may contribute to resistance. Studies have shown that EVO can target tumor stem cell-like cells,34 and EVO in combination with other drugs may offer synergistic inhibition of cancer cells.35,36 Therefore, EVO may target tumor stem cells and survival-related pathways, but its effects on ABC transporter are unclear. EVO may synergize with other drugs to overcome MDR without increasing toxicity and could be used as adjuvant chemotherapy for tongue squamous cell carcinoma.
Activation of the NF-κB signaling pathway regulates antiapoptotic and survival activity in tumor cells. Studies have shown that an in vitro colon cancer model of 5-fluorouracil chemotherapy resistance is related to NF-κB activation.37 In addition, resistance in acute myeloid leukemia is associated with the activation of NF-κB, and it can be used as a target for treatment.38 Overexpression of NF-κB is positively correlated with chemotherapeutic resistance and poor prognosis in serous ovarian epithelial carcinoma.39 Thus, its overactivation may cause minimal residual disease (MRD) in tumor cells, and in some cancers such as ovarian cancer, NF-κB has dual regulatory effects.40 Hong et al41 showed that high NF-κB expression was correlated with poor differentiation and high metastasis of tongue squamous cell carcinoma. Su et al42 concluded that the overactivation of NF-κB was correlated with the invasiveness of tongue cancer according to NF-κB expression in clinical specimens of tongue cancer. Similarly, Wang et al’s43 showed that NF-κB was correlated with epithelial–mesenchymal transition (EMT) in SCC9 and SCC25 cell lines. Thus, tongue squamous cell carcinoma has NF-κB activiation itself, promoting the survival of cancer cells and increasing metastasis. Our Western blotting data were consistent with this hypothesis that CAL-27 and Tca8113 cells have the activation of NF-κB p65.
Some studies have suggested that drugs that inhibit NF-κB combined with GEM can improve the therapeutic effects of GEM on pancreatic and gallbladder cancers.30,44 Small interfering RNA knockout of NF-κB improved GEM’s effects on pancreatic cancer cells;45 thus, NF-κB may be a promising target for reducing MRD.
The Bcl-2 family includes antiapoptotic and pro-apoptotic proteins, which are major regulators of the mitochondrial membrane potential.46 The antiapoptotic proteins are Bcl-2 and Bcl-xl, whereas Bax and Bid are distributed in the cytoplasm to promote apoptosis. There is a significant positive correlation between Bcl-2 family and NF-κB.47,48 Many tumor-associated signaling pathways are associated with Bcl-2 family proteins, and data have shown that EVO plus GEM significantly reduced the expression of Bcl-2 and Bcl-xl, suggesting increased apoptosis. EVO may not only inhibit NF-κB but also may regulate the apoptotic pathways of cancer cells.
Studies have indicated that GEM alone in gallbladder cancer cells can activate NF-κB signaling, causing chemotherapeutic resistance,49 which may also occur after lengthy treatment of pancreatic cancer with GEM, whereby the NF-κB signaling is activated and promotes the invasion and metastasis of pancreatic cancer cells.50 Thus, GEM treatment alone may induce NF-κB, leading to diminished therapeutic effects.
In this study, GEM treatment alone in tongue cancer cells did not cause the activation of NF-κB in either cell line, possibly due to the lower concentration of GEM or shorter treatment time. EVO alone significantly reduced NF-κB activity in CAL-27 cells, and NF-κB p65 expression in the combination treatment group was significantly reduced compared with the controls, but these effects were not obvious in Tca8113 cells. Figures 6 and 7 show that mouse weight decrease did not detect, so toxicity did not increase with dual therapy.
Conclusion
EVO plus GEM may be effective for treating tongue cancer. In vitro data were confirmed in a mouse model in which EVO and GEM significantly blocked tumor growth compared with treatment with either agent alone. In conclusion, the results of this study showed that EVO can potentiate the antitumor activity of GEM in human tongue cancer cells both in vitro and in vivo, and this may be due to EVO-induced suppression of NF-κB and NF-κB-regulated gene products. More studies are needed to confirm these data and improve tongue cancer treatment options.
Ethics approval and consent to participate
This article does not contain any studies with human participants. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Acknowledgment
The present study was supported by the grants from the National Natural Science Foundation of China (No 81773942) and the International Scientific and Technological Cooperation Project of Gansu Province (No 17YF1WA165).
