Back to Journals » Medical Devices: Evidence and Research » Volume 17
Evaluation of the Puritan Bennett™ 980 Ventilator System Safety and Performance in the Real-World Setting
Authors Roshon M, Khandhar PB, Biniwale M , Ramanathan R, Frazier TP, Xu F, Zhang L, Guan X, Wenling D, Lambermont B
Received 8 August 2023
Accepted for publication 12 January 2024
Published 23 January 2024 Volume 2024:17 Pages 37—45
DOI https://doi.org/10.2147/MDER.S433900
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Michael Roshon,1 Paras B Khandhar,2 Manoj Biniwale,3 Rangasamy Ramanathan,3 T Patrick Frazier,4 Feng Xu,5 Linlin Zhang,6 Xiangdong Guan,7 Dai Wenling,8 Bernard Lambermont9
1Department of Emergency Medicine, Penrose-St. Francis Health Services, Colorado, Springs, CO, USA; 2Pediatric Critical Care Medicine, Beaumont Children’s Hospital, Royal Oak, MI, USA; 3Division of Neonatology, University of Southern California Keck School of Medicine, Los Angeles, CA, USA; 4Department of Medicine, University of Alabama at Birmingham, Heersink School of Medicine, Birmingham, AL, USA; 5Department of Intensive Care, Children’s Hospital of Chongqing Medical University, Chongqing, People’s Republic of China; 6Department of Critical Care Medicine, Beijing Tiantan Hospital, Capital Medical University, Beijing, People’s Republic of China; 7Department of Critical Care Medicine, the First Affiliated Hospital of Sun Yat-Sen University, Guangzhou, People’s Republic of China; 8Department of Critical Care Medicine, Yancheng First People’s Hospital, Yancheng, People’s Republic of China; 9Department of Intensive Care, University Hospital of Liege, Liege, Belgium
Correspondence: Michael Roshon, Email [email protected]
Purpose: Mechanical ventilation is a life-supporting intervention but is associated with known risks and complications. To improve the efficacy and safety profile of mechanical ventilation, manufacturers have developed advanced ventilator settings, modes, and alarm strategies to optimize ventilation for patient needs while avoiding complications. However, there is little real-world data published on the deployment of ventilator technology. The main objective of this study was to assess the clinical safety and performance of the Puritan Bennett™ 980 Ventilator System (PB980) using real-world clinical data collected from a diverse, global patient population.
Methods: This was a multi-center, post-market registry study that included nine sites: four in the United States of America, one in Europe, and four in China. Patients were enrolled into the registry if they were intended to be treated with a PB980. Data collection began at the start of ventilation and continued until extubation off the ventilator or up to seven days of ventilation, whichever occurred first. Subjects were divided by age into three categories: infants (0– 365 days), pediatric (1– 17 years), and adult (18 years and older). The primary outcome was device-related complication rate.
Results: Two-hundred-and-eleven subjects were enrolled (41 infants, 48 pediatric, and 122 adults). Sixteen deaths, unrelated to device deficiency, occurred during the data collection timeframe (relative frequency: 7.58, 95% CI: 4.40, 12.0). Only one device-related adverse event was reported (relative frequency: 0.47% 95% CI: 0.01%, 2.61%).
Conclusion: Ventilation by the PB980 was delivered safely in this multi-center observational study, which included a diverse sample of patients with broad ventilatory needs.
Plain Language Summary: Mechanical ventilation is a life supporting intervention. Much progress has been made in this field thanks to a better knowledge of respiratory physiopathology and improved ventilation delivered by modern ventilators.
In this global, post-market registry study, ventilation by the Puritan Bennett™ 980 Ventilator was delivered safely to a diverse sample of patients.
Keywords: ventilation, PB980, safety, complications, respiratory distress, critical care
Introduction
Mechanical ventilation is a life-supporting intervention for patients with respiratory failure or critical illness. However, mechanical ventilation also carries the risk of significant complications, including ventilator-associated pneumonia, neuromuscular weakness from sedative therapies, volutrauma, and barotrauma.1,2 Ventilation devices have advanced to employ a variety of settings and modes that can be manipulated in response to patient ventilation needs to improve the efficacy and safety profile.3–5
Ventilator design has become more sophisticated and refined over the past century, with improvements from negative-pressure ventilators that required the patient’s entire body to be encased in the 19th and early 20th centuries to today’s positive-pressure ventilators that boast a plethora of settings and modes to provide responsive ventilation to a large spectrum of patient needs.6,7 Ventilation solutions are intended to protect the airway and support the pulmonary system while minimizing ventilator-induced complications.8 To optimize patient safety and comfort, ventilator advancements aim to make mechanical ventilation synchronous with patient demands and less intermittent mandatory delivery of positive pressure.
