Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Evaluation of the Performance and Tolerance of the Combination of an HA-based Filler with Tri-Hyal Technology and a Skin Biorevitalizer on Skin Aging Parameters
Authors Fanian F , Philippon V, Gorj M, Rumyantseva Mathey E , Caillens M , Goorochurn R, Curic S, Humbert P
Received 14 July 2022
Accepted for publication 19 January 2023
Published 24 April 2023 Volume 2023:16 Pages 1095—1105
DOI https://doi.org/10.2147/CCID.S372490
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Ferial Fanian,1 Valerie Philippon,2 Mihai Gorj,3 Elena Rumyantseva Mathey,3 Maxence Caillens,4 Ranesha Goorochurn,5 Shannel Curic,1 Philippe Humbert6
1FILLMED Laboratories, Paris, France; 2Private Clinic, Boulogne Billancourt, France; 3Private Clinic, Paris, France; 4Private Clinic, Sarcelles, France; 5Dermo Dev Partners, Paris, France; 6Private Clinic, Ornans, France
Correspondence: Ferial Fanian, Scientific Department, FILLMED Laboratories, 2 rue de Lisbonne, Paris, 75008, France, Tel +33 6 73 76 18 20, Email [email protected]
Background: Biorevitalization solutions contain numerous nutritive compounds to improve skin quality. Dermal fillers like HA (hyaluronic acid), depending on rheological characteristics, are used to fill large static defaults with a sustained long-term efficacy. Treatments with either dermal filler or biorevitalization solutions alone are not enough to bring a global facial aging approach.
Objective: To demonstrate the anti-aging performance and safety of a new protocol, BIONUTRILIFT, which combined an HA-based filler with Tri-Hyal technology and a skin biorevitalizer, to target the skin quality and wrinkles correction at the same time.
Materials and Methods: Eligible subjects were enrolled based on a score of 2, 3, 4, or 5 on Bazin cheek folds wrinkle scale. Safety outcomes measured were immediate and local tolerability. Performance outcomes measured included: proportion of subjects in whom the severity of cheeks folds, nasolabial folds, marionette lines, upper lip wrinkles and skin radiance remained at least one point below the baseline measurement (Bazin scale) Global Aesthetic Improvement Scale scores by subjects and investigators.
Results: In performance analyses with the combined protocol, skin radiance and cheek folds wrinkle correction sustained during the four-month study and decrease by 61% and 55%, respectively. 96% and 77% of subjects respectively showed at least a one-point decrease in the mean skin radiance score and Bazin score compared with baseline. Interestingly, the BIONUTRILIFT protocol showed the distance effect of vector A (cheek injection) and vector B (mandibular injection) on perioral zone and remained significant even 120 days after injections. Adverse events (AEs) were consistent with the expected AEsthat occurred after dermal injections. No serious AEswere recorded.
Conclusion: BIONUTRILIFT may satisfy the subjects’ demand by obtaining in the same session a simple, personalized, noninvasive, atraumatic, and reproductible technique.
Keywords: dermal filler, biorevitalization, combined protocol, distance effect
Introduction
Aging is a complex phenomenon including, over time, many structural changes. Face aging is characterized by a loss of skin volume, a decrease and a redistribution of fat, mainly in the orbital and malar areas. In parallel, there is a decrease in collagen production, reduced fibroblastic activity, dermal atrophy, and alteration of elastin. At the level of the skin a reduction in cell renewal, dehydration, loss of radiance, elasticity, firmness, and the appearance of fine lines and wrinkles are observed.
In order to slow down facial aging, nonsurgical solutions enable patients to have simple and efficient treatments, such as injectable anti-aging products: biorevitalization products, dermal fillers and hyaluronic acid (HA)-based formulation for skin rejuvenation. The most commonly biorevitalization solutions are vitamin blends, trace elements, organic silicon, and HA. HA can be combined with a moisturizing agent, most often glycerol, but also mannitol or sorbitol.1 The greatest immediately clinical effects observed after facial biorevitalization are reduction of wrinkles, the improvement of deep hydration, firmness, uniformity, and radiance of the complexion.2,3 In recent years, dermal fillers that became the material of choice are HA-based fillers for use in soft tissue and dermal correction and mostly replacing collagen fillers.4,5
Hyaluronic acid is widely used in anti-aging products, either in native form in products to be injected by skin biorevitalization techniques or cross-linked in dermal fillers. Naturally abundant in the human body, this biopolymer is one of the most important biological constituents of the extracellular matrix of the dermis and it forms the elastoviscous fluid matrix in which collagen and elastin fibers are embedded in a proper configuration. In the aged skin, the lack of connections with HA may contribute to the disorganization of collagen and elastin fibers and lead to the presence of fine lines, wrinkles, and nasolabial folds.6–8 Due to its high hydrophilic properties and its significant advantage of structural conservation which explain the reason of HA’s volumizing potential after injection and its nonallergenic field.7 Native non-cross-linked HA used in skin biorevitalization is a linear polysaccharide chain similar to HA in the dermis. This native HA injected into the dermis enables hydration of the skin through a higher water-binding capacity and builds up the extracellular matrix and reactivates fibroblasts and induces the synthesis of new collagen, elastin, and endogenous HA.9,10
Because of the high solubility of native HA and its half-life of a few days in natural form, HA must be chemically stabilized by a cross-linking process to reduce its natural degradation. Depending on molecular weight of HA, the process and degree of cross-linking, the HA molecule thus modified comes in the form of a highly viscous, insoluble gel, usable as a dermal filler. The clinical effect and duration of presence in the skin are directly associated with the rheological characteristics of the HA gel, its cohesiveness and its elasticity, the injected amount, and the area treated.
