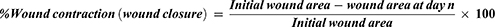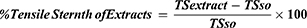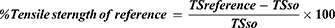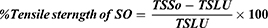Back to Journals » Infection and Drug Resistance » Volume 15
Evaluation of Wound Healing and Antibacterial Activities of Solvent Fractions of 80% Methanol Leaf Extract of Brucea antidysenterica J.F. Mill (Simaroubaceae)
Authors Wolde B , Abay SM , Nigussie D , Legesse B , Makonnen E , Mengie Ayele T
Received 4 February 2022
Accepted for publication 26 March 2022
Published 5 April 2022 Volume 2022:15 Pages 1517—1531
DOI https://doi.org/10.2147/IDR.S360761
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Befekadu Wolde,1 Solomon Mequanente Abay,2 Dereje Nigussie,3 Belete Legesse,4 Eyasu Makonnen,2,4 Teklie Mengie Ayele5
1Department of Pharmacy, Addis Ababa Health Bureau, Addis Ababa, Ethiopia; 2Department of Pharmacology and Clinical Pharmacy, School of Pharmacy, College of Health Science, Addis Ababa University, Addis Ababa, Ethiopia; 3Vaccines and Diagnostic Research Directorate, Ethiopian Public Health Institute, Addis Ababa, Ethiopia; 4Center for Innovative Drug Development and Therapeutics Trial in Africa (CDT-Africa), College of Health Science, Addis Ababa University, Addis Ababa, Ethiopia; 5Department of Pharmacy, College of Health Science, Debre Tabor University, Debre Tabor, Ethiopia
Correspondence: Teklie Mengie Ayele, Department of Pharmacy, College of Health Science, Debre Tabor University, P.O. Box: 272, Debre Tabor, 6300, Ethiopia, Tel +251 910111531, Email [email protected]
Background: Brucea antidysenterica is a well-known medicinal plant that has traditionally been used to treat a variety of ailments, including wound healing. Supporting the traditional claims, wound healing, antibacterial, anti-inflammatory, and antioxidant activities of the crude extracts of different parts of the plant were reported. The aim of this study was to evaluate the wound healing and antibacterial activities of solvent fractions of the menthol leaf extract of Brucea antidysenterica.
Methods: Methanol (80%) leaf extract of Brucea antidysenterica was fractionated using three solvents; water, n-butanol and chloroform. An ointment containing 2% and 4% of each fraction was formulated and applied to wounds inflicted on rats topically. The wound contraction rate, period of epithelialization, and breaking strength were analysed. In vitro antibacterial activities were tested using the agar diffusion method. The macro-tube dilution technique was used to determine the minimum inhibitory concentration (MIC), and the minimum bactericidal concentration (MBC) was determined by sub-culturing the MIC and concentrations below the MIC.
Results: The 2% and 4% aqueous fractions (AF) significantly increased wound contraction (p 0.001) compared to the negative control and increased tensile strength compared to untreated (p 0.001). Among the three fractions, the n-butanol fraction showed the highest antibacterial growth inhibition, ranging from 8 mm (E. coli) to 16 mm (S. aureus).
Conclusion: Data obtained from this study collectively indicated that the aqueous fraction of 80% methanol leaf extract of B. antidysenterica possesses wound healing and antibacterial activities.
Keywords: Brucea antidysenterica, wound healing activity, antibacterial activity, excision, incision, agar well diffusion, MIC, MBC
Introduction
A wound is defined as damage or disruption to the normal anatomical structure and function of a living tissue.1 This ranges from a simple disruption in the epithelial integrity of the skin to deeper subcutaneous tissue involvement and also damage to other structures, like muscle and bone.2 Wounds can arise from physical, chemical, thermal, microbial, or immunological damage to a tissue or can be the result of a disease process like diabetes mellitus.
Wounds have considerable humanistic and economic burdens, both at individual and societal levels. A wound deters individual quality of life and productivity; and is associated with major economic burdens on the health care system.3,4 The current situation worldwide estimate of people with chronic wounds rises to 6 million each year. In developed countries, 1–2% of individuals in a population acquire a chronic wound during their lifetime.5 Globally, the economic burden of chronic wound is estimated to be nearly 2–4% of the health budgets.6
Wound healing is the complex and dynamic process of restoring the structure and function of damaged tissues. It follows coordinated interactions between diverse immunological and biological systems.7,8 The interaction involves a cascade of ordered and precisely regulated steps and events, which are divided into four overlapping but distinct phases, ie, the hemostasis/coagulation phase, the inflammation phase, the proliferation phase, and the remodelling phase.9–11
Physiological wound healing process effectively restores the structural and functional states of injured tissues. However, the healing process may be disrupted and wounds may be delayed from healing, heal incompletely, or become chronic wounds.12 Wound infections are one of the most common causes of impaired wound healing. Even though the existence of polymicrobial communities is common, bacteria are the most prevalent contaminants of wound healing.13
Traditional and complementary medicines are widely used globally for a number of disease conditions. Wounds and skin disorders are among the major uses. It is reported that, one-third of all traditional medicines in use are indicated for the treatment of wounds and skin disorders.14 Medicinal plants are the major remedies used in traditional medicine. Moreover, antibacterial activity was found in essential oils and plant extracts from medicinal plants against resistant fungal and bacterial strains.15–17 B. antidysenterica is a well-known medicinal plant with numerous reported uses, such as treatments against scabies and wounds, leprosy, dysentery, gonorrhoea, eczema, fever, malaria, haemorrhoids, trypanosomiasis, and others.5 The traditional use of this plant has been supported by scientific experiments for its wound healing properties,5,18 anti-inflammatory,5,19 and antibacterial activities.19–22 The crude methanol extract of the leaves of this plant has been studied to have anti-bacterial and wound healing activities. The effect of its solvent fractions on wounds and bacteria, however, has not yet been evaluated. The aim of this study was, therefore, to evaluate the in vivo wound healing and in vitro antibacterial activity of solvent fractions of methanol extract of B. antidysenterica.
