Back to Journals » Nature and Science of Sleep » Volume 14
Evaluation of Vestibular Function in Patients Affected by Obstructive Sleep Apnea Performing Functional Head Impulse Test (fHIT)
Authors Pace A , Milani A, Rossetti V, Iannella G, Maniaci A , Cocuzza S , Alunni Fegatelli D, Vestri A, Magliulo G
Received 1 November 2021
Accepted for publication 18 February 2022
Published 17 March 2022 Volume 2022:14 Pages 475—482
DOI https://doi.org/10.2147/NSS.S346241
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ahmed BaHammam
Annalisa Pace,1,2 Alessandro Milani,1 Valeria Rossetti,1 Giannicola Iannella,1– 3 Antonino Maniaci,4 Salvatore Cocuzza,4 Danilo Alunni Fegatelli,5 Annarita Vestri,5 Giuseppe Magliulo1
1Organi di Senso Department, Sapienza University, Rome, Italy; 2Scienze Chirurgiche Department, Sapienza University, Rome, Italy; 3Department of Head-Neck Surgery, Morgagni Pierantoni Hospital, Forlì, Italy; 4Otorinolaringoiatria Department, University of Catania, Catania, Italy; 5Department of Public Health and Infectious Diseases, Sapienza University, Rome, Italy
Correspondence: Annalisa Pace, Organi di Senso Department, Sapienza University, Viale dell’Università 33 Rome, 00165, Italy, Tel +393203581431, Fax +390649976817, Email [email protected]
Purpose: Obstructive sleep apnoea (OSA) is a common disease with significantly related complications. Since a connection between the vestibular nucleus and sleep regulator pathways has been demonstrated, vestibular evaluation in OSA patients was partially studied and none used functional head impulse test (fHIT) for this purpose. This paper aimed at evaluating the vestibular function in patients affected by OSA using fHIT, selecting patients who did not present any other related to cardiovascular, neurological, or metabolic diseases.
Patients and Methods: Patients enrolled had a diagnosis of OSA by polysomnography type III and were cataloged according to American Association of Sleep Medicine criteria. Each patient underwent fHIT. Statistical significance was set at 0.05.
Results: A total of 85 patients were enrolled in the study of which 50 had a diagnosis of OSA and were included in the case group, while 35 belonged to the control group. In 88.6% of subjects of the case group was evidenced a vestibular impairment with a substantial difference between the two study groups (p< 0.05).
Conclusion: The results show that the incidence of vestibular lesions in patients with obstructive sleep apnoea is underestimated and that fHIT can identify these lesions early.
Keywords: obstructive sleep apnea, vestibular system, functional head impulse test, vestibular ocular reflex, sleep regulator pathways
Introduction
Obstructive sleep apnoea (OSA) is a common disease that involves about 9% of women and 24% of men in the general population with a high economic impact.1 In the last decades, it has become a highly relevant topic in international literature owing to its significant complications. OSA is characterized by intermittent hypoxia due to upper airways collapse and consequent cardiovascular, metabolic, and neurological disorders.2
Currently, the main investigations are focused on OSA complications (metabolic, neurological, and cardiovascular), and on the relationship between the poor quality of sleep and an increased risk of falls, especially in older patients.3 In contrast, a limited number of studies investigated the relationship with vestibular impairment, since a connection between the vestibular nucleus (VN) and sleep regulator pathways has been demonstrated.4,5
In the literature, vestibular evaluation in OSA patients was performed only with vHIT (video Head Impulse Test) or VEMPs (vestibular evoked myogenic potentials) and, to the best of our knowledge, none used fHIT (functional Head Impulse Test) for this purpose. Moreover, none studied the association between vestibular disorders and OSA patients, avoiding the bias of other chronic simultaneous comorbidities.6–9
This paper aimed at evaluating the vestibular function in patients affected by OSA using fHIT, selecting patients who did not present any other related cardiovascular, neurological, or metabolic diseases.
Patients and Methods
This descriptive cross-sectional study was designed at the Organi di Senso Department of Sapienza University of Rome between April 2020 and April 2021 using a convenience sampling strategy. It was approved by the local Sapienza University ethical committee (RIF: 6267) following the principles of the Declaration of Helsinki. Informed consent was signed by each patient enrolled in the study.