Author contributions
Professor Jing Wang and Jian Chen contributed to the design of the study. Qi Guo and Yanmei Liu participated in the sequence alignment and drafted the manuscript. Jiayuan Zhao carried out the animal experiments. Jing Wang participated in the cell culture work. Yue Li performed the statistical analysis. Yunqing Pang conceived of the study, participated in its design and coordination, and helped to draft the manuscript. All the authors read and approved the final manuscript. All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Scully C, Bagan J. Oral squamous cell carcinoma: overview of current understanding of aetiopathogenesis and clinical implications. Oral Dis. 2009;15(6):388–399. | ||
Bello IO, Soini Y, Salo T. Prognostic evaluation of oral tongue cancer: means, markers and perspectives (II). Oral Oncol. 2010;46(9):636–643. | ||
Ghantous Y, Yaffi V, Abu-Elnaaj I. Oral cavity cancer: epidemiology and early diagnosis. Refuat Hapeh Vehashinayim (1993). 2015;32(3):55–63, 71. Hebrew. | ||
Bai X, Meng H, Ma L, Guo A. Inhibitory effects of evodiamine on human osteosarcoma cell proliferation and apoptosis. Oncol Lett. 2015;9(2):801–805. | ||
Chien CC, Wu MS, Shen SC, et al. Activation of JNK contributes to evodiamine-induced apoptosis and G2/M arrest in human colorectal carcinoma cells: a structure-activity study of evodiamine. PLoS One. 2014;9(6):e99729. | ||
Hong JY, Park SH, Min HY, Park HJ, Lee SK. Anti-proliferative effects of evodiamine in human lung cancer cells. J Cancer Prev. 2014;19(1):7–13. | ||
Wang KL, Hsia SM, Yeh JY, Cheng SC, Wang PS, Wang SW. Anti-proliferative effects of evodiamine on human breast cancer cells. PLoS One. 2013;8(6):e67297. | ||
Wei L, Jin X, Cao Z, Li W. Evodiamine induces extrinsic and intrinsic apoptosis of ovarian cancer cells via the mitogen-activated protein kinase/phosphatidylinositol-3-kinase/protein kinase B signaling pathways. J Tradit Chin Med. 2016;36(3):353–359. Chinese. | ||
Zhao LC, Li J, Liao K, et al. Evodiamine induces apoptosis and inhibits migration of HCT-116 human colorectal cancer cells. Int J Mol Sci. 2015;16(11):27411–27421. | ||
Feng R, He J, Guo J, Sun K, Yang X, Wang J. Evodiamine combined with radiation inhibits proliferation, adhesion and migration of human tongue squamous cell carcinoma cell line Tca-8113. Tumor. 2011;31(5):417–423. Chinese. | ||
Li J. Effect of evodiamine combined with radiation on the growth of Tca-8113 nude mice xenografts. Master Degree Thesis of Lanzhou University. 2014. | ||
Takada Y, Kobayashi Y, Aggarwal BB. Evodiamine abolishes constitutive and inducible NF-kappaB activation by inhibiting IkappaBalpha kinase activation, thereby suppressing NF-kappaB-regulated antiapoptotic and metastatic gene expression, up-regulating apoptosis, and inhibiting invasion. J Biol Chem. 2005;280(17):17203–17212. | ||
Li F, Sethi G. Targeting transcription factor NF-kappaB to overcome chemoresistance and radioresistance in cancer therapy. Biochim Biophys Acta. 2010;1805(2):167–180. | ||
Berkovich L, Earon G, Ron I, Rimmon A, Vexler A, Lev-Ari S. Moringa Oleifera aqueous leaf extract down-regulates nuclear factor-kappaB and increases cytotoxic effect of chemotherapy in pancreatic cancer cells. BMC Complement Altern Med. 2013;13:212. | ||
Gupta SC, Sundaram C, Reuter S, Aggarwal BB. Inhibiting NF-κB activation by small molecules as a therapeutic strategy. Biochim Biophys Acta. 2010;1799(10–12):775–787. | ||
Sui H, Pan SF, Feng Y, et al. Zuo Jin Wan reverses P-gp-mediated drug-resistance by inhibiting activation of the PI3K/Akt/NF-κB pathway. BMC Complement Altern Med. 2014;14:279. | ||