Methods
Collection, Extraction and Fractionation of Plant Material
Fresh leaves of B. antidysenterica (Figure 1) were collected around Atawi, Wara Jarso woreda, Semen Shoa Zone, Oromia Region, Ethiopia, around 170km north-west of Addis Ababa. Identification and authentication of the plant material was done by a taxonomist at the National Herbarium unit of the Ethiopian Public Health Institute (EPHI), Addis Ababa, Ethiopia, and a voucher specimen was deposited (BA012019) for future reference.
 |
Figure 1 Picture of Brucea antidysenterica (photograph taken by principal investigator during sample collection). |
The powdered leaf was extracted using cold maceration. Methanol (80% (V/V)) was chosen as a solvent for its better yield, obtained in previous studies on this plant.19,20 Erlenmeyer conical flasks, containing the extraction mixture, were fixed on mini orbital shakers adjusted at 145 revolutions per minute (rpm) with periodic agitation for three days. Then, the mixture was filtered with Whatman’s filter No.-1 paper, and the mark was re-macerated to have additional yield. The combined filtrate was concentrated by a rotary evaporator at 40 oC and a water bath at 40 oC. The concentrate was frozen and dried by lyophilisation, and a brownish crude extract was obtained.
The crude extract was further fractionated by successive solvent-solvent fractionation using water, chloroform, and n-butanol. The crude extract was dissolved in 1/3 (W/V) of each solvent successively and separated in a separatory funnel. The yields of the aqueous (AF), n-butanol (BF), and chloroform (CF) fractions were 73gm (61%), 25gm (21%), and 16gm (13%), respectively.
Experimental Animals
Adult, healthy Wistar albino rats (Rattus norvegicus) of both sexes weighing 180–250g and aged 4–6 months were obtained from the Ethiopian Public Health Institute (EPHI) and used for excision and incision wound models. Healthy adult female Swiss albino mice (29–40 g) were obtained from the School of Pharmacy, Addis Ababa University, and used for an acute toxicity study. The animals were housed under standard conditions with 12-hour light and dark cycles and had free access to standard food and water ad libitum. Animals were acclimatized to the laboratory conditions for a week before being subjected to any experiment. All the way through the experiment, the animals were handled according to international laboratory animal use and care guidelines.23
Microbial Organisms
In vitro antibacterial activity of each solvent fraction was evaluated against gram positive bacteria, S. aureus and S. pyogenes, and gram negative bacteria, E. coli, P. aeruginosa, and K. Pneumoniae. All bacterial strains were standard strains (American Type Culture Collections (ATCC)) and obtained from the Department of Microbiology, Parasitology, and Immunology, School of Medicine, Addis Ababa University, Addis Ababa.
Ointment Formulation
Simple ointments of each fraction were made according to the formula (Table 1) described in the British Pharmacopoeia.24 The reduced formula was used to create 50 grams of 2% w/w and 4% w/w solvent fractions ointments (Table 1).To prepare the simple ointment, the calculated amount of hard paraffin and cecostearyl alcohol were mixed and melted in a beaker. Using a separate beaker, the mixture of wool fat and white soft paraffin was melted. After the two mixtures were thoroughly mixed in the water bath, the former was added to the latter and then stirred until cooled. Then 2% and 4% ointments of each fraction were formulated by mixing 2g and 4g powders of each fraction with 98 g and 96 g of the ointment bases, respectively.
 |
Table 1 Formula Used to Formulate Simple Ointment and Medicated Ointment |
Grouping and Dosing of Experimental Animals
For the excision wound model, nine groups of healthy adult rats (each of five rats) were used. Group I was treated with simple ointment and used as a control. Groups II, III, IV, V, VI, and VII were treated with 2%AF, 4%AF, 2%BF, 4%BF, 2%CF, and 4%CF, respectively. The last two groups served as positive controls: Group VIII with 0.2% nitrofurazone (NF) and Group IX with MEBO.
For the incision model of wound healing, ten groups of rats with five rats each were used. Groups I to IX were treated in the same way as described for the Excision model, and Group X was left untreated and served as a negative control.
Acute Oral and Dermal Toxicity Studies
An acute toxicity study was carried out in accordance with the OECD guidelines for chemical testing OECD/OCDE-425.23 For each fraction of the test samples, a group of three healthy female Wistar albino rats (180–200 kg) were used for the test. The animals were then followed for clinical signs of toxicities like changes in skin and fur, eyes, mucus membranes, and respiratory system, autonomic system, gross weight change, motor activity, and behavioral patterns.