The enrolled subjects were older than 18 years with a suspicion of OSA and did not present any cardiovascular, metabolic, or neurological disease. They had never previously undergone any ENT surgical procedure and did not refer to any history of audiological or vestibular disorders or current imbalance symptomatology (Dizziness Inventory Scale <40).8 Patients who received domiciliary therapy for OSA (continuous positive airway pressure, oral appliance, nose device) were also excluded.
Data of patients were collected in a database: sex, height, weight, age. Body mass index (BMI) was calculated. All patients underwent ENT examination by type III sleep study device, according to the 2017 AASM classification.10 Devices was assembled and set by an ENT specialist who tested its functioning.
The device collected the following data: time of sleep, respiratory movement of thorax and abdomen, respiratory airflow, heart rate, arterial oxygen saturation, and patients’ position.
The data were analyzed following AASM criteria.10 Apnea was defined as a ≥ 10-seconds reduction in the airflow by at least 90% from baseline.10
Hypopnea was defined as ≥ 10-seconds reduction in the airflow by at least 30% from baseline associated with either arousal or a 3% O2 saturation drop. AHI score (Apnea-Hypopnea Index)10 was estimated by the mean number of apnea and hypopnea events in an hour of sleep.
Two different authors (AP e AM) checked and reviewed all recordings and a third author (GM) performed random quality checks reanalyzing all data codified in the database.
A considerable amount of data was collected from each PSG recording: AHI (supine and not supine); oxygen desaturation index (ODI; supine and not supine); mean SpO2 (supine and not supine).
Finally, the AHI score (mean between supine and not supine) was considered as the index of severity of OSA disease, following AASM criteria.10
Healthy patients were considered those with an AHI value <5/h. On the contrary, OSA patients were classified into three groups: mild OSA (AHI 5/h and <15/h); moderate OSA (AHI 15/h and <30/h); severe OSA (AHI >30/h).
Each patient underwent Functional Head Impulse test (BeOnSolution Society, Zero Branco, Italy) (Figures 1–3). This test is based on the ability of a patient to recognize an optotype that is presented during impulse head rotations at varying angular accelerations.11
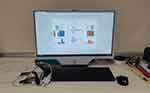 |
Figure 1 functional Head Impulse Test (fHIT) setting. |
 |
Figure 2 Hardware of fHIT. (A) Gyroscope; (B) keyboard with 8 Landolt C Optotypes. |
 |
Figure 3 Hardware of fHIT mounted on patient. |
The patient was located in a dark room seated at a distance of 150 cm from a monitor connected to the fHIT device. Before the beginning of the test, visual acuity was checked using a distance-scaled white Landolt C Optotypes display on the screen. Only patients with normal visual acuity of 0.2logMAR or with a correcting value ≤0.5 logMAR were enrolled.
Mounted gyroscope was placed on the head of the patient to measure the angular velocity of movement. One trained operator (AM) operated the fHIT. He put both hands on the patient’s head dispensing impulses in a specific direction, that consisted of brief, small rotatory movements in the plane of each semicircular canal pair (left and right horizontal; left anterior-right posterior; right anterior-left posterior). The patient had a keyboard with 8 Landolt C Optotypes to push following the one which appeared on the black screen monitor for 80ms. The operator performed 20 head impulses for each direction for each semicircular canal plane.12
fHIT software identified the percentage of correct response (%CA) for each and a value <80% of correct responses was considered abnormal.12–14
Numerical variables were summarized as mean and standard deviation or median and interquartile range, respectively, according to the distribution of the data; categorical variables were represented as frequencies and proportions. A Scatter plot was used to describe the relationship between AHI and ODI and the strength of the linear relationship was measured using Pearson’s correlation coefficient. Box plots were used to compare Z scores between healthy and pathological subjects, followed by Wilcoxon Rank-Sum tests to assess statistical significance.
Logistic regression was used to evaluate the association between the diagnosis of OSA and the AHI classification adjusted by age and BMI. Statistical significance was set at 0.05. All the analyses were performed using the statistical software R (version 4.0.4).
