Back to Journals » Clinical Ophthalmology » Volume 17
Evaluation of Visual Outcomes and 3-Month Refractive Stability of a New Hydrophobic Acrylic Intraocular Lens
Received 2 April 2023
Accepted for publication 22 June 2023
Published 3 July 2023 Volume 2023:17 Pages 1859—1864
DOI https://doi.org/10.2147/OPTH.S415400
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Scott Fraser
Clayton Blehm,1 Brad Hall2
1Gainesville Eye Associates, Gainesville, GA, USA; 2Sengi, Penniac, NB, Canada
Correspondence: Clayton Blehm, Gainesville Eye Associates, 2061 Beverly Road, Gainesville, GA, 30501, USA, Tel +1 770-532-4444, Email [email protected]
Purpose: To determine the refractive stability of a new hydrophobic acrylic intraocular lens (IOL) when implanted bilaterally.
Methods: This was a prospective, evaluator masked, single surgeon study of 58 eyes of 29 patients. Patients were bilaterally implanted with the Clareon monofocal IOL (CNA0T0, Alcon Vision LLC). Refractive stability was evaluated between 1 and 3 months postoperatively. At 3 months postoperatively, data were also collected for binocular uncorrected and distance corrected visual acuities at distance (4 m) and intermediate (80 cm and 66 cm) and binocular defocus curve.
Results: Postoperative refraction was statistically equivalent between 1 and 3 months postoperatively (p < 0.001). Mean postoperative uncorrected distance visual acuity was − 0.01 ± 0.10 logMAR, and mean corrected distance visual acuity was − 0.04 ± 0.06 logMAR. Mean postoperative uncorrected intermediate visual acuity was 0.16 ± 0.13 logMAR and 0.24 ± 0.14 logMAR at 80 cm and 66 cm, respectively. With distance correction in place, mean visual acuity at 80cm and 60cm was 0.16 ± 0.13 logMAR and 0.23 ± 0.14 logMAR, respectively.
Conclusion: The Clareon monofocal IOL can provide stable refraction, excellent distance vision, and functional intermediate vision postoperatively.
Keywords: hydrophobic acrylic IOL, cataract surgery, Clareon
Plain Language Summary
Cataract surgery involves removing the natural opaque lens and replacing it with an artificial clear intraocular lens (IOL). There are many different types of IOLs to choose from; however, the most often implanted type of IOL is monofocal. Traditionally, these lenses provide good vision when viewing objects at a distance (such as oncoming traffic), but may not provide clear vision at near (such as reading a book) or intermediate (such as viewing a digital device). The purpose of this study was to evaluate a new monofocal IOL. Specifically, the primary purpose was to determine the refractive stability of a new monofocal IOL when implanted in both eyes. The secondary purpose was to assess the visual outcomes at distance and intermediate.
Introduction
Cataract surgery patients have high expectations of excellent visual outcomes following intraocular lens (IOL) implantation. Visual outcomes are influenced by the type of IOL, such as a monofocal, multifocal, or extended depth of focus (EDOF) IOL. Monofocal IOLs provide good distance vision and are the standard of care to which other types of IOLs are compared. Presbyopia correcting IOLs (multifocal and EDOF) are able to offer patients improved visual acuity at intermediate and near distances compared to a monofocal IOL, but at the cost of higher visual disturbances, especially using IOLs with diffractive optics.1–4 New optical designs are available in monofocal IOLs, with the aim to provide similar distance visual acuity to a standard monofocal IOL, but also improved intermediate visual acuity without the compromise of dysphotopsias.5 Functional intermediate visual acuity is increasingly important in the day-to-day lives of patients.6
The Clareon IOL is comprised of a novel hydrophobic acrylic material (Alcon Vision, LLC). Instead of phenylethyl methacrylate (PEMA), which is used in the Acrysof material (Alcon Vision, LLC), the Clareon material incorporates hydroxyethyl methacrylate (HEMA). The Clareon material also contains phenylethyl acrylate and a UV absorber, and has an increased water content (1.5% at 35°C) compared to the Acrysof material (0.4% at 35°C).7 Glistenings, first reported in 1984,8 are water filled vacuoles within the IOL material, approximately 1 to 30 μm in size.9 Although glistenings have been reported with all types of IOL materials, they were observed to be higher with hydrophobic acrylic materials.10 Studies to date have reported minimal glistenings with this new Clareon material.11,12 Distance visual acuity has also been demonstrated to be excellent.13 However, as this is a relatively new IOL, there are minimal real-world US data on the clinical outcomes with the Clareon monofocal IOL. The primary purpose of this study was to determine the refractive stability of the Clareon monofocal IOL when implanted bilaterally. The secondary purpose was to assess the visual outcomes at distance and intermediate.