The Puritan Bennett™ 980 Ventilator System (PB980) is a CE-marked and FDA-cleared medical device designed to provide continuous ventilation for patients who require respiratory support, delivered via invasive mechanical ventilation (IMV) or noninvasive ventilation (NIV). Care can be administered to neonatal, pediatric, and adult patients weighing at least 0.3 kg, with tidal volumes for mandatory volume-controlled breaths from 2 mL to 2500 mL. The PB980 is intended for use in hospitals, and intra-hospital transport.
Several bench studies have been published utilizing lung models to simulate a variety of ventilation needs to support the safety and performance of the PB980.9–16 However, despite the advances in ventilator technology, real-world experience and the safety performance of these devices have yet to be fully explored in the medical literature.
The PB980 Post-Approval Registry study aimed to assess the clinical safety and performance of the PB980 using real-world clinical data collected from a diverse, global patient population. The primary objective was to obtain direct clinical evidence to support the safety of the ventilator system.
Materials and Methods
Study Design
Data was collected from the PB980 Post-Approval Registry, a prospective, observational, multinational study conducted from June 2020 to April 2022 in the United States of America (USA), Europe, and China (Table 1). Enrollment in the study did not impact the care patients received before, during, or after participation. Study procedures were performed in accordance with the Declaration of Helsinki under a protocol that was duly approved by the Institutional Review Board or Ethics Committee (IRB/EC), as applicable, at each site. This study was sponsored and supported by Medtronic (Minneapolis, MN).
 |
Table 1 Participating Sites and Enrollments |
Patient and Procedures
Patients receiving IMV or NIV using the PB980, as ordered by the treating physician, were eligible for study enrollment. Enrollment took place prior to ventilation or up to 30 days following the start of ventilation. Data were collected for the duration of mechanical ventilation to extubation or seven ventilation days, whichever occurred first.
Subjects were divided based on age into three cohorts: infants (0–365 days), pediatric (1 year – 17 years), and adult (18 years and above). Due to the population under study, many subjects presented with trauma and/or were intubated, therefore a variety of methods were used to obtain consent. Per local IRB/EC requirements, consent was obtained in one of the following ways, depending on clinical situation: 1) prior to receiving care, 2) after receiving care (up to 30 days following start of ventilation), or 3) waiver of consent. Waiver of consent was provided at the discretion of each site’s IRB/EC, with only sites in the USA utilizing this option. For pediatric subjects who were not enrolled under waiver of consent, assent and parental and/or guardian consent was obtained, per local IRB/EC requirements. For infant subjects, who were not enrolled under waiver of consent, parental and/or guardian consent was obtained.
The PB980 Post-Approval Registry was designed to collect real-world clinical data on various patients using the ventilator as intended for routine medical care. Ventilator settings were managed by the attending clinician(s), as determined by patient clinical need and routine care. Trained research staff recorded ventilator settings every four hours for the duration of ventilation or up to seven ventilation days, whichever occurred first. All system-related adverse events and device deficiencies that occurred during the data collection period were promptly reported.
Data Collection
The primary endpoint was defined as the device complication rate of the PB980 during the duration of the study. Complications were defined as any event related to the ventilator or the ventilation therapy that impacted the patient during study participation. Site investigators assessed all complications and complication details, and investigator-determined relatedness was reported to the study sponsor and IRB/EC, as applicable.
In addition to the primary endpoint, subject demographics, including age, sex, weight, and height were collected. A brief medical history was obtained by reviewing the medical record to determine the history or presence of brain/neurological disease, cardiac disease, diabetes, renal disease, respiratory disease, stroke, or musculoskeletal disease. Admission diagnosis and reason for surgery, if applicable, were noted, along with an indication for respiratory intervention. The type of ventilation mode, endotracheal tube use, date and time of initial ventilation, date and time of extubation, and date and time of admission to and discharge from the intensive care unit were also recorded. Ventilation settings were collected every four hours and included ventilation type, ventilation mode, fractional inspired oxygen (FiO2), blood oxygen saturation (SpO2), tidal volume, peak inspiratory pressure, inspiration time, and positive end-expiratory pressure (PEEP). Additionally, physiologic measures were collected every four hours and included respiratory rate, heart rate, and blood gas results, if available, including blood pH, partial pressure of carbon dioxide (PaCO2), partial pressure of oxygen (PaO2), and bicarbonate (HCO3). Reason for study exit, including death, was also collected.
