Back to Journals » Clinical Ophthalmology » Volume 14
Evaluation of Vision-Related Quality of Life after Autologous Internal Limiting–Membrane Transplantation for Refractory Macular Holes
Authors Yuan D , Zhang W, Yuan S, Xie P, Liu Q
Received 2 May 2020
Accepted for publication 2 July 2020
Published 23 July 2020 Volume 2020:14 Pages 2079—2085
DOI https://doi.org/10.2147/OPTH.S259642
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Dongqing Yuan, Weiwei Zhang, Songtao Yuan, Ping Xie, Qinghuai Liu
Department of Ophthalmology, First Affiliated Hospital of Nanjing Medical University, Nanjing, Jiangsu Province, People’s Republic of China
Correspondence: Qinghuai Liu
Department of Ophthalmology, First Affiliated Hospital of Nanjing Medical University, 300 Guangzhou Road, Nanjing, Jiangsu Province 210029, People’s Republic of China
Email [email protected]
Purpose: To evaluate the vision-related quality of life of vitrectomy combined with autologous internal limiting membrane (ILM) transplantation for refractory macular holes (MHs).
Methods: There were 40 eyes with refractory MHs included, and all eyes received 23 G vitrectomy and ILM peeling with autologous ILM transplantation. Preoperative and postoperative basic conditions were recorded. The Chinese version of the vision-related quality-of-life scale was used to evaluate patients after operation. Quality of life, postoperative visual acuity, and size of MHs before operation were assessed with Spearman rank correlations.
Results: All patients were followed up for 3 months after surgery. Mean postoperative best-corrected visual acuity had significantly improved after surgery. Vision-related quality of life of patients after surgery was closely related to the MH index, but negatively correlated with best-corrected visual acuity before and after surgery.
Conclusion: The anatomical structure of refractory MHs with ILM peeling combined with autologous ILM transplantation was largely reduced, and the visual acuity of patients improved significantly.
Keywords: refractory macular hole, internal limiting membrane transplantation, vision-related quality of life
Introduction
Macular hole (MH) refers to the continuous interruption of the retinal neuroepithelial layer in the macular zone, which causes metamorphopsia and decreased vision. Presently, MHs are generally treated with vitrectomy combined with internal limiting membrane (ILM) peeling.1 However, for patients with complex traumatic MHs, large MHs (diameter >600 µm), high-myopia MHs with retinal detachment, and other refractory MHs, it may be difficult with simple ILM peeling to achieve stage I rupture closure, and postoperative visual function improvement is limited.2 In view of these refractory MHs, Morizane et al first reported that autologous transplantation of the ILM may contribute to improved anatomic and visual outcomes in the treatment of refractory MHs.3 De Novelli et al also found that the methods of ILM tamponade, inverted ILM flap, and autologous ILM transplantation have relatively better effects on hole closure.4 Our previous study reported a new surgical technique of uninverted pedicle ILM transposition for treatment of eyes with large MHs.5 Our findings indicated that transposition resulted in a high incidence of anatomic closure with good visual outcome for the treatment of large MHs. However, for patients with refractory MHs, vision-related quality of life (QoL) was not just about the closure rate of MHs: it was about visual activity and quality, such as contrast sensitivity. Many studies have reported that the tiled transplantation ILM pedicle flap technique is more advantageous than the inverted ILM flap technique, because the microenvironment of the former is more similar to normal physiological conditions, eg, the MHs directly contact the same surface of the ILM in the same way as normal physiological conditions.6,7 The tiled transplantation ILM pedicle flap technique means the ILM flap is covered in the same direction. Our results have also indicated that visual acuity (VA) increases with autologous ILM transplantation, but vision-related QoL was not evaluated. In this study, patients with refractory MHs, including MHs with large diameter, high-myopia MHs, and secondary MHs, were treated with vitrectomy of ILM peeling combined with autologous ILM transplantation. The Chinese version of the vision-related QoL 25 (CVRQoL-25) chart was used to evaluate the effect of the operation on visual function improvement in patients.8
Methods
This was a retrospective observational study at the First Affiliated Hospital of Nanjing Medical University. This study was performed following the guidelines of the Declarations of Helsinki and Tokyo for humans and approved by the Ethics Committee of the First Affiliated Hospital of Nanjing Medical University (approval 2017-SR-223). Forty patients with refractory MHs were enrolled from January 2017 to December 2018. Among them, 25 patients had large-diameter MHs (>600 µm), 12 high-myopia MHs, and three secondary traumatic MHs. There were 17 male (17 eyes) and 23 female (23 eyes). The average age of patients was 57.6±7.8 years. All patients received tests for best-corrected VA (BCVA), slit-lamp examinations, ophthalmoscopy after mydriasis, A/B ultrasound examination, eye-ground photography stack examination, and spectral-domain optical coherence tomography (OCT; Cirrus; Carl Zeiss Meditec, Dublin, CA) before and after operation. Visual examination was conducted with Snellen visual chart, and results converted into logarithm of theminimum angle of resolution (logMAR). After OCT scana of the macular region, central retinal thickness (CRT), base diameter, and minimum diameter for MHs were measured and MH index (MHI) values calculated afterward. MHI equals the ratio of the edge thickness of hole and the diameter of the hole base.