Xia YZ, Ni K, Guo C, et al. Alopecurone B reverses doxorubicin-resistant human osteosarcoma cell line by inhibiting P-glycoprotein and NF-kappa B signaling. Phytomedicine. 2015;22(3):344–351. | ||
Dai X, Zhang J, Arfuso F, et al. Targeting TNF-related apoptosis-inducing ligand (TRAIL) receptor by natural products as a potential therapeutic approach for cancer therapy. Exp Biol Med (Maywood). 2015;240(6):760–773. | ||
Dai Y, Lawrence TS, Xu L. Overcoming cancer therapy resistance by targeting inhibitors of apoptosis proteins and nuclear factor-kappa B. Am J Transl Res. 2009;1(1):1–15. | ||
Wei WT, Chen H, Wang ZH, et al. Enhanced antitumor efficacy of gemcitabine by evodiamine on pancreatic cancer via regulating PI3K/Akt pathway. Int J Biol Sci. 2012;8(1):1–14. | ||
Braakhuis BJ, van Dongen GA, Vermorken JB, Snow GB. Preclinical in vivo activity of 2′,2′-difluorodeoxycytidine (Gemcitabine) against human head and neck cancer. Cancer Res. 1991;51(1):211–214. | ||
Miyazaki Y, Yamabe K, Hayashi N, et al. A case of liver metastasis of ampullary carcinoma that developed postoperatively that was effectively treated with gemcitabine plus cisplatin. Gan To Kagaku Ryoho. 2016;43(12):2080–2082. Japanese. | ||
Guilbault C, Garant A, Faria S, et al. Long-term outcomes of induction carboplatin and gemcitabine followed by concurrent radiotherapy with low-dose paclitaxel and gemcitabine for Stage III non-small-cell lung cancer. Clin Lung Cancer. 2017;18(5):565–571. | ||
Kim GP, Parisi MF, Patel MB, Pelletier CL, Belk KW. Comparison of treatment patterns, resource utilization, and cost of care in patients with metastatic pancreatic cancer treated with first-line nab-paclitaxel plus gemcitabine or FOLFIRINOX. Expert Rev Clin Pharmacol. 2017;10(5):559–565. | ||
Dhanapal V, Gunasekara M, Lianwea C, et al. Outcome for patients with relapsed/refractory aggressive lymphoma treated with gemcitabine and oxaliplatin with or without rituximab; a retrospective, multicentre study. Leuk Lymphoma. 2017;58(9):1–9. | ||
Ramaswamy A, Ostwal V, Pinninti R, et al. Gemcitabine-cisplatin versus gemcitabine-oxaliplatin doublet chemotherapy in advanced gallbladder cancers: a match pair analysis. J Hepatobiliary Pancreat Sci. 2017;24(5):262–267. | ||
Du QH, Xu YB, Zhang MY, Yun P, He CY. Propofol induces apoptosis and increases gemcitabine sensitivity in pancreatic cancer cells in vitro by inhibition of nuclear factor-κB activity. World J Gastroenterol. 2013;19(33):5485–5492. | ||
Iwase R, Haruki K, Fujiwara Y, et al. Combination chemotherapy of nafamostat mesylate with gemcitabine for gallbladder cancer targeting nuclear factor-κB activation. J Surg Res. 2013;184(1):605–612. | ||
Zhang Z, Duan Q, Zhao H, et al. Gemcitabine treatment promotes pancreatic cancer stemness through the Nox/ROS/NF-κB/STAT3 signaling cascade. Cancer Lett. 2016;382(1):53–63. | ||
Maliandi MV, Mato-Berciano A, Sobrevals L, Roué G, José A, Fillat C. AduPARE1A and gemcitabine combined treatment trigger synergistic antitumor effects in pancreatic cancer through NF-κB mediated uPAR activation. Mol Cancer. 2015;14:146. | ||
Zhou L, Qi L, Jiang L, et al. Antitumor activity of gemcitabine can be potentiated in pancreatic cancer through modulation of TLR4/NF-κB signaling by 6-shogaol. AAPS J. 2014;16(2):246–257. | ||
Feng H, Guo B, Kong X, Wu B. Evodiamine enhances the radiosensitivity of esophageal squamous cell cancer Eca-109 cells. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2016;32(7):940–944. Chinese. | ||