Many practitioners treat their patients with dermal fillers and biorevitalizing products in the same session but not in the same skin layer. These personalized treatments showed that this approach increases the effect of each product alone but there is no evidence-based information to prove it. The aim of this study was to demonstrate the anti-aging performance and safety of a new protocol, which targets the skin quality and wrinkle correction at the same time. This protocol is based on injection of a biorevitalizing solution (NCTF 135HA, FILLMED Laboratories, France) which contains 5 mg/mL of native HA in combination with one or two cross-linked HA-based dermal fillers, in the same session: ART FILLER Fine lines (AFFL, FILLMED Laboratories, Paris, France; HA 20 mg/mL) or/and ART FILLER Universal (AFUni, FILLMED Laboratories, Paris, France; HA 25 mg/mL). Both AFFL and AFUni are formulated with a unique combination of three sizes of hyaluronic acid chains, Tri-Hyal technology, to achieve the desired rheological characteristics, and a sustained efficacy for at least 18 months.11
Materials and Methods
Subject Selection
The inclusion criteria were male or female subjects older than 19 years of age with a Fitzpatrick phototype of I to IV and a score of 2, 3, 4, or 5 on the Bazin cheek folds wrinkle scale. All subjects agreed not to expose themselves to the sun or UV during the entire study. Female subjects agreed to do a pregnancy test and keep a reliable contraception method during whole study. The noninclusion criteria included the participants with any allergy to the study product, history of dermal fillers during last one year, history of keloid scars, any facial pathologies such as facial herpes, any systemic diseases, medication and condition which may interfere with the results of the study including auto-immune diseases, coagulation disorders.
Study Design
The study adhered to the Declaration Helsinki with the authorization of the ethics committee (Southeast V, Medical University of Grenoble) on November 13, 2019 under registration number 2019-A00144-53. All participants signed a written consent form, accepting not modifying their lifestyle and avoiding the sun exposure during the whole study. This study is a nonrandomized, noncomparative, prospective multicentric study at four centers in France for 120 days. The aim of this study was to demonstrate the anti-aging performance and safety of a new combined protocol with injection of NCTF 135HA in combination with one or two other medical devices, either ART FILLER Fine lines and/or ART FILLER Universal (based on the score at baseline) through a 25G × 55 mm cannula (BIONUTRILIFT cannula, Softfil France) in the same session. The protocol consisted of one injection session at day 0 and three follow-up visits at days 30, 60, and 120.
Study Products
NCTF 135HA, ART FILLER Fine lines and ART FILLER Universal are CE marked Class III medical devices according to Directive 93/42EEC and have been freely marketed in the European Union, since 2007 and 2016, respectively.
ART FILLER Fine lines and ART FILLER Universal are the HA-based dermal fillers prepared in prefilled sterile graduated disposable syringe of 1.0 mL and 1.2 mL, respectively. They contain the cross-linked hyaluronic acid viscoelastic gel as well as 0.3% by weight of lidocaine hydrochloride for its anesthetic properties. AF Fine Lines is a superficial HA filler which contains 20 mg of HA with a good cohesivity and G′ approximately 70–80 with a degree of modification of 3% (MoD%). AF Universal is a medium to deep dermal filler which contains 25 mg of HA with a good cohesivity and G′ approximately 120–130 with a MoD of 4% according to the laboratory data. NCTF 135HA is a HA-based microfiller which contains 5 mg/mL of non-cross-linked sodium hyaluronate and a polyrevitalising solution described in Table 1. It is prepared in 3 mL sterile disposable vials and should be prepared in a sterile way in a 3 mL sterile syringe before injection.