The acute dermal toxicity test was done according to the OECD guideline for the test of chemicals OECD: 434 with slight modification.25 Twenty-four hours prior to the application of test ointment, dorsal fur (around 10% of body surface area) was shaved and the animals were housed in individual cages. On the test day, 2% and 4% w/w ointment preparations of each solvent fraction were applied as thin films uniformly to the shaved area. After 24 hours of exposure, residual ointment was removed, and animals were observed for a day periodically for any sign of dermal toxicity, then daily for the development of any delayed toxicity for two weeks.
Wound Healing Studies
Excision Wound Model
Before wound formation, animals were anesthetized with intraperitoneal (IP) administration of 80 mg/kg of ketamine HCl. The dorsal fur was then shaved, and a circular area of 314 mm was marked with a standard material, and the full thickness of the marked circular area was excised out with sterile surgical scissors (Figure 2A and B). Rats were housed in individual cages on the wounding day, which was considered day 0. After 24 hours of wounding, tests and control ointments were applied and continued once daily until the wound healed. Wound closure was monitored and wound area was measured on the 2nd, 4th, 6th, 8th, 10th, and 22nd days. The wound contraction for each day of measurement was calculated based on the initial wound size as stated in the formula below.26–29 In addition; the number of days required for the fall of dead tissue remnants without any residual raw wound was measured as the epithelialization period.30
 |
Figure 2 Excision wounding of animals. 3A- circularly marked area to be excised, 3B - excised wound area. |
Where “n” = number of measurement days (2nd, 4th, 8th, 12th … 0.22nd.
Incision Wound Model
On wounding day, animals were anesthetized using the same technique described for the excision model. Then, the dorsal fur was shaved and a three-cm long longitudinal paravertebral incision was made through the skin and subcutaneous tissue (Figure 3A). The skin parted was sutured one centimetre apart (Figure 3B). Starting from day 1 of post-wounding, animals were treated with test and control ointments, except the last group of animals, which were left untreated and used as references. On day eight post-wounding, sutures were removed and treatment continued. Tensile strength was measured on the 10th post-wounding day using a continuous water flow technique.31 (Figure 3C). Based on the tensile strength measured, the percent of strength was calculated using the following formulas.32
Where; “SO” is an ointment without active substance (vehicle), and “LU” is left untreated (negative control).
Antibacterial Activity
Agar Well Diffusion Method
Agar (MHA) was prepared according to the manufacturer’s recommendation, and bacterial cultures were prepared to a density of 108 cells mL−1 of 0.5 McFarland standards.33 The aliquot was spread evenly on to MHA or MHA enriched with 5% sheep blood for S. Pyogenes. The medium was allowed to dry for 30 minutes at room temperature. After the media hardened, the required numbers of wells were made with a sterile cork borer of 6 mm in diameter.
Test samples (solvent fractions) were prepared at a concentration of 500 mg/mL and 100 mg/mL using DMSO as the solvent. Using a micropipette, 20µL of fraction samples and the negative control (DMSO) were loaded into the respective wells as labeled using permanent marker on the Petri plates. A Ciprofloxacin 5µg/mL disc was used as a control. Plates were left at room temperature for two hours and then incubated at 37 oC for 24 hours. After the incubation period, plates were observed for inhibition of bacterial growth, and inhibition zones were measured using a scale.34,35
Determination of Minimum Inhibitory Concentration (MIC)
For fractions which showed a zone of inhibition greater than or equal to 7 mm for a certain organism, MIC was determined using the broth macro-dilution method.34,35 For fractions which showed activity at a concentration of 500 mg/mL, the concentration was further diluted to 1:2 and 1:4, to have 250 mg/mL and 125 mg/mL, and fractions that showed activity at 100 mg/mL were further diluted to 75 mg/mL, 50 mg/mL (1:2), 25 mg (1:4), 12.5 mg/mL (1:8), and 6.25 mg/mL (1:16).
Each fraction concentration was diluted in broth media by a factor of two, and 1mL was added to a standard test tube.The standard inoculums were diluted in broth media as (1:150), and 1mL of the aliquot was added to each tube, containing different concentrations of fractions. A tube without any test samples was used as a control for bacterial growth. The tubes were covered and incubated at 37 oC for 24 hours. After incubation, bacterial growth was analysed; the minimum concentration that did not show any visible bacterial growth was taken as MIC.34
Determination of the Minimum Bactericidal Concentration (MBC)
MBC was determined by sub-culturing solvent fractions having a value of less than or equal to the MIC value. From the MIC test solutions, the contents were streaked using sterile cotton swabs on an agar plate and incubated at 37°C for 24 hours. The lowest concentration that yielded no single bacterial colony was taken as MBC.35
Preliminary Phytochemical Screening
Qualitative screening for the presence of secondary metabolites in each solvent fraction was performed using standard tests described previously.5,19,21,36–38 The presence of alkaloids, saponins, flavonoids, terpenoids, phenols, glycosides, and tannins were tested.
Statistical Analysis
Raw data were expressed as mean ± SEM (standard error of the mean). The results were statistically analyzed using one-way analysis of variance (ANOVA) followed by Post Hoc Tukey -tests and data were considered significant at p < 0.05. Statistical analysis was done using SPSS version 23.
Results
Toxicity Study
Aqueous, n-butanol and chloroform fractions of 80% methanol extract of B. antidysenterica leaves appeared to be safe up to the dose of 2000 mg/kg, which was established by the absence of mortality and any sign of gross or behavioral toxicity in mice up to two weeks. The LD50 of all three solvent fractions was greater than 2000 mg/kg.