Wu Q, Yang Z, Nie Y, Shi Y, Fan D. Multi-drug resistance in cancer chemotherapeutics: mechanisms and lab approaches. Cancer Lett. 2014;347(2):159–166. | ||
Han S, Woo JK, Jung Y, et al. Evodiamine selectively targets cancer stem-like cells through the p53-p21-Rb pathway. Biochem Biophys Res Commun. 2016;469(4):1153–1158. | ||
Khan M, Bi Y, Qazi JI, Fan L, Gao H. Evodiamine sensitizes U87 glioblastoma cells to TRAIL via the death receptor pathway. Mol Med Rep. 2015;11(1):257–262. | ||
Li YL, Pan YN, Wu WJ, et al. Evodiamine induces apoptosis and enhances apoptotic effects of erlotinib in wild-type EGFR NSCLC cells via S6K1-mediated Mcl-1 inhibition. Med Oncol. 2016;33(2):16. | ||
Körber MI, Staribacher A, Ratzenböck I, Steger G, Mader RM. NFκB-Associated Pathways in Progression of Chemoresistance to 5-Fluorouracil in an In Vitro Model of Colonic Carcinoma. Anticancer Res. 2016;36(4):1631–1639. | ||
Zhang RR, Zhang H, Chen FX. Research progress on chemoresistance mechanism of nuclear factor Kappa B signalling pathway in acute myeloid leukemia. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2015;23(6):1785–1790. Chinese. | ||
Shuang T, Wang M, Zhou Y, Shi C. Over-expression of nuclear NF-κB1 and c-Rel correlates with chemoresistance and prognosis of serous epithelial ovarian cancer. Exp Mol Pathol. 2016;100(1):139–144. | ||
Yang G, Xiao X, Rosen DG, et al. The biphasic role of NF-kappaB in progression and chemoresistance of ovarian cancer. Clin Cancer Res. 2011;17(8):2181–2194. | ||
Hong ZH, Shao F, Zhu GG, Su T. Expression of NF-kappaB and COX-2 in tongue squamous cell carcinoma. Shanghai Kou Qiang Yi Xue. 2010;19(3):335–338. Chinese. | ||
Su X, Wang J, Chen W, Li Z, Fu X, Yang A. Overexpression of TRIM14 promotes tongue squamous cell carcinoma aggressiveness by activating the NF-κB signaling pathway. Oncotarget. 2016;7(9):9939–9950. | ||
Wang Y, Lin Z, Sun L, et al. Akt/Ezrin Tyr353/NF-κB pathway regulates EGF-induced EMT and metastasis in tongue squamous cell carcinoma. Br J Cancer. 2014;110(3):695–705. | ||
Yang MH, Lee KT, Yang S, et al. Guggulsterone enhances antitumor activity of gemcitabine in gallbladder cancer cells through suppression of NF-κB. J Cancer Res Clin Oncol. 2012;138(10):1743–1751. | ||
Cao LP, Song JL, Yi XP, Li YX. Double inhibition of NF-κB and XIAP via RNAi enhances the sensitivity of pancreatic cancer cells to gemcitabine. Oncol Rep. 2013;29(4):1659–1665. | ||
Hardwick JM, Soane L. Multiple functions of BCL-2 family proteins. Cold Spring Harb Perspect Biol. 2013;5(2):a008722. | ||
Liu Y, Gao X, Deeb D, et al. Mycotoxin verrucarin A inhibits proliferation and induces apoptosis in prostate cancer cells by inhibiting prosurvival Akt/NF-kB/mTOR signaling. J Exp Ther Oncol. 2016;11(4):251–260. | ||
Zheng X, Koropatnick J, Chen D, et al. Silencing IDO in dendritic cells: a novel approach to enhance cancer immunotherapy in a murine breast cancer model. Int J Cancer. 2013;132(4):967–977. | ||
Yu Y, Wang J, Xia N, Li B, Jiang X. Maslinic acid potentiates the antitumor activities of gemcitabine in vitro and in vivo by inhibiting NF-κB-mediated survival signaling pathways in human gallbladder cancer cells. Oncol Rep. 2015;33(4):1683–1690. | ||
Arora S, Bhardwaj A, Singh S, et al. An undesired effect of chemotherapy: gemcitabine promotes pancreatic cancer cell invasiveness through reactive oxygen species-dependent, nuclear factor κB- and hypoxia-inducible factor 1α-mediated up-regulation of CXCR4. J Biol Chem. 2013;288(29):21197–21207. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.