Results
A total of 85 patients were enrolled in the study (53 males and 32 females) of which 50 (28M and 22 F; mean age 45.8) had a diagnosis of OSA and were included in the “case group”. On the contrary, 35 patients (25M and 10F; mean age 51.2) without OSA diagnosis were enrolled in the “control group”. The data collected about weight, height, and BMI are reported in Table 1.
 |
Table 1 Patients Characteristics, Results of PSG and fHIT |
Patients belonging to the case group were sub-classified according to AASM: 7 patients (14.0%) had mild OSA; 18 patients (36.0%) had moderate OSA and 25/50 (50.0%) had severe OSA.
fHIT analysis showed an impaired vestibular value in 38/85 patients (44.7%): 4/35 (11.4%) belonging to Control Group and 34/38 (88.6%) to Case one (Table 1). The Z score allow a good discrimination between healthy and OSA patients. However, it does not allow a good discrimination between the different levels of OSA. A larger sample may confirm or deny this evidence.
The box plots in Figure 4 showed a substantial difference between the “Z-score” distributions of the two study groups (all the p-values were lower than 0.001).
 |
Figure 4 Box plots of the “Z-score” distributions of the two study groups. |
Results from the multiple logistic model showed a significant association between the OSA diagnosis and the AHI classification (see Table 2). In particular, the adjusted odds ratio showed a positive association between the diagnosis of OSA and the presence of AHI.
 |
Table 2 Logistic Model (Adjusted for Age and BMI) |
Discussion
OSA is a common chronic disease that involves about 9% of women and 24% of men in the general population1 of the general population and has a high economic impact. It is characterized by multiple episodes of air-flow reduction due to a collapse in the upper respiratory airways.
Many studies conducted between 1998–2008 have reported that OSA produces a chronic inflammatory state related to cardiovascular, metabolic, and neurological complications.14,15
Nocturnal intermittent hypoxia induces oxidative stress, sympathetic hyperactivity, endothelial dysfunction, and metabolic impairment. However, although over recent years many studies have examined and reported an association between OSA and cognitive deficits,16,17 few have focused on the relation with vestibular impairment.
This association may be based on the relationship between the vestibular nucleus (VN) and sleep regulator pathways. Animal and human clinical reports pointed out an interconnection between the suprachiasmatic hypothalamic nucleus (SCN) and VN through the lateral intergeniculate area.18,19 SCNs are the principal structures for biological circadian sleep-awakens rhythm control. These nuclei have a cholinergic afferent function that stimulates the cortex to maintain an awake status. This pathway is strengthened by afferent orexinergic input produced by a small group of neurons placed in the lateral hypothalamus (perifornical region; posterior nucleus; lateral area),5 while the ventrolateral preoptic nucleus (VLPO) has a GABAergic connection, maintaining the sleepiness state. Therefore, in rats, an impairment of the connection between VN and SCNs induced a deficit in the wake-sleep rhythm causing narcolepsy with cataplexy phenotype. This condition was confirmed in an astronaut population who reported sleep disorders during the mission in the absence of gravity, possibly due to the lack of vestibular stimulation.5
Another theory was proposed by Gallina et al who suggested a relationship between the vestibular system and OSAS due to the contiguous position of the VN and the respiratory one (parabrachial). Their results in OSA patients, evaluated using PSG video-oculo-nystagmography and caloric testing, evidenced a quali-quantitative alteration of vestibular function worse in patients with elevated AHI. Moreover, they assumed that lack of sleepiness induced an anomalous posture and consequent dearth of vestibular-proprioceptive balance.7
Currently, the vestibular system is being studied with different methods that test specific portions of the posterior labyrinth. Many exploit the analysis of the Vestibular Ocular Reflex (VOR). This is an indirect sign of labyrinthine function since it is a reflex arc that connects the semicircular canals of the labyrinths on both sides with VNs and then oculomotor muscles.20 The functional head impulse test (fHIT) is one of the most recent instruments introduced for evaluating VOR function. It is based on the ability of a patient to recognize an optotype presented during impulse head rotations at varying angular accelerations. In this way, all the semicircular canals are tested and, indirectly also the superior and inferior vestibular nerve function.
Our results showed a similar incidence of vestibular impairment in comparison to Gallina et al in the case group (68% vs 65%).7
Moreover, in accordance with Micarelli et al,6 a significant alteration of VOR activity (researched through vHIT) and posturographic value in OSA patients were observed. However, in their study, the correlation between the severity of OSA and vestibular disease is not well expressed but these authors preferred to point out the relationship between vestibular impairment, ESS (Epworth sleepiness scale), and Dizziness Handicap Inventory (DHI).