All the patients received 23 G vitrectomy with ILM peeling combined with autologous ILM transplantation successfully. The surgical method5 was three channels through pars plana corporis ciliaris being established and the vitreous body being excised. Indocyanine green staining was performed for 15 seconds. Afterward, intraocular forceps were used to tear the ILM at least two papillary diameters in the macular area, and a pedicle attached to the superior temporal retina was left. The free temporal edge of the ILM was grasped and the whole pedicle ILM rotated till the nasal part of the ILM fully covered the MH. Cover the ILM was covered directly on MHs in the former direction in an uninverted fashion. A little perfluorodecalin was injected to fix the ILM to avoid floating and moving. Then, the perfluorodecalin was sucked out after fluid–air exchange. At the same time, the peeled ILM was fixed in the macular region. Finally, the vitreous chamber was filled with silicone oil. The patient was maintained in a prone position after surgery. Phacoemulsification combined with vitrectomy treatment was performed in 36 patients with cataracts.
Silicone oil was removed 3–6 months after surgery and all patients followed until at least it had been removed. Parameters mainly included postoperative BCVA, intraocular pressure, postmydriasis funduscopy, and OCT for closure of the MHs. Evaluation of the CVRQoL-25) performed to investigate the influence of transplantation of ILM on vision-related life quality of patients with refractory MHs.
The CVRQoL-25 questionnaire is composed of 12 dimensions and 26 items.9 The 12 dimensions are “holistic health conditions”, “general vision”, “ophthalmodynia”, “close-range activity”, “remote activity”, “drive”, “surrounding vision”, “colour vision”, “limitation of social role”, “degree of dependence”, “social function”, and “mental health conditions”. There are six grades (A, B, C, D, E, and F) for each dimension, the first five are scoring 100, 75, 50, 25, and 0, respectively, while F is deemed “no response”. The higher the score, the better the survival quality of the project. No corresponding situation was regarded as deficient or not counted in final-score statistics. For example, if colour perception dimension were missing, the general score was the average value of the other dimensions. SPSS 17.0 was used for statistical analysis. Measurement data was expressed as means ± SD and count data as rate (%). Descriptive statistics and independent-sample t-tests were used to compare measurement data and Spearman rank-correlation analysis used to compare correlations between parameters. P<0.05 was taken as statistically significant.
Results
After the last follow-up, 38 patients with MHs had them anatomically closed, with a holes-closure rate of 95%. The two unclosed eyes had significant MHI improvement (0.64–0.93 and 0.39–0.78, respectively). One of the two unclosed eyes had a large-diameter MH (1,000 μm), and the other had a high-myopia MH. Neither patient had further surgery after our treatment, as they both had improved MHI scores. Preoperative logMAR BCVA was 1.52±0.29 and MHI 0.51±0.18. At 3 months after surgery, logMAR BCVA was 1.09±0.33 and CRT 160.05±14.88 μm. Overall average scores on the CVRQoL-25 before surgery and 3 months after were 57.28±6.63 and 71.50±8.81, respectively (Table 1). The independent t-test result showed that postoperative VA had improved significantly (t=6.234, P=0). Mean general CVRQoL-25 score had also increased (t=−8.162, P=0). Spearman rank-correlation analysis showed that postoperative CVRQoL-25 scores were negatively correlated with preoperative logMAR BCVA (r=−0.495, P=0.001; Figure 1) and postoperative logMAR BCVA (r=−0.760, P=0; Figure 2). It was also positively correlated with preoperative MHI (r=0.375, P=0.017; Figure 3) and postoperative CRT (r=0.414, P=0.008; Figure 4) values.