It is revealed that neither 2% nor 4% ointment preparations of all solvent fractions showed any sign of inflammation, redness, rash or irritation 24 hours after applications. No overt signs of toxicity were observed during monitoring for a further 14 days.
Wound Healing Effects
Effect on Excision Wound Model
Only the aqueous fraction, as shown in Tables 2 and 3, appeared to have a promising effect on wound contraction and epithelialisation period, whereas the n-butanol and chloroform fractions appeared to have a negative effect on healing.
 |
Table 2 The Effect of Solvent Fractions of 80% Methanol Extract of B. antidysenterica Leaves on Wound Contraction of Excision Wound in Rat |
 |
Table 3 The Effect of Solvent Fractions of 80% Methanol Extract of B. Antidysenterica Leaves on Epithelialization Period of Excision Wound in Rats |
At day 10, 2% and 4% showed significant wound contraction compared to simple ointment at day 10 (p 0.001) and day 8 (p 0.05), respectively, and all measurement days afterward. On the last day of measurement, 2% and 4% AF showed 97.5% and 98.39% of wound contraction, respectively (Figure 4). There was no discernible difference between the standard drug and the AF-treated animals.
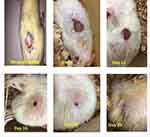 |
Figure 4 Pictograph of wound contraction of animal treated with aqueous fraction of 80% methanol extract of B. antidysenterica leaf on different days of measurement. |
The period of epithelialization was shown to be short in AF and standard drug-treated groups compared to control (Table 3). Although 2% AF ointment failed to produce a statistically significant difference in the period of epithelialization, those treated with 4% AF had a significantly shorter (p ˂ 0.001) epithelialization period compared to control.
Effect on Incision Wound Model
The aqueous fraction of B. antidysenterica 80% methanol extract was found to increase the breaking strength of incision wounds, ie, tensile strength compared to the negative control group. As shown in Table 4, 2% AF (p 0.05) and 4% AF (p 0.001) significantly increased the tensile strength compared to simple ointment. However, BF and CF did not show any effect on tensile strength.
 |
Table 4 The Effect of Solvent Fractions of 80% Methanol Extract of B. antidysenterica Leaves on the Breaking Strength of the Incision Wound Site in Rats |
Antibacterial Activity
Bacterial Growth Inhibition
The three fractions showed moderate bacterial growth inhibition of different standard organisms tested. Bacterial growth inhibitory activity, measured as the zone of inhibition, of the fractions ranged from 7 mm (chloroform fraction against K. pneumoniae) to 16 mm (butanol fraction against S. aureus). The butanol fraction inhibited the most growth of the gram positive bacteria S. aureus and S. pyogenes, as well as the gram negative bacteria E. coli and P. aeruginosa (Table 5).
 |
Table 5 The Mean Bacterial Growth Inhibition Zone (mm) of Solvent Fractions of 80% Methanol Extract of B. antidysenterica Leaves in Agar Well Diffusion Method (20µL Sample Volume) |
Minimum Inhibitory and Bactericidal Concentrations
The MIC and MBC values were determined for fractions that showed growth inhibitions in the agar well diffusion method. The lowest MIC (75mg/mL) was observed with the n-butanol fraction against S. Aureus (Table 6). The same table shows that the lowest MBC observed (125mg/mL) with n-butanol fraction against S. pyogenes and S. aureus and chloroform fraction against S. aureus.
 |
Table 6 The MIC (mg/mL) and MBC (mg/mL) of Solvent Fractions of 80% Methanol Extract of B. antidysenterica Leaves Using Tube Dilution Method |
Phytochemical Screening
The results of qualitative phytochemical screening of solvent fractions of B. antidysentrica leaves methanol extract showed the presence of different secondary metabolites (Table 7).
 |
Table 7 Phytochemical Screening of Solvent Fractions of 80% Methanol Extract of B. antidysenterica Leaves |
Discussion
Wound healing and antibacterial activities of B. antidysenterica have been reported in a range of ethnobotanical studies. In the previous study, the wound healing activity of 80% methanol leaf extract of B. antidysenterica showed significant wound contraction (99.9%) compared to simple ointment, and it shortened the epithelialization period and increased the tensile strength of wounds.5 In addition to wound healing effects, extracts of B. Antidysenterica were reported to show bacteriostatic and bactericidal activity against different wound-causing bacterial strains.21,22 The present study was intended to evaluate the wound healing and anti-bacterial activities of solvent fractions of a methanol extract of B. antidysenterica.
The results of a toxicity study on the solvent fractions showed that the extracts were safe at effective wound healing and antibacterial doses. In the present acute toxicity study, aqueous, n-butanol and chloroform fractions of the 80% crude leaf extract were revealed to have an oral median lethal dose (LD50) of more than 2000 mg/kg, indicating the safety nature of the fractions. Similarly, acute dermal toxicity showed that 2% and 4% ointment preparations were non-irritants and caused no rash, redness, or other unexpected reactions. This was in line with the recommendation that products used for topical wound treatments should be non-toxic, biocompatible, and have intended clinical activity without adversely affecting the physiological healing process.