 |
Table 1 Composition of NCTF 135HA (Approved CE Marking Medical Device) |
Injection Method
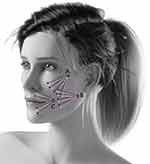
The physician disinfected the treated area with chlorhexidine and decided to inject 1, 2, or 3 vectors for each side of the face with a combination of NCTF135HA with ART FILLER Fine lines and/or ART FILLER Universal. The number of vectors varies according to the skin aging grade of the face (Table 2). The injection procedure is based on the superficial subcutaneous injection by cannula according to the fan technique (Figure 1).
 |
Table 2 Amount of Product Injected According to the Skin Aging Grade (Bazin Cheek Folds Score) |
The injectors should inject the dermal fillers (ART FILLER Fine Lines or ART FILLER Universal in five lines with a single-entry point (fan technique, vector). Then, the NCTF 135HA which has been previously prepared in a 3 mL sterile syringe, injected through with canula the same entry point in four lines between the lines previously injected by dermal fillers.
Evaluation Methods
The clinical assessment was performed by a visual scoring system regarding the skin wrinkles and skin radiance in different zones on the face (Table 3).
 |
Table 3 Clinical Assessments Used by the Investigators to Obtain Objective Evaluations of the Treatment Evolution |
The Global Aesthetic Improvement Scale (GAIS) was assessed by the investigators and the subjects, on a 7 grades of satisfaction level including: very much improved, much improved, improved, no change, worse, much worse, and very much worse.
Safety
Safety analysis includes all the subjects who received at least one injection of one of the devices under study. Immediate tolerance was assessed by measuring the pain based on an analog 10-grade scale from absence to 10 for very intense pain and recording all local adverse events associated with the injection. They were scored based on a 0–3 scale by the investigator at each visit from the first injection until the end of the study for erythema, ecchymosis, hematoma, edema, dyschromia, nodule/papule, and pruritus. In parallel, the patients recorded any local or systemic reactions or disorders on a daily log.
Statistical Methodology
The main criterion for this study is based on Bazin 8-point scale clinical scoring of the cheek folds (Figure 2).12 For this scale, a decrease of one point of the score considered as successful esthetic evolution. Assuming a standard deviation of the before-after differences=2; then 35 patients are required to have a 90% chance of detecting a difference between means of 1.0 with a significance level (alpha) of 5% (calculated with the sample size for paired t-test (one-tailed)).
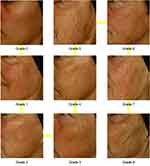 |
Figure 2 Bazin cheek wrinkles scale. |
However practical experience with such procedure shows that a notified body expects safety data on at least 100 follow-up subjects. For this reason, it was finally proposed to include 100 subjects. Statistical analysis was performed with R 4.0.2, MATLAB R2020b, GraphPad InStat and Excel 2016.
Descriptive statistics are provided for each parameter (ie number of observations, mean, standard deviation, minimum, maximum, median) with 95% confidence interval. The repartition of the sample size is provided for the evolution of the clinical scores in the different classes of scores under the form of n/percentage. Analyses were performed per area and per treatment.
The mean and median changes of the values between D0 and other time points (D60 and D120) were calculated, and the statistical significance of the changes were calculated by a Student’s t-test for paired series or its nonparametric equivalent, the Wilcoxon test. A decrease of one grade is considered a satisfactory esthetic improvement. The percentage of success was calculated by the ratio of satisfactory responses.
Results
Study Population
Ninety-nine healthy subjects between 45 and 79 years old (mean age: 63.9 years) were enrolled in the study in Paris, France including 1 male and 98 female volunteers. The Fitzpatrick phototyping distribution was 54% phototype II, 37% phototype III, 5% phototype IV, and 3% phototype I. Some patients were not able to come back for the D60 follow-up session visit due to the COVID-19 lockdown period in France and for this reason the results were analyzed only on D30 and D120.
Performance Analyses for Each Clinical Assessment
Cheek Folds
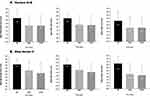
A significant improvement of skin wrinkles score was obtained for cheek folds at D30 and D120 versus baseline, after the combined injection protocol (p<0.0001). Thirty days after the injection (D30), the cheek folds score decreased by 45% (mean score 1.8±1.4 vs 3.3±1.1 at baseline; p<0.0001) and this score continued to decrease until D120 by 55% (mean score 1.5±1.4 vs 3.3±1.1 at D0; p<0.0001), with a success rate of 77% (at least one grade diminution) (Figure 3).
Radiance
A significant improvement of skin radiance score was observed for all time points by 52% at D30 and by 61% at D120 vs baseline (1.5±0.79 and 1.2±1.0 at D30 and D120 respectively compared to 3.1±0.76 for baseline, p<0.0001 for all). At D120, a 96% success rate was observed (at least one grade improvement) (Figure 3).