 |
Table 1 Comparison of the Visual Acuity, MHI, CRT, and CVRQoL-25 Scores Before and After Surgery |
 |
Figure 1 Correlation between postoperative CVRQoL-25 scores and preoperative logMAR BCVA. |
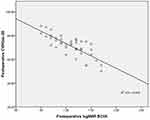 |
Figure 2 Correlation between postoperative CVRQoL-25 scores and postoperative logMAR BCVA. |
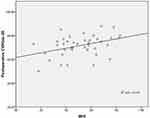 |
Figure 3 Correlation between postoperative CVRQoL-25 and preoperative MHI scores. |
 |
Figure 4 Correlation between postoperative CVRQoL-25 scores and postoperative CRT. |
Discussion
ILM peeling combined with uninverted pedicle autologous ILM transplantation is a new option to treat refractory MHs. Ding et al recently indicated that this method can significantly increase the closure rate of MHs.10 However, only clinical anatomical reduction of MHs cannot completely reflect the visual functional recovery of patients. Therefore, improvement in VA and patients’ subjective sensation of QoL after surgery are also key factors in evaluating the success of surgery. In this research, CVRQoL-25 scores and recovery status of MHs were assessed to evaluate the efficacy of ILM peeling combined with transplantation on refractory MHs and provide a new direction for prognosis assessment of such diseases.
Many studies have reported changes in the severity of metamorphopsia were significantly relevant to changes in CVRQoL-25 composite score, but changes in other variables were not, including BCVA and contrast sensitivity in MH and epiretinal membrane.11,12 A later study indicated that visual function and CVR-QoL had improved significantly after successful MH surgery.13 We found that CVRQoL-25 was negatively correlated with pre- and postoperative logMAR BCVA. In addition, postoperative logMAR BCVA was also significantly improved. Single ILM peeling for refractory MHs might be ineffective for anatomical reduction, owing to the complicated pathological structure and/or complications (such as choroidal atrophy, retinoschisis, traumatic choroidal rupture) for refractory MHs. In recent years, ILM flap surgery or autologous ILM transplantation has been increasingly applied in refractory MH treatment. In 2010, Michalewska et al first reported ILM flap surgery and compared its curative effect with traditional surgery on treating large-diameter MHs >400 μm.14 Their results showed that the ILM flap–surgery group had a higher MH-closure rate and better vision prognosis than the traditional surgery group. Compared with ILM flap, autologous ILM transplantation has a wider range of applications, especially in patients with MHs not closed after the first ILM peeling and where ILM flap surgery cannot be performed. Morizane et al used this method to treat ten patients with refractory MHs, of which nine patients had MH closure and postoperative VA was significantly improved.3 About 80% of patients had VA improved by more than 0.2. Repeated surgery with the inverted ILM flap technique was published for the first time in 2018 by Michalewska et al.15 They indicated that repeat surgery with the inverted ILM flap technique was an effective method of treatment and silicone oil improved anatomical outcome after second surgery, but did not influence visual results. In our technique, we peeled but did not remove the ILM around the MH. Instead, the pedicle of the ILM attached to the retina allows us to cover the MH in an uninverted way, which is more physiologically natural. The ILM is characterized by a smooth vitreal side and an undulated retinal side, and the retinal side is found with Müller cell debris on removed ILM specimens.16 Therefore, the retinal side of the ILM, if it is to cover the MH, would theoretically provide a more favorable structure for glia proliferation and macular closure. We found that 38 of the 40 patients achieved anatomical reduction of MH successfully — 95%. Also, postoperative VA had significantly improved. We also found that vision-related QoL was closely associated with patients’ VA. The surgery improved the patients’ VA and greatly improved their QoL.