On the contrary to acute dermal toxicity results, application of 5% chloroform ointment at the wound site during the pilot study showed wound exacerbation and 2 out of 5 animals treated within six days of continuous treatment were died. The same concentration of n-butanol fraction also adversely affected wound healing, though no death was recorded up to the 10th day of treatment. Additionally, 2% and 4% of n-butanol and chloroform fractions revealed irritation, inflammation, and adversely affected wound healing. The present findings are supported by those of the previous study, which reported that 10% ointment of 80% methanol extract had a lethal effect when applied to excision wounds. The study also reported that 50% of the animals tested with 10% methanol leaf extract and 4% of methanol fruit extract killed 50% of the treated animals.5 The toxic effect might be attributed to the non-polar compound/s which is not present in the aqueous fraction because no adverse effect was observed in mice treated with the aqueous fraction in the current experiment. The negative effects of n-butanol and chloroform fractions may be attributed to the increased or prolonged inflammation, considering that the effect revealed after two or three days of treatment is characterized by redness, swelling, and pain around the wound site, and defected scarring that may suggest pathological fibrosis. Fibrosis is a natural process of physiological wound healing to repair tissue function. Defected fibrosis, however, leads to suboptimal healing, dysfunctional tissue, and detrimental scarring. The main cause of pathological fibrosis is chronic inflammation, which is caused by persistent activation of TGF-signaling.39
Wound repair involves different processes, including contraction, formation of epithelialization, and fibrosis.39 As there is no single representative model or reference standard for studying wound healing effects, two in vivo models, excision and incision, wound healing models, were used in this study.
The excision wound model is popularly used to examine effective wound closure; contraction of wound area and epithelialization (restoring the epidermis) being the reference parameters. In the present study, 2% and 4% ointments of the aqueous fraction of an 80% methanol extract of B. antidysenterica leaves enhanced wound contraction rate compared to simple ointment treated animals. The effect, however, was less than that of the standards. On day 20 of wounding, AF showed a dose dependent wound closure effect, though not a complete wound closure effect, unlike the standard. This may show wound healing activity of the fraction. Prolonged inflammation may lead to the generation of reactive oxygen species (ROS) which in turn damages cells and tissues in the wound site, augmented by impaired antioxidant activity, leading to worsening of the condition and impaired healing.40 The wound closure effect of AF observed in our study might be associated with its anti-inflammatory5 and antioxidant properties,19,21 exerted by the secondary metabolites like flavonoids present in the fraction.
Wound contraction facilitates healing in a short time as it decreases the size of the wound and reduces the amount of extracellular matrix needed to repair defected tissues. Contraction also facilitates healing by promoting epithelialization. Epithelialization (re-epithelialization) is achieved by epithelial cells’ (keratinocytes’) migration from the basement membrane upward or from the wound edge. Contraction shortens this distance keratinocytes must travel.39 The wound contraction observed by the aqueous fraction of B. antidysenterica 80% methanol leaf extract may be associated with B. antidysenterica’s anti-inflammatory activity, and potent antioxidant property.5,20 Prolonged inflammations may lead to the generation of ROS, which in turn damages cells and tissues in the wound site. In addition, an increase in free radical production augmented by impaired antioxidant activity may worsen the condition, resulting in impaired healing.41 In this regard, the potent antioxidant activity of the aqueous fraction, as well as the high content of antioxidant phytochemicals such as vitamin C in B. antidysenterica leaf,18 may be mentioned as one contributing property to the wound healing activities demonstrated in this study.
The epithelialization period is another parameter examined by the excision wound model. Successful wound closure is considered to be a defining parameter of successful wound closure, and impaired re-epithelialization is associated with chronic non-healing wounds.40 In addition, loss of skin barrier function might cause dehydration, infection, or even death. In the present study, 4% AF shortened the epithelialization period compared to simple ointment.
Closure of the wound site and regeneration of the epithelium is not enough for better wound healing. Strength or durability of the wounded area is important and is achieved by increased formation of collagen and concentration and stabilization of fibers.42
In the incision wound model, measuring the tensile strength of the wound is an important parameter that implies durable healing. The aqueous fraction of B. antidysenterica 80% methanol leaf extract increased the tensile strength of incision wounds at the strength of both 2% and 4% ointment preparations. Breaking strength or tensile strength was achieved by aqueous fraction treated animals and was significantly higher than those left untreated or animals treated by simple ointment.
Enhanced strength of the wound matrix implies that cell to cell and cell to matrix interactions are regained and shows repair of the framework for angiogenesis, which in turn facilitates blood circulation that provides oxygen and nutrients for the healing tissue. Increasing strength in the process of wound healing, such as observed by the aqueous fraction of B. antidysenterica leaf extract treatment in this study, may suggest functional recovery of injured tissue.
This study examined whether treatment with an aqueous fraction of 80% methanol leaf extract of B. antidysenterica leaf facilitated wound contraction, shortened the period of epithelialization, and enhanced breaking strength. These effects may be related to induction or stimulation of cellular proliferation, enhancement of collagen synthesis and cross-linking of proteins, increased anti-inflammatory activity and potent antioxidant property of the fraction.
In the incision wound model, measuring the tensile strength of the wound is an important parameter that implies durable healing.41 The aqueous fraction of B. antidysenterica 80% methanol leaf extract increased the tensile strength of incision wounds at both concentrations.