Methods
This was a single arm, prospective, evaluator masked, single surgeon study of the visual outcomes and refractive stability of a new hydrophobic acrylic IOL. An institutional review board (IRB) reviewed and approved the study (Salus IRB, approval number CB-20-001). This study was registered on clinicaltrials.gov (NCT04936256), and was conducted in compliance with Good Clinical Practice (GCP), the tenets of the Declaration of Helsinki, and International Harmonization (ICH) guidelines. All subjects gave written informed consent. Data are not available for sharing.
To be eligible for the study, subjects had to be 50 years or older, candidates for bilateral cataract surgery with implantation of a non-toric IOL, and in good ocular health other than residual refractive error and cataract. Subjects were excluded if they had irregular astigmatism, previous corneal surgery, previous anterior or posterior chamber surgery, diabetic retinopathy, macular pathology, or a history of retinal detachment.
Preoperative assessments were performed using Argos (Movu, a Santec Company) for biometry, and Atlas 9000 (Carl Zeiss Meditec) for topography. All eyes were targeted for plano or the first minus power using the Barrett Universal II formula and implanted with the non-toric Clareon® monofocal IOL (CNA0T0). Preoperative planning was confirmed with intraoperative aberrometry (the ORA System® with Verifeye+ TechnologyTM, Alcon Vision LLC). Sutureless microincision phacoemulsification was performed in eyes by one experienced surgeon (CB), and their standard of care was the postoperative regimen. Refractive and visual outcomes were assessed at 1 and 3 months postoperatively. The primary endpoint of this study was refractive stability, defined as a spherical equivalent change ≤0.50 D between the 1 and 3 month postoperative visits. Secondary endpoints included binocular uncorrected and distance corrected visual acuities at distance (UDVA and CDVA at 4 m) and intermediate (UIVA and DCIVA at 80 cm and 60 cm). Binocular defocus curve was also performed at the 3 month postoperative visit. Evaluators (person conducting refraction) were masked and did not have access to the 1 month data when conducting and evaluating refractive outcomes at the 3 month visit.
All statistical analyses were performed using the statistical software R (version 4.1.2; The R Foundation for Statistical Computing, Vienna, Austria). The two one-sided equivalence test (TOST) was used to assess refractive stability (manifest refraction spherical equivalent; MRSE), using equivalence bounds of −0.5 D and 0.5 D. Comparisons of parametric data were performed using a paired t-test, while comparisons of non-parametric data were performed using the Wilcoxon signed rank test. A p-value ≤0.05 was considered significant for all statistical tests. We estimated that the study would require a sample size of at least 23 patients (46 eyes) to achieve a power of 90% and a level of significance of 5% (two sided), for detecting a mean of the differences of 0.50 D between pairs, and assuming the standard deviation of the differences to be 0.5. For additional power of the study and for any possible patients that leave the study prematurely, we aimed to recruit a total of 30 patients (60 eyes).