The MHI is commonly used to assess the degree of deformation degree in MHs. The larger the MHI value, the smaller the preoperative deformation of the MH is, suggesting that postoperative visual recovery should be good. Kusuhara et al showed that the MHI was closely related to postoperative BCVA.17 The visual prognosis of the MHI >0.5 group was significantly better than that of the MHI <0.5 group. In this study, we found that patients’ vision-related QoL was positively correlated with preoperative MHI, and the larger the MHI value, the higher the patients’ postoperative vision-related QoL. Therefore, it is believed that the MHI of MHs can affect vison-related QoL of patients to a certain extent. The MHI can also be used as a prognostic indicator for refractory MHs.
The thickness of macula foveae in patients that accepted autologous ILM transplantation was evaluated by OCT. Postoperative CRT was positively correlated with postoperative visual quality. It is suggested that the increase in postoperative CRT thickness caused improvement in visual quality. Recent studies have demonstrated that during MH closure, Müller cells and other glial cells proliferate and close holes in a bridge-like proliferation to repair damaged photoreceptor cells, leading to recovery of macula foveae.18 Other research has suggested that closure of MHs is due to the removal of traction from photoreceptor cells by surgery, such that cells can be repositioned to achieve the closure.19 Vieregge et al evaluated long-term changes in functional and structural outcomes after successful repair of large MHs with ILM flap techniques.20 They found further improvement in BCVA as further microstructural regeneration of the retina, and a decrease in ellipsoid-zone defects over time. In this study, we speculated that the ILM was implanted as a scaffold above the MHs and photoreceptors repositioned by the proliferation of glial cells to promote repair of the retinal neuroepithelial layer, thus promoting MH healing. In addition, some studies have suggested that the transplanted ILM can reconstruct the lacuna between the retinal neuroepithelial layer and the pigment epithelial layer, improving the pump function of the pigment epithelial cells and promoting the healing of MHs.21
In conclusion, ILM peeling combined with autologous ILM transplantation can relieve the traction of MHs, and its mechanism might provide support for proliferation of Müller cells and restoring function to photoreceptor cells, significantly increasing patients’ VA and improving their vision-related QoL. It is preferable to treat refractory MHs with a combination of ILM peeling and autologous ILM transplantation. However, there is a lack of large-scale clinical trials at present. In addition, further research is needed to get to know the prognosis of ILM transplantation and damage of surgery to the retina to evaluate its clinical efficacy.
Abbreviations
ILM, internal limiting membrane; BCVA, best-corrected visual acuity; CRT, central retinal thickness; OCT, optical coherence tomography; MHI, MH index; CVRQoL-25, Chinese version of vision-related quality of life 25 (questionnaire).
Ethics Approval and Consent to Participate
This study was approved by the Ethics Committee of the First Affiliated Hospital of Nanjing Medical University (approval 2017-SR-223) and conducted in accordance with the Declarations of Helsinki and Tokyo for humans. Written informed consent was obtained from all enrolled participants.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data, took part in drafting the article or revising it critically for important intellectual content, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors have no conflicts of interest to declare.
References
1. Chatziralli IP, Theodossiadis PG, Steel DHW. Internal limiting membrane peeling in macular hole surgery; why, when, and how? Retina. 2018;38(5):870–882. doi:10.1097/IAE.0000000000001959
2. Pires J, Nadal J, Gomes NL. Internal limiting membrane translocation for refractory macular holes. Br J Ophthalmol. 2017;101(3):377–382. doi:10.1136/bjophthalmol-2015-308299
3. Morizane Y, Shiraga F, Kimura S, et al. Autologous transplantation of the internal limiting membrane for refractory macular holes. Am J Ophthalmol. 2014;157(4):861–9.e1. doi:10.1016/j.ajo.2013.12.028
4. De Novelli FJ, Preti RC, Ribeiro Monteiro ML, Pelayes DE, Junqueira Nóbrega M, Takahashi WY. Autologous internal limiting membrane fragment transplantation for large, chronic, and refractory macular holes. Ophthalmic Res. 2015;55(1):45–52. doi:10.1159/000440767
5. Hu Z, Ye X, Lv X, et al. Non-inverted pedicle internal limiting membrane transposition for large macular holes. Eye. 2018;32(9):1512–1518. doi:10.1038/s41433-018-0107-2
6. Wang LP, Sun WT, Lei CL, Deng J. Clinical outcomes with large macular holes using the tiled transplantation internal limiting membrane pedicle flap technique. Int J Ophthalmol. 2019;12(2):246–251. doi:10.18240/ijo.2019.02.10
7. Takai Y, Tanito M, Sugihara K, Ohira A. The role of single-layered flap in temporal inverted internal limiting membrane flap technique for macular holes: pros and cons. J Ophthalmol. 2019;2019:5737083. doi:10.1155/2019/5737083
8. Ge LN, Zhang X, Shen LJ. Metamorphopsia and vision-related quality of life and its influencing factor after surgical treatment of idiopathic macular hole. Chinese J Ocular Fundus Dis. 2017;33(2):153–156.