Chronic non-healing wounds are among the major causes of wound infections. Most infections are caused by bacteria that may reside in the wound area, migrate from other body parts, or colonize the wound from the environment. Because of this, effective antimicrobial therapy has been one of the most important wound care mechanisms.34
Medicinal plants with antibacterial activities have been reported to be alternative sources for anti-infective medications and for fighting emerging resistant microbial strains. In the present study, all the three fractions showed activity against different gram-positive and gram-negative standard bacterial strains. The present finding is supported by previous findings.19,21 S. aureus was observed to be the most sensitive bacteria among tested organisms to all three fractions. The butanol and chloroform fractions showed bactericidal activity against S. aureus at a concentration of 125 mg/mL. The most active fraction that had activity against most of the bacteria strains tested was the n-butanol fraction. In the present study, no association was observed between antibacterial and wound healing activities.
Medicinal plants have been recognized to have a broad biological activity. In the process of wound healing, they may stimulate the production of critical cytokines such as PDGF, FGF, VEGF, TGF-, and IL-1 to accelerate epithelialization, angiogenesis, granulation tissue formation, proliferation of fibroblasts, and collagen deposition in the wounds. These comprehensive healing activities and other broad biological properties of medicinal plants may be attributed to their phytochemical contents.21,41
Qualitative phytochemical tests of aqueous, n-butanol and chloroform fractions of 80% methanol leaf extract of B. antidysenterica showed that all three fractions are rich in different phytochemicals such as alkaloids, flavonoids, terpenoids and phenols, which is in agreement with the findings of the previous studies.20,43 The biological activities of B. antidysenterica leaf shown in the present study may be attributed to these phytochemicals. Phenols, for example, have potent antioxidant and free radical scavenging activities. In this study, the aqueous fraction of 80% methanol extract of B. antidysenterica leaf was shown to have phenols, and a higher amount of phenols was reported to be present in the leaf extract.20 This high phenolic content may have contributed to the better wound healing property of this fraction through suppressing ROS and other oxidants and promoting cell proliferation. The other phenolic molecules, flavonoids, are also potential natural antioxidants shown to be present in the fractions of B. antidysenterica leaf methanol extract and with a higher amount in the aqueous fraction.19,42
Phenols, including flavonoids and terpenoids, have been shown to have antibacterial, anti-inflammatory, and astringent properties,44 which may have contributed to the wound contraction, epithelialization rate, and antibacterial activities observed in this study. Furthermore, essential oils with alkaloids in their appearance are used in a variety of medicinal settings. Essential oil extracted from Thymus vulgaris–red thyme geraniol, for example, has been shown to have cytotoxic effect against clinically isolated pseudomonas aeruginosa in investigations.45 Essential oils extracted from medicinal plants have been shown in clinical studies to have anti-acne and anti-inflammatory properties, as well as the ability to reduce in vivo erythematous lesions.46 Furthermore, essential oils from Ruta graveolens showed antifungal efficacy against clinically isolated fungus strains.47
In addition to alkaloids, other pharmacologically important phytochemicals were shown to be present in all fractions of the plant in the present study. Some of the pharmacological activities of alkaloids include antibacterial, antifungal, vasoconstriction, analgesic, and cytotoxic properties.13,48,49 The wound healing, antibacterial, and even toxic effects observed in the present study may be associated with the alkaloid content of the fractions. Therefore, both wound healing and antibacterial activities might be attributed to the secondary metabolites present in the fractions as shown by the phytochemical screening.
In conclusion, all three fractions are safe at antibacterial and wound healing doses. The aqueous fraction of 80% methanol B. antidysenterica leaf extract has wound healing activity. The study also showed that all the three fractions have antibacterial activity, with the n-butanol fraction being the most active. Furthermore, the findings from the current study support the previous reports of in-vivo wound healing and antibacterial activities of the crude extracts of B. antidysenterica. Further studies, however, should be carried out on the mechanism of wound healing and the antibacterial activities of the fractions.
Ethics Approval
Ethical clearance and permission was obtained from Addis Ababa University Research and Ethical Review Committee.
Consent for Publication
Not applicable.
Acknowledgments
The authors would like to acknowledge Addis Ababa Universities and CDT- Africa for the financial and material support to conduct this study.
Author Contributions
All authors made a significant contribution to the work reported, that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agreed to be accountable for all aspects of the work.
Disclosure
This paper is our thesis which is presented at Addis Ababa University, College of health Science, school of pharmacy, department of pharmacology and clinical pharmacy for partial fulfilment of Master’s degree in pharmacology and it is submitted in Addis Ababa university electronic thesis and dissertation repository to avoid duplication of the work by others and it is not published yet in any journal. It is possible to check the following link: [https://etd.aau.edu.et/] in Department of Pharmacology and Clinical Pharmacy School of Pharmacy College of Health Sciences Addis Ababa University. The authors report no conflicts of interest for this work.
References
1. Hemamalini K, Ramu A, Mallu G, et al. Evaluation of wound healing activity of different crude extracts of anogeissus acuminata and gymnosporia emerginata. Rasayan J Chem. 2011;4(2):466–471.
2. Velnar T, Bailey T, Smrkolj V. The wound healing process: an overview of the cellular and molecular mechanisms. J Int Med Res. 2009;37(5):1528–1542. doi:10.1177/147323000903700531
3. Olsson M, Järbrink K, Divakar U, et al. The humanistic and economic burden of chronic wounds: a systematic review. Wound Repair Regen. 2019;27(1):114–125. doi:10.1111/wrr.12683
4. Shedoeva A, Leavesley D, Upton Z, Fan C. Wound healing and the use of medicinal plants. Evid Based Complement Alternat Med. 2019;2019:1–30. doi:10.1155/2019/2684108
5. Tessema Z, Makonnen E, Debella A, Molla Y. Evaluation of in vivo wound healing and anti-inflammatory activity of 80% methanolic extract of the leaves of B. antidysentrica J. F. Mill (Simaroubaceae) in mice. Asian J Complement Altern Med. 2019;7(1):1–8.