Our choice to include only patients with a DHI< 40 (not pathological) was adopted to evaluate the latent peripheral impairment. Moreover, our results showed that there was no correlation between the severity of OSA and vestibular impairment, which was mainly expressed in moderate OSA. However, the lack of a good discrimination between the different vestibular damages and OSA severity prompted us to revalue the relationship between these two parameters in a larger sample to confirm or deny it. Moreover, it should be emphasized a possible explanation for this can be attributed to the different ability of each patient to adopt a central compensation,7,21,22 which may be inadequate for totally regulating the reduction of VOR gain while allowing the patient to be asymptomatic.
In the Case and Control groups, the comparison between fHIT results of each semicircular canal always showed a statistical difference without a defined predominance. Hence, indirectly, it is possible to affirm that intermittent hypoxia defaces the function of the superior and inferior vestibular nerves and their connection (VN). Therefore, the absence of other concomitant diseases strengthens the hypothesis that OSA is a favoring cause of dizziness and risk of falls.
Limitation of the Study
One of the limitations was the performer of a type III sleep study and not a type I (polysomnography) since it was easy to administer. Another limit is represented by the absence of a complete evaluation of the posterior labyrinth. VEMPs are the most objective method for testing the function of the saccule and utricle. All the authors who tested VEMPs in OSA patients are unanimous in affirming that these patients have impaired values.21–24
The relatively small sample may be a probable cause of the wide OR range and so all the results should be evaluated within the framework of these limitations. The study is designed as a first exploratory analysis to investigate the relationship between OSA and vestibular impairment. On the basis of these results the authors intend to carry out a formally dimensioned study with adequate statistical power.
Conclusion
This evaluated the vestibular function in patients affected by OSA using fHIT, in OSA patients without related cardiovascular, neurological, or metabolic diseases. Results showed that latent vestibular impairment may be presented in OSA patients and it could be early diagnosed by fHIT. A possible explanation could be addicted to the relationship between the vestibular nucleus (VN) and sleep regulator pathways. The data found highlight how an early vestibular diagnosis may give us information about a latent microvascular and neurological deficit in obstructive sleep apnea patients that increases the risk of falls and altered drive performance. Moreover, vestibular evaluation may represent an additional method to follow the evolution of OSA disease during treatment.
Abbreviations
OSA, Obstructive Sleep Apnoea; VN, vestibular nucleus; vHIT, video Head Impulse Test; VEMPs, vestibular evoked myogenic potentials; fHIT, functional Head Impulse Test; ENT, Ear Nose Throat; BMI, body mass index; PSG, polysomnography; AASM, American Academy sleep medicine; AHI, Apnea-Hypopnea Index; ODI, Oxygen desaturation index; SCN, suprachiasmatic hypothalamic nucleus; VLPO, ventrolateral preoptic nucleus; VOR, Vestibular Ocular Reflex; ESS, Epworth sleepiness scale; DHI, Dizziness Handicap Inventory.
Acknowledgments
The authors thank the society Beon Solution Srl in Zero Branco (Treviso, Italy) for leading the functional head impulse test—fHIT 1.0 (www.beonsolutions.it) and in particular Alessandro Florian for the technical support during the experimentation.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Salman LA, Shulman R, Cohen JB. Obstructive sleep apnea, hypertension, and cardiovascular risk: epidemiology, pathophysiology, and management. Curr Cardiol Rep. 2020;22:6. doi:10.1007/s11886-020-1257-y
2. Maniaci A, Iannella G, Cocuzza S, et al. Oxidative stress and inflammation biomarker expression in obstructive sleep apnea patients. J Clin Med. 2021;10:277. doi:10.3390/jcm10020277
3. Stevens D, Jackson B, Carberry J. The impact of obstructive sleep apnea on balance, gait, and falls risk: a narrative review of the literature. J Gerontol A Biol Sci Med Sci. 2020;75:2450–2460. doi:10.1093/gerona/glaa014
4. Besnard S, Tighilet B, Chabbert C, et al. The balance of sleep: role of the vestibular sensory system. Sleep Med Rev. 2018;42:220–228. doi:10.1016/j.smrv.2018.09.001
5. Komada Y, Inoue Y, Mizuno K, et al. Effects of acute simulated microgravity on nocturnal sleep, daytime vigilance, and psychomotor. performance: comparison of horizontal and 6 degrees head-down bed rest. Percept Mot Skills. 2006;103:307–317. doi:10.2466/pms.103.2.307-317
6. Micarelli A, Liguori C, Viziano A, Izzi F, Placidi F, Alessandrini M. Integrating postural and vestibular dimensions to depict impairment in moderate-to-severe obstructive sleep apnea syndrome patients. Sleep Res. 2017;26:487–494. doi:10.1111/jsr.12516
7. Gallina S, Dispenza F, Kulamarva G, Riggio F, Speciale R. Obstructive sleep apnoea syndrome (OSAS): effects on the vestibular system. Acta Otorhinolaryngol Ital. 2010;30:281–284.