9. Huang J, Liu XL. Development and evaluation of chinese version vision-related quality of life questionnaire-25. Chin J Optom Ophthalmol Vis Sci. 2016;18(11):660–664.
10. Ding C, Li S, Zeng J. Autologous neurosensory retinal transplantation for unclosed and large macular holes. Ophthalmic Res. 2019;61(2):88–93. doi:10.1159/000487952
11. Okamoto F, Okamoto Y, Fukuda S, Hiraoka T, Oshika T. Vision-related quality of life and visual function after vitrectomy for various vitreoretinal disorders. Invest Ophthalmol Vis Sci. 2010;51(2):744–751. doi:10.1167/iovs.09-3992
12. Fukuda S, Okamoto F, Yuasa M, et al. Vision-related quality of life and visual function in patients undergoing vitrectomy, gas tamponade and cataract surgery for macular hole. Br J Ophthalmol. 2009;93(12):1595–1599. doi:10.1136/bjo.2008.155440
13. Wang Y, Liang X, Gao M, Liu J, Liu L, Liu W Vision-related quality of life after pars plana vitrectomy with or without combined cataract surgery for idiopathic macular hole patients. Int Ophthalmol. 39 2775–2783 doi:10.1007/s10792-019-01124-6
14. Michalewska Z, Michalewski J, Adelman RA, Nawrocki J. Inverted internaI limiting membrane fIap technique for large macular holes. Ophthalmology. 2010;117(10):2018–2025. doi:10.1016/j.ophtha.2010.02.011
15. Michalewska Z, Nawrocki J. Repeat surgery in failed primary vitrectomy for macular holes operated with the inverted ILM flap technique. Ophthalmic Surg Lasers Imaging Retina. 2018;49(8):611–618. doi:10.3928/23258160-20180803-09
16. Steel DHW, Dinah C, White K, Avery PJ. The relationship between a dissociated optic nerve fibre layer appearance after macular hole surgery and Muller cell debris on peeled internal limiting membrane. Acta Ophthalmol. 2017;95:153–157. doi:10.1111/aos.13195
17. Kusuhara S, Teraoka Escaño MF, Fujii S, et al. Prediction of postoperative visual outcome based on hole configuration by optical coherence tomography in eyes with idiopathic macular holes. Am J Ophthalmol. 2004;138(5):709–716. doi:10.1016/j.ajo.2004.04.063
18. Bringmann A, Pannicke T, Grosche J, et al. Müller cells in the healthy and diseased retina. Prog Retin Eye Res. 2006;25(4):397–424. doi:10.1016/j.preteyeres.2006.05.003
19. Hangai M, Ojima Y, Gotoh N, et al. Three-dimensional imaging of macular holes with high-speed optical coherence tomography. Ophthalmology. 2007;114(4):763–773. doi:10.1016/j.ophtha.2006.07.055
20. Vieregge M, Valmaggia C, Scholl HPN, Guber J. Microstructural retinal regeneration after internal limiting membrane flap surgery for repair of large macular holes: a 1-year follow-up study. Int Ophthalmol. 2019;39(6):1277–1282. doi:10.1007/s10792-018-0941-z
21. Alkabes M, Mateo C. Macular buckle technique in myopic traction maculopathy: a 16-year review of the literature and a comparison with vitreous surgery. Graefes Arch Clin Exp Ophthalmol. 2018;256(5):863–877. doi:10.1007/s00417-018-3947-3
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