Results
A total of 31 subjects (62 eyes) were enrolled in the study, of which 29 subjects (58 eyes) completed the study. One subject was lost to follow up and one subject passed away (unrelated to treatment). Table 1 outlines the preoperative and patient demographics for eyes that completed the study.
 |
Table 1 Preoperative and Demographic Data |
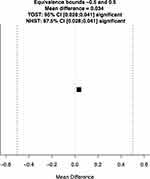
The primary endpoint of this study was refractive stability, and refractive outcomes are shown in Table 2. Sphere and MRSE were not significantly different between postoperative months 1 and 3 (p > 0.05). Differences for cylinder between postoperative month 1 (0.31 ± 0.48) and postoperative month 3 (0.16 ± 0.26) were statistically significant, but not clinically significant. Results of the TOST equivalence test are summarized in Figure 1. The mean of the differences in MRSE between 1 and 3 months postoperatively was minimal at 0.03 D. This mean difference was statistically significant using the TOST equivalence test (p < 0.001), but not clinically relevant. The MRSE at 1 and 3 months postoperatively was statistically equivalent (p < 0.001). The distribution of residual refractive errors is summarized in Table 3.
 |
Table 2 Postoperative Refractive Outcomes (n = 58) |
 |
Table 3 Distribution of Residual Refractive Error at 3 Months Postoperative (n = 58) |
Binocular uncorrected and distance corrected visual acuities at 3 months postoperatively are summarized in Table 4. Postoperative UDVA was good with 97% of subjects 20/25 or better. Postoperative UIVA was also acceptable at 80 cm and 66 cm, with 83% and 72% of subjects 20/40 or better. Visual acuities were slightly improved with distance correction in place and 100%, 86%, and 72% of subjects had CDVA (4 m), DCIVA (80 cm), and DCIVA (66 cm) 20/40 or better, respectively. Figure 2 shows the binocular defocus curve at 3 months postoperatively. At −1.25 D (80 cm equivalent), visual acuity was approximately 0.25 logMAR, while at −1.5 D (66 cm equivalent), visual acuity was approximately 0.3 logMAR.
 |
Table 4 Visual Acuities at 3 Months Postoperative (n = 29) |
 |
Figure 2 Binocular defocus curve at 3 months postoperatively. Abbreviations: D, diopters; logMAR, log of minimum angle of resolution. |
Discussion
Cataract surgery is a refractive procedure, and success of any IOL is partially determined by its refractive stability. In this study, MRSE at 1 month and 3 months postoperative were statistically equivalent, which indicates good refractive stability with the Clareon monofocal IOL. Other studies have also reported good refractive stability of the Clareon monofocal IOL, and for up to 12 months12–14 and 36 months.15 There was a slight improvement to refractive cylinder between 1 month (0.31 D) and 3 months (0.16 D) postoperatively, which was statistically significant but does not appear to be clinically relevant. In addition to providing good refractive outcomes, implantation with the Clareon monofocal IOL resulted in excellent visual acuity at distance. The percentage of subjects with binocular UDVA and CDVA 20/20 or better was 69% and 90%, respectively. Other studies of the Clareon monofocal IOL have reported similar results.11–13,15
To the best of our knowledge, this study is the first report of intermediate visual acuity with the Clareon monofocal IOL. Visual acuity at intermediate distances is attracting increased attention, as it is important for the day-to-day activities of patients.6 The slight myopia in the postoperative refractions improved UIVA compared to DCIVA, although both the binocular UIVA and DCIVA in our study were acceptable for a monofocal IOL. A recent US Food and Drug Administration (FDA) trial, using the Acrysof monofocal as a control, reported mean binocular DCIVA of 0.20 logMAR at 66 cm,16 compared to 0.23 logMAR in this study. A recent meta-analysis of enhanced monofocal IOLs5 reported that the ranges for mean binocular UIVA and DCIVA were 0.03 to 0.17 logMAR17,18 and 0.01 to 0.14 logMAR,17,19 respectively. The mean UIVA and DCIVA observed in our study fall outside the range reported by the meta-analysis, however we are not able to draw any definitive conclusions without conducting a comparative study.