6. Nussbaum SR, Carter MJ, Fife CE, et al. An economic evaluation of the impact, cost, and medicare policy implications of chronic nonhealing wounds. Value Health. 2018;21(1):27–32. doi:10.1016/j.jval.2017.07.007
7. Davoodi-Roodbordeii F, Afshar M, Haji Abas Tabrizi F, et al. Topical hydrogel containing Fumaria vaillantii Loisel extract enhances wound healing in rats. BMC Complement Altern Med. 2019;19(1):1–9. doi:10.1186/s12906-019-2645-y
8. Guo S, DiPietro LA. Critical review in oral biology & medicine: factors affecting wound healing. J Dent Res. 2010;89(3):219–229. doi:10.1177/0022034509359125
9. Chen D, Hou Q, Zhong L, Zhao Y, Li M, Fu X. Bioactive molecules for skin repair and regeneration: progress and perspectives. Stem Cells Int. 2019.doi:10.1155/2019/6789823
10. Rodero MP, Khosrotehrani K. Skin wound healing modulation by macrophages. Int J Clin Exp Pathol. 2010;3(7):643–653.
11. Rodrigues M, Kosaric N, Bonham CA, Gurtner GC. Wound healing: a cellular perspective. Physiol Rev. 2019;99(1):665–706. doi:10.1152/physrev.00067.2017
12. Frykberg RG, Banks J. Challenges in the treatment of chronic wounds. Adv Wound Care. 2015;4(9):560–582. doi:10.1089/wound.2015.0635
13. Ayele TT, Regasa MB, Delesa DA. Evaluation of antimicrobial activity of some traditional medicinal plants and herbs from Nekemte District against wound causing bacterial pathogens. Sci Technol Arts Res J. 2016;4(2):199.
14. Hosseinkhani A, Falahatzadeh M, Raoofi E, Zarshenas MM. An evidence-based review on wound healing herbal remedies from reports of traditional Persian medicine. J Evid Based Complementary Altern Med. 2017;22(2):334–343. doi:10.1177/2156587216654773
15. Chaves-López C, Usai D, Donadu MG, et al. Potential of Borojoa patinoi Cuatrecasas water extract to inhibit nosocomial antibiotic resistant bacteria and cancer cell proliferation in vitro. Food Funct. 2018;9(5):2725–2734. doi:10.1039/C7FO01542A
16. Bua A, Usai D, Donadu MG, et al. Antimicrobial activity of Austroeupatorium inulaefolium (H.B.K.) against intracellular and extracellular organisms. Nat Prod Res. 2018;32(23):2869–2871. doi:10.1080/14786419.2017.1385014
17. Kozics K, Bučková M, Puškárová A, Kalászová V, Cabicarová T, Pangallo D. The effect of ten essential oils on several cutaneous drug-resistant microorganisms and their cyto/genotoxic and antioxidant properties. Molecules. 2019;24(24):4570. doi:10.3390/molecules24244570
18. Mekonnen Z, Amuamuta A, Abere Y. Wound healing effect of aqueous extracts of Brucea antidysenterica and Croton marcostachyus from Northwest Ethiopia in albino mice. Afr J Pharma Ther. 2019;8(1):14–19.
19. Dilnesa A, Mekonon A, Abebe A. Phytochemical screening and antioxidant activity investigations on the crude extracts of Brucea antidysenterica leaves. Int J Res Dev. 2016;1(10):1–15.
20. Dilnesa A, Alemayehu M, Atakilt A. Green synthesis of silver nanoparticles and their antibacterial activities of the crude extracts of Brucea antidysenterica leaves. Int J Math Phys Sci Res. 2016;4(1):90–95.
21. Fentahun M, Ayele Y, Amsalu N, Alemayehu A. Antibacterial evaluation and phytochemical analysis of selected medicinal plants against some pathogenic enteric bacteria in Gozamin District, Ethiopia. J Pharmacovigil. 2017;5:6.
22. Taye B, Giday M, Animut A, Seid J. Antibacterial activities of selected medicinal plants in traditional treatment of human wounds in Ethiopia. Asian Pac J Trop Biomed. 2011;1(5):370–375. doi:10.1016/S2221-1691(11)60082-8
23. Organization for Economic Cooperation and Development (OCDE). (2008). OECD guidelines for the testing of chemicals.
24. British Pharmacopoeia. Department of Health and Social Security Scottish Home and Health Department.
25. Organization for Economic Cooperation and Development (OCDE). (2004). Organization for economic cooperation and development guidelines for testing of chemicals 434: acute dermal toxicity – fixed dose procedure.
26. Fikru A, Makonnen E, Eguale T, Debella A, Abie G. Evaluation of in vivo wound healing activity of methanol extract of Achyranthes aspera L. J Ethnopharmacol. 2012;143(2):469–474. doi:10.1016/j.jep.2012.06.049
27. Wesley JJ, Christina AJM, Chidambaranathan N, Ravikumar K. Wound healing activity of the leaves of Tribulus terrestris (Linn) aqueous extract in rats. J Pharm Res. 2009;2(5):841–843.