Statistical Analysis
The data was analyzed using SAS© statistical software, Version 9.4 (TS1M7) (SAS Institute Inc., Cary, NC, USA). Categorical variables are presented as counts and percentages. Descriptive statistics are provided for continuous variables. As this was an observational study, no treatment groups were assigned, or statistical significance testing completed.
A minimum sample size of 160 subjects total, with at least 30 subjects in each cohort, was determined necessary to detect a complication rate between 5% and 10%, with a precision of 1.7% to 2.4% standard error. This sample size allowed for a 95% chance of detecting a complication rate as low as 2%.
Results
Participants
Two-hundred-eleven subjects were enrolled in the registry across the three age groups (Table 2), exceeding the minimum sample size calculated to achieve study goals. The most commonly reported medical history in adult subjects included cardiac, brain/neurological, and respiratory disease (Table 3). Pediatric patients most frequently reported brain/neurological disease and respiratory disease, while infants most commonly had a history of respiratory disease. Across all three age cohorts, the most common indication for ventilation was related to presenting medical conditions as opposed to trauma or surgery.
 |
Table 2 Subject Characteristics |
 |
Table 3 Subject Medical History and Indication for Ventilation |
Procedural Characteristics
Only one device-related complication was reported during the study across all 211 subjects (relative frequency: 0.47%, 95% CI: 0.01%, 2.61%). The device deficiency occurred in a 56-year-old female who presented with trauma due to a fall. At baseline, the patient had an ASA status of 4 and a medical history positive for brain or neurological disease, respiratory disease, and musculoskeletal disease. The subject received IMV using the PB980 20 days after initial admission to the intensive care unit, after being transferred from another ventilator (no data collection occurred while receiving ventilation support using non-PB980 ventilator). While the subject was on the PB980, the most common ventilation setting used was spontaneous mode set to PAV+ and flow trigger mode. The complication was an expiratory filter blockage that occurred after the subject had been on the PB980 for approximately one day. The ventilator alarm appropriately indicated an expiratory filter warning, and the subject was immediately transferred to another ventilator (not PB980) to avoid further obstruction. The blockage did not appear to result in any short or long-term detriment to the subject’s health and did not prolong hospital length of stay. Sixteen deaths (relative frequency: 7.58, 95% CI: 4.40, 12.0), none related to device deficiency, occurred during the study (Table 4). Reported reasons for death included: respiratory failure (n=6), cardiogenic shock (n=3), sepsis (n=1), stroke (n=1), cardiac arrest (n=1), and incomplete description given (n=4). Six patients were reported to have been removed from the ventilator as part of comfort care.
 |
Table 4 Summary of Deaths Reported During Study |
The most frequently used ventilator settings during study enrollment are presented in Table 5. All pediatric and adult subjects, except one, received IMV, whereas approximately half of the infants received NIV. During the study period, three adults and two infants were noted to have alternated between IMV and NIV. The most frequently used ventilator settings are reported. Assist control was the most frequently reported breath control mode for adult patients, while pediatric and infant patients were most frequently treated using synchronized, intermittent mandatory ventilation (SIMV). SIMV mode is available for both IMV and NIV. Infants received pressure support more frequently than adult or pediatric patients. Flow triggering was the most frequently used trigger type across all cohorts. Table 6 depicts physiologic measures collected during study procedures and reflects the range of physiologies and patient states included in this registry.
 |
Table 5 Most Frequently Used Ventilator Settings by Age Group |
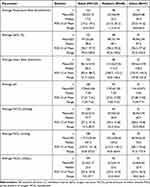 |
Table 6 Physiologic Measures by Age Group |
Discussion
Previous studies have explored ventilator safety and performance using lung models to simulate patient scenarios.9–16 These studies have concentrated primarily on bench studies and have demonstrated superior or comparative performance of the PB980 to other ventilators.9–12,14,16 There is robust clinical literature examining patient outcomes and ventilator use. However, less is explicitly reported on ventilator safety and performance in the clinical setting. Case reports have documented ventilator malfunction and its effects on individual patient outcomes,17–22 with some case reports specifically describing ventilator filter blockage. In previous reports, filter blockage due to patient secretions led to bilateral tension pneumothorax, presumably secondary to defective anesthesia breathing circuit filter.23,24 If the filter on a ventilator is obstructed, oxygen may not flow properly to the patient. Hypoxia can have serious adverse effects, including death. Obstruction can be detected by the ventilator with changes in pressure which would cause the ventilator to alarm. In response to the filter blockage during the current study, the alarm went off and the ventilator was changed, resulting in no further complications.