To the best of our knowledge, this is the first report of a binocular defocus curve for the Clareon monofocal IOL. From the defocus curve, we would expect mean distance corrected visual acuities at 4 m (0.00 D defocus), 80 cm (−1.25 D defocus), and 66 cm (−1.5 D defocus) to be −0.01 logMAR, 0.25 logMAR, and 0.3 logMAR, respectively. However, mean, CDVA, DCIVA (80 cm), and DCIVA (66 cm) were −0.04 logMAR, 0.16 logMAR, and 0.23 logMAR, respectively. Binocular defocus curve testing was the last measurement subjects had at the 3 month postoperative visit. It is possible that dry eye reduced the visual acuity of subjects during defocus curve testing, which could explain this difference. However, subjects were given rewetting drops as needed. The binocular defocus curve results at 0 D, −1.25 D, and −1.5 D in our study also do appear to be within the ranges reported in studies of other monofocal IOLs.15,20 Other studies have reported differences between defocus curves at −1.50 D compared to the actual visual acuity measurement at 66 cm.21,22
We acknowledge that having a single-arm is a limitation of our study. Secondary endpoints were intended to be descriptive, with no statistical inference. Future studies comparing visual acuities after implantation with the Clareon monofocal to implantation with another monofocal or enhanced monofocal are warranted. Another limitation of this study was the follow-up time period. We are not able to draw conclusions about the long-term refractive stability of this lens, although our results suggest that refractive is stable up to 3 months postoperatively.
In conclusion, the Clareon monofocal IOL provides stable refraction, excellent distance vision, and functional intermediate vision postoperatively. This IOL may be a good option for patients desiring good distance vision and functional intermediate vision.
Funding
This study was supported with an investigator-initiated study grant (64146113) from Alcon Vision, LLC, Fort Worth, TX, USA.
Disclosure
Brad Hall reports that he has received consulting fees from Ace Vision Group. The authors report no other conflict of interest for this work.
References
1. Schallhorn JM. Multifocal and extended depth of focus intraocular lenses: a comparison of data from the United States food and drug administration premarket approval trials. J Refract Surg. 2021;37:98–104. doi:10.3928/1081597X-20201111-02
2. Cao K, Friedman DS, Jin S, et al. Multifocal versus monofocal intraocular lenses for age-related cataract patients: a system review and meta-analysis based on randomized controlled trials. Surv Ophthalmol. 2019;64:647–658. doi:10.1016/j.survophthal.2019.02.012
3. Pedrotti E, Carones F, Aiello F, et al. Comparative analysis of visual outcomes with 4 intraocular lenses: monofocal, multifocal, and extended range of vision. J Cataract Refract Surg. 2018;44:156–167. doi:10.1016/j.jcrs.2017.11.011
4. Pedrotti E, Carones F, Talli P, et al. Comparative analysis of objective and subjective outcomes of two different intraocular lenses: trifocal and extended range of vision. BMJ Open Ophthalmol. 2020;5:e000497. doi:10.1136/bmjophth-2020-000497
5. Wan KH, Au ACK, Kua WN, et al. Enhanced monofocal versus conventional monofocal intraocular lens in cataract surgery: a meta-analysis. J Refract Surg. 2022;38:538–546. doi:10.3928/1081597X-20220707-01
6. MacRae S, Holladay JT, Glasser A, et al. Special report: American Academy of Ophthalmology task force consensus statement for extended depth of focus intraocular lenses. Ophthalmology. 2017;124:139–141. doi:10.1016/j.ophtha.2016.09.039
7. Maxwell A, Suryakumar R. Long-term effectiveness and safety of a three-piece acrylic hydrophobic intraocular lens modified with hydroxyethyl-methacrylate: an open-label, 3-year follow-up study. Clin Ophthalmol. 2018;12:2031–2037. doi:10.2147/OPTH.S175060