8. Jacobson GP, Newman CW. The development of the dizziness handicap inventory. Arch Otolaryngol Head Neck Surg. 1990;116:424–427. doi:10.1001/archotol.1990.01870040046011
9. Iannella G, Magliulo G, Lo Iacono CAM. Positional obstructive sleep apnea syndrome in elderly patients. Int J Environ Res Public Health. 2020;17:1120. doi:10.3390/ijerph17031120
10. Kapur VK, Auckley DH, Chowdhuri S, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine Clinical practice guideline. J Clin Sleep Med. 2017;13:479–504. doi:10.5664/jcsm.6506
11. Caldani S, Baghdadi M, Moscoso A. Vestibular functioning in children with neurodevelopmental disorders using the functional head impulse test. Brain Sci. 2020;10:887. doi:10.3390/brainsci10110887
12. Versino M, Colnaghi S, Corallo G, Mandalà M, Ramat S. The functional head impulse test: comparing gain and percentage of correct answers. Prog Brain Res. 2019;248:241–248.
13. van Dooren TS, Lucieer FMP, Duijn S. The functional head impulse test to assess oscillopsia in bilateral vestibulopathy. Front Neurol. 2019;10:365. doi:10.3389/fneur.2019.00365
14. Trayhurn P, Wang B, Wood IS. Hypoxia in adipose tissue: a basis for the dysregulation of tissue function in obesity? Br J Nutr. 2008;100:227–235. doi:10.1017/S0007114508971282
15. Jurkovicová I, Celec P. Sleep apnea syndrome and its complications. Acta Med Austriaca. 2004;31:45–50.
16. Macey PM, Kumar R, Woo MA, Valladares EM, Yan-Go FL, Harper RM. Brain structural changes in obstructive sleep apnea. Sleep. 2008;31:967–977.
17. Morrell MJ, McRobbie DW, Quest RA, Cummin AR, Ghiassi R, Corfield DR. Changes in brain morphology associated with obstructive sleep apnea. Sleep Med. 2003;4:451–454. doi:10.1016/S1389-9457(03)00159-X
18. Horowitz SS, Blanchard J, Morin LP. Medial vestibular connections with the hypocretin (orexin) system. J Comp Neurol. 2005;487:127–146. doi:10.1002/cne.20521
19. Cavdar S, Onat F, Aker R, Sehirli U, San T, Yananli HR. The afferent connections of the posterior hypothalamic nucleus in the rat using horseradish peroxidase. J Anat. 2001;198:463–472. doi:10.1017/S0021878201007555
20. Fetter M. Vestibulo-ocular reflex. Dev Ophthalmol. 2007;40:35–51.
21. Sowerby L, Rotenberg B, Brine M, George CF, Parnes LS. Sleep apnea, daytime somnolence, and idiopathic dizziness—a novel association. Laryngoscope. 2010;120:1274–1278. doi:10.1002/lary.20899
22. Gao T, Zhang Q, Gao JH, et al. Vestibular-evoked myogenic potentials in patients with severe obstructive sleep apnea. J Int Med Res. 2020;48:300060520909717. doi:10.1177/0300060520909717
23. Ulusoy B, Gül O, Elsürer C, et al. The relationship between the findings of vestibular evoked myogenic potentials and severity of obstructive sleep apnea syndrome. Eur Arch Otorhinolaryngol. 2020;277:37–46. doi:10.1007/s00405-019-05654-8
24. Mutlu M, Bayır O, Yüceege MB, et al. Vestibular evoked myogenic potential responses in obstructive sleep apnea syndrome. Eur Arch Otorhinolaryngol. 2015;272:3137–3141. doi:10.1007/s00405-014-3294-x
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