Three studies examining reported ventilator-related adverse events using national registries found that the most common cause for ventilator-related adverse events was associated with user error.25–27 A large screen displaying respiratory curves on the PB980 allows for monitoring and storing the curves during inspiratory and expiratory pauses, allows for the calculation and monitoring of intrinsic PEEP, total respiratory system resistance, and compliance. Those measurements can be helpful when determining the delivery of respiratory support. Data from the UK National Reporting and Learning System (2006–2008) found that of the 1029 incidents, 17.9% were related to the ventilator, though specific information about ventilator type, settings, or patient condition was not reported.27 To our knowledge, this is the first study to report PB980 ventilator-related safety and performance as a primary outcome in a real-world setting on a large sample of diverse patients.
Limitations
This study was designed to collect information on ventilator safety and performance and did not collect information on additional clinical parameters or outcomes. This limits the clinical conclusions that can be derived from the data. Any future study or expansion of the registry should include a collection of clinically relevant outcomes. Further, subjects were only enrolled in the study if they received ventilation with the PB980 and only up to seven days of ventilation, thus potentially excluding large swaths of ventilatory care provided to patients. Efforts were made to create a diverse sample; however, the data do not include all patient or disease types who receive ventilation across the globe. Additionally, data was collected every four hours with the most frequent ventilator settings reported, obscuring any changes to ventilation settings that may have taken place between data collection intervals.
Conclusion
Using real-world, global data, this study supports the safety and performance of the PB980 across a diverse group of patients with a wide variety of ventilation settings and physiologic parameters.
Acknowledgments
Study execution support was provided by Katherine Schiller, Naomi Wang, Demarcus Williams, and Ryan Zhou of Medtronic, Elizabeth Kring and Christine Batchelder of Beaumont Children’s, and Nathan Dureg and Kate Ramm of University of Southern California General Medical Center. Biostatistical analysis and support was provided by Anne Sexter, Guang Yang, Hui Xiong, and Julia Yang of Medtronic. Medical writing support was provided by Hanan Zavala of Medtronic in accordance with Good Publication Practice (GPP 2022) guidelines.28
An abstract of this work was accepted and presented at the Society of Critical Care Medicine 2024 Critical Care Congress (January 21 – 23, Phoenix Arizona).
Disclosure
The study was sponsored and funded by Medtronic. All authors (or their institutions) received research support from Medtronic to conduct this study.
Michael Roshon serves as a director on USACS’ National Clinical Governance Board.
References
1. Klompas M, Kleinman K, Murphy MV. Descriptive epidemiology and attributable morbidity of ventilator-associated events. Infect Control Hosp Epidemiol. 2014;35(5):502–510. doi:10.1086/675834
2. Silva PL, Ball L, Rocco PR, Pelosi P. Physiological and Pathophysiological Consequences of Mechanical Ventilation. Thieme Medical Publishers, Inc.; 2022.
3. Dexter AM, Clark K. Ventilator graphics: scalars, loops, & secondary measures. Respiratory Care. 2020;65(6):739–759. doi:10.4187/respcare.07805
4. Dos Santos Rocha A, Habre W, Albu G. Novel ventilation techniques in children. Pediatr Anesthesia. 2022;32(2):286–294. doi:10.1111/pan.14344