8. Ballin N. Glistenings in injection-molded lens. J Am Intraocul Implant Soc. 1984;10:473. doi:10.1016/S0146-2776(84)80052-X
9. Kato K, Nishida M, Yamane H, Nakamae K, Tagami Y, Tetsumoto K. Glistening formation in an AcrySof lens initiated by spinodal decomposition of the polymer network by temperature change. J Cataract Refract Surg. 2001;27:1493–1498. doi:10.1016/S0886-3350(01)00895-1
10. Hayashi K, Hirata A, Yoshida M, Yoshimura K, Hayashi H. Long-term effect of surface light scattering and glistenings of intraocular lenses on visual function. Am J Ophthalmol. 2012;154(2):240–251 e242. doi:10.1016/j.ajo.2012.03.011
11. Stanojcic N, O’Brart D, Hull C, et al. Visual and refractive outcomes and glistenings occurrence after implantation of 2 hydrophobic acrylic aspheric monofocal IOLs. J Cataract Refract Surg. 2020;46:986–994. doi:10.1097/j.jcrs.0000000000000201
12. Lehmann R, Maxwell A, Lubeck DM, Fong R, Walters TR, Fakadej A. Effectiveness and safety of the Clareon monofocal intraocular lens: outcomes from a 12-month single-arm clinical study in a large sample. Clin Ophthalmol. 2021;15:1647–1657. doi:10.2147/OPTH.S295008
13. Oshika T, Sasaki N; Clinical Study Group on New Intraocular L, Delivery S. One-year multicenter evaluation of a new hydrophobic acrylic intraocular lens with hydroxyethyl methacrylate in an automated preloaded delivery system. J Cataract Refract Surg. 2022;48(3):275–279. doi:10.1097/j.jcrs.0000000000000746
14. Negishi K, Masui S, Torii H, Nishi Y, Tsubota K, Mohan RR. Refractive stability of a new single-piece hydrophobic acrylic intraocular lens and corneal wound repair after implantation using a new automated intraocular lens delivery system. PLoS One. 2020;15:e0238366. doi:10.1371/journal.pone.0238366
15. Nuijts R, Bhatt U, Nanavaty MA, Roberts TV, Peterson R, Teus MA. Three-year multinational clinical study on an aspheric hydrophobic acrylic intraocular lens. J Cataract Refract Surg. 2023. doi:10.1097/j.jcrs.0000000000001173
16. McCabe C, Berdahl J, Reiser H, et al. Clinical outcomes in a United States registration study of a novel extended depth of focus intraocular lens with a nondiffractive design. J Cataract Refract Surg. 2022;48(11):1297–1304. doi:10.1097/j.jcrs.0000000000000978
17. Huh J, Eom Y, Yang SK, Choi Y, Kim HM, Song JS. A comparison of clinical outcomes and optical performance between monofocal and new monofocal with enhanced intermediate function intraocular lenses: a case-control study. BMC Ophthalmol. 2021;21:365. doi:10.1186/s12886-021-02124-w
18. Lopes D, Loureiro T, Carreira R, et al. Comparative evaluation of visual outcomes after bilateral implantation of an advanced or conventional monofocal intraocular lens. Eur J Ophthalmol. 2022;32:229–234. doi:10.1177/1120672121995343
19. Nanavaty MA, Ashena Z, Gallagher S, Borkum S, Frattaroli P, Barbon E. Visual acuity, wavefront aberrations, and defocus curves with an enhanced monofocal and a monofocal intraocular lens: a prospective, randomized study. J Refract Surg. 2022;38:10–20. doi:10.3928/1081597X-20211109-02
20. Monaco G, Gari M, Di Censo F, Poscia A, Ruggi G, Scialdone A. Visual performance after bilateral implantation of 2 new presbyopia-correcting intraocular lenses: trifocal versus extended range of vision. J Cataract Refract Surg. 2017;43:737–747. doi:10.1016/j.jcrs.2017.03.037
21. US FDA. AcrySofTM IQ VivityTM extended vision Intraocular Lens (IOL): summary of safety and effectiveness data. Available from: https://www.accessdata.fda.gov/cdrh_docs/pdf/P930014S126B.pdf.
22. US FDA. TECNIS® symfony extended range of vision IOL. Available from: https://www.accessdata.fda.gov/cdrh_docs/pdf/p980040s065d.pdf.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.