28. Liu H, Lin S, Xiao D, Zheng X, Gu Y, Guo S. Evaluation of the wound healing potential of Resina Draconis (Dracaena cochinchinensis) in animal models. Evid Based Complement Altern Med. 2013;(2013:1–10.
29. Wang PH, Huang BS, Horng HC, Yeh CC, Chen YJ. Wound healing. J Chin Med Assoc. 2018;81(2):94–101. doi:10.1016/j.jcma.2017.11.002
30. Kundu A, Ghosh A, Singh NK, et al. Wound healing activity of the ethanol root extract and polyphenolic rich fraction from Potentilla fulgens. Pharm Biol. 2016;54(11):2383–2393. doi:10.3109/13880209.2016.1157192
31. Barua CC, Talukdar A, Begum SA, et al. In vivo wound-healing efficacy and antioxidant activity of Achyranthes aspera in experimental burns. Pharm Biol. 2012;50(7):892–899. doi:10.3109/13880209.2011.642885
32. Hudzicki J. Kirby-Bauer disk diffusion susceptibility test protocol. Am Soc Microbiol. 2016;15:1–13.
33. Mostafa AA, Al-Askar AA, Almaary KS, Dawoud TM, Sholkamy EN, Bakri MM. Antimicrobial activity of some plant extracts against bacterial strains causing food poisoning diseases. Saudi J Biol Sci. 2018;25(2):361–366. doi:10.1016/j.sjbs.2017.02.004
34. Ullah N, Parveen A, Bano R, et al. In vitro and in vivo protocols of antimicrobial bioassay of medicinal herbal extracts: a review. Asian Pac J Trop Dis. 2016;6(8):660–667. doi:10.1016/S2222-1808(16)61106-4
35. Krishnaveni A, Thaakur SR. Pharmacognostical and preliminary phytochemical studies of Passiflora foetida. Anc Sci Life. 2008;27(3):19–23.
36. Oumer A, Bisrat D, Mazumder A, Asres K. A new antimicrobial anthrone from the leaf latex of Aloe trichosantha. Nat Prod Commun. 2014;9(7):949–952. doi:10.1177/1934578X1400900717
37. El Ayadi A, Jay JW, Prasai A. Current approaches targeting the wound healing phases to attenuate fibrosis and scarring. Int J Mol Sci. 2020;21(3):1105. doi:10.3390/ijms21031105
38. Malviya N, Jain S. Wound healing activity of aqueous extract of Radix paeoniae root. Acta Pol Pharm. 2009;66(5):543–547.
39. Beshir K, Shibeshi W, Ejigu A, Engdawork E. In vivo wound healing activity of 70% ethanolic leaf extract of Becium grandiflorum Lam. (Lamiaceae) in mice. Ethiop Pharm J. 2016;32(2):117–130. doi:10.4314/epj.v32i2.3
40. Patel S, Srivastava S, Singh MR, Singh D. Mechanistic insight into diabetic wounds: pathogenesis, molecular targets and treatment strategies to pace wound healing. Biomed Pharmacother. 2019;112:108615. doi:10.1016/j.biopha.2019.108615
41. Heil N, Bravo K, Montoya A, Robledo S, Osorio E. Wound healing activity of Ullucus tuberosus, an Andean tuber crop. Asian Pac J Trop Biomed. 2017;7(6):538–543. doi:10.1016/j.apjtb.2017.05.007
42. Pastar I, Stojadinovic O, Yin NC, et al. Epithelialization in wound healing: a comprehensive review. Adv Wound Care. 2014;3(7):445–464. doi:10.1089/wound.2013.0473
43. Inbaneson SJ, Ravikumar S, Suganthi P. In vitro antiplasmodial effect of ethanolic extracts of coastal medicinal plants along Palk Strait against Plasmodium falciparum. Asian Pac J Trop Biomed. 2012;2(5):364–367. doi:10.1016/S2221-1691(12)60057-4
44. Bahramsoltani R, Farzaei MH, Rahimi R. Medicinal plants and their natural components as future drugs for the treatment of burn wounds: an integrative review. Arch Dermatol Res. 2014;306:601–617. doi:10.1007/s00403-014-1474-6
45. Veronica A, Matthew D, Donatella U, et al. In vitro activity of essential oils against Pseudomonas aeruginosa isolated from infected hip implants. J Infect Dev Ctries. 2018;12(11):996–1001. doi:10.3855/jidc.10988
46. Mazzarello V, Gavini E, Rassu G, et al. Clinical assessment of new topical cream containing two essential oils combined with tretinoin in the treatment of acne. Clin Cosmet Investig Dermatol. 2020;13:233–239. doi:10.2147/CCID.S236956
47. Donadu MG, Peralta- Ruiz Y, Usai D, et al. Colombian essential oil of Ruta graveolens against nosocomial antifungal resistant candida strains. J Fungi. 2021;7:383. doi:10.3390/jof7050383
48. Juneja K, Mishra R, Chauhan S, Gupta S, Roy P, Sircar D. Metabolite profiling and wound-healing activity of Boerhavia diffusa leaf extracts using in vitro and in vivo models. J Tradit Complement Med. 2020;10(1):52–59. doi:10.1016/j.jtcme.2019.02.002
49. Walton EW. Topical phytochemicals: applications for wound healing. Adv Skin Wound Care. 2014;27(7):328–332. doi:10.1097/01.ASW.0000450101.97743.0f
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.