5. Pham T, Brochard LJ, Slutsky AS. Mechanical ventilation: state of the art. Elsevier. 2017;1382–1400.
6. Kacmarek RM. The mechanical ventilator: past, present, and future. Respiratory Care. 2011;56(8):1170–1180. doi:10.4187/respcare.01420
7. MacIntyre N, Rackley C, Khusid F. Fifty years of mechanical ventilation—1970s to 2020. Crit Care Med. 2021;49(4):558–574. doi:10.1097/CCM.0000000000004894
8. Egbuta C, Easley RB. Update on ventilation management in the Pediatric Intensive Care Unit. Pediatr Anesthesia. 2022;32(2):354–362. doi:10.1111/pan.14374
9. Beloncle F, Piquilloud L, Olivier PY, et al. Accuracy of P0.1 measurements performed by ICU ventilators: a bench study. Ann Intensive Care. 2019;9(1):104. doi:10.1186/s13613-019-0576-x
10. Itagaki T, Bennett DJ, Chenelle CT, Fisher DF, Kacmarek RM. Performance of Leak Compensation in All-Age ICU Ventilators During Volume-Targeted Neonatal Ventilation: a Lung Model Study. Respiratory Care. 2017;62(1):10–21. doi:10.4187/respcare.05012
11. Itagaki T, Chenelle CT, Bennett DJ, Fisher DF, Kacmarek RM. Effects of Leak Compensation on Patient-Ventilator Synchrony During Premature/Neonatal Invasive and Noninvasive Ventilation: a Lung Model Study. Respiratory Care. 2017;62(1):22–33. doi:10.4187/respcare.04825
12. Koyama Y, Uchiyama A, Yoshida J, Yoshida T, Yamashita T, Fujino Y. A Comparison of the Adjustable Ranges of Inspiratory Pressurization During Pressure Controlled Continuous Mandatory Ventilation of 5 ICU Ventilators. Respiratory Care. 2018;63(7):849–858. doi:10.4187/respcare.05286
13. Krieger TJ, Wald M. Volume-targeted ventilation in the neonate: benchmarking ventilators on an active lung model. Pediatr Crit Care Med. 2017;18(3):241–248. doi:10.1097/PCC.0000000000001088
14. Moon K, Takeuchi M, Tachibana K, Mizuguchi S, Hirano S. Accuracy of Reported Tidal Volume During Neonatal Ventilation With Airway Leak: a Lung Model Study. Pediatr Crit Care Med. 2019;20(1):e37–e45. doi:10.1097/PCC.0000000000001752
15. Morita PP, Weinstein PB, Flewwelling CJ, et al. The usability of ventilators: a comparative evaluation of use safety and user experience. Crit Care. 2016;20(1):263. doi:10.1186/s13054-016-1431-1
16. Marjanovic NS, De Simone A, Jegou G, L’Her E. A new global and comprehensive model for ICU ventilator performances evaluation. Ann Intensive Care. 2017;7(1):68. doi:10.1186/s13613-017-0285-2
17. Di Paolo M, Evangelisti L, Ambrosino N. Unexpected death of a ventilator-dependent amyotrophic lateral sclerosis patient. Revista Portuguesa de Pneumologia. 2013;194:175–178. doi:10.1016/j.rppneu.2012.12.001
18. Kim K. Ventilator malfunction due to water condensation during low flow anaesthesia. Anaesthesia Intensive Care. 2011;39(6):1155.
19. Kumar BK, Ravi M, Dinesh K, Nanda A. Ventilator malfunction. J Anaesthesiol Clin Pharmacol. 2011;27(4):576. doi:10.4103/0970-9185.86623
20. Sripriya R, Parthasarathy S, Ravishankar M. Ventilator dysfunction: role of graphics in detection. Ain-Shams J Anaesthesiol. 2016;9(3):465. doi:10.4103/1687-7934.189103
21. Olayode A, Mashriqi N, Liu C, et al. 882: A Case of Ventilator Malfunction-Induced Pneumothorax. Crit Care Med. 2023;51(1):434. doi:10.1097/01.ccm.0000909256.95446.33
22. le Noble JL, Terpoorten FJ, der Snoek M, Foudraine N. Ventilator dysfunction due to unexpected salbutamol crystallisation while using a nebuliser: a practical advice for intensivists. Intensive Care Med Exp. 2022;10(1):1–3. doi:10.1186/s40635-022-00441-y
23. McEwan AI, Dowell AL, Karis JH. Bilateral tension pneumothorax caused by a blocked bacterial filter in an anesthesia breathing circuit. Anesthesia Analg. 1993;76(2):440–442.
24. Smith CE, Otworth JR, Kaluszyk P. Bilateral tension pneumothorax due to a defective anesthesia breathing circuit filter. J Clin Anesthesia. 1991;3(3):229–234. doi:10.1016/0952-8180(91)90166-K
25. Kamio T, Masamune K. Mechanical ventilation-related safety incidents in general care wards and ICU settings. Respiratory Care. 2018;63(10):1246–1252. doi:10.4187/respcare.06109
26. Pham JC, Williams TL, Sparnon EM, Cillie TK, Scharen HF, Marella WM. Ventilator-related adverse events: a taxonomy and findings from 3 incident reporting systems. Respiratory Care. 2016;61(5):621–631. doi:10.4187/respcare.04151
27. Cassidy C, Smith A, Arnot‐Smith J. Critical incident reports concerning anaesthetic equipment: analysis of the UK National Reporting and Learning System (NRLS) data from 2006–2008. Anaesthesia. 2011;66(10):879–888. doi:10.1111/j.1365-2044.2011.06826.x
28. DeTora LM, Toroser D, Sykes A, et al. Good Publication Practice (GPP) Guidelines for Company-Sponsored Biomedical Research: 2022 Update. Ann Intern Med. 2022;175(9):1298–1304. doi:10.7326/m22-1460
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