Perioral Scores
Thirty-five subjects among 99 were injected for vector C, which directly treated the perioral zone. The results revealed that although there was not the direct injection in the perioral zone on 64 subjects, there is a distance effect on nasolabial folds, marionette lines and upper lips score. For this reason, the results on the perioral scores were calculated separately for the population injected or not for vector C (Figure 1 and Figure 5).
Marionette Lines
Regardless the number of vectors, the data showed a significant improvement of wrinkles score for marionette lines at D30 and D120 vs baseline (2.5±1.4 at D30 and 2.4±1.5 at D120 vs 3.5±1.2 at baseline, p<0.0001 for all) (Figure 4). However, the improvement was also significant for the population not be injected with vector C as follows: 2.2±1.3 and D30 and D120 vs 3.2±1.1 at baseline (p<0.0001 for all). This result shows the distance effect of vector A (cheek injection) and vector B (mandibular injection) on perioral zone. In parallel, injection of vector C (perioral injection) has also a local effect on the same area as follows: 3.1±1.4 at D30 and 2.7±1.6 at D120 vs 4.1±0.98 baseline (p<0.0001 for all) (Figure 5).
NSL Folds
A significant improvement of wrinkles score was observed for nasolabial folds at D30 and D120 vs baseline (2.5±1.1 at D30 and 2.4±1.2 at D120 vs 3.4±0.88 at baseline, p<0.0001 for all) (Figure 4).
Moreover, the amelioration was also significant for the population without vector C injection as follows: 2.3±1.0 at D30 and 2.2±1.2 at D120 vs 3.2±0.82 at baseline (p<0.0001 for all). This result supports the distance lifting effect of vector A and vector B on perioral zone. Furthermore, injection of vector C has also a local effect on the same area as follows: 2.9±1.2 at D30 and 2.6±1.2 at D120 vs 3.7±0.88 baseline (p<0.0001 for all) (Figure 5).
Upper Lips
A significant improvement of wrinkles score was achieved for upper lips wrinkles at D30 and D120 vs baseline (2.2±1.5 at D30 and 2.1±1.6 at D120 vs 3.5±1.4 at baseline, p<0.0001 for all) (Figure 4). In addition, the improvement was significant for the population without vector C treatment as follows: 2.1±1.5 at D30 and 2.1±1.7 at D120 vs 3.1±1.4 at baseline (p=0.0005 and p<0.0001, respectively). This result confirms the distance lifting effect of vector A and vector B on perioral zone. Finally, injection of vector C has also a local effect on the same area: 2.4±1.4 at D30 and 2.3±1.3 at D120 vs 4.2±1.2 baseline (p=0.0005 and p<0.0001, respectively) (Figure 5).
Global Aesthetic Improvement Assessment
The satisfaction rate was evaluated by investigator GAIS (IGAIS) and also by subject GAIS (SGAIS).
IGAIS: The satisfaction rate evaluated by the physician nearly reached 100% of improvement for D30 and D120: 99% and 98%, respectively.
GAIS: The patients observed an excellent global esthetic improvement and close to the satisfaction rate obtained by the investigator: 86% both for D30 and D120.
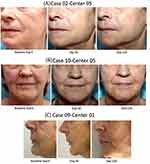
The satisfaction rate by GAIS was confirmed between the subjects and investigators (Figure 6). The before-after clinical photos revealed the visible esthetic changes demonstrated in Figure 7.
 |
Figure 6 Investigator and subject satisfaction rate (%) of the new protocol BIONUTRILIFT evaluated by GAIS. |
Safety and Tolerance
No serious adverse events have been reported during the study. Sixty-seven adverse events were reported and among them, 57 AEs were relating to expected adverse events that occurred frequently after dermal injections: the skin reactions were mostly concerned as ecchymosis (n=27, 40%), swelling (n=11, 16%), erythema (n=5, 7%), pain (n=4, 6%), irregularities on palpation (n=4, 6%), pain at palpation (n=2, 3%), pruritus (n=2, 3%), stinging (n=2, 3%), which disappeared later (none of the reported local reactions were still present at D60 n=69 nor D120 n=93 among the data collected). There was only one subject with palpable nodule and one subject with moderate pigmentation which disappeared before D30. The nonrelated adverse events were limited to one subject suffered from zona on one arm approximately two months after injections and one case had eyes dryness and stinging. The overall safety was very good and acceptable adverse events were summarized in Figure 8.
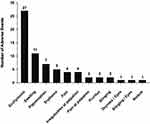 |
Figure 8 Local adverse events. |
Discussion
Nonsurgical cosmetic medicine procedures for the face are developing considerably to improve the quality and appearance of the skin. Several practitioners use the association of dermal fillers and biorevitalizing products to treat their patients to bring a global approach of facial aging.13 This personalized approach increases the effect of each product alone but there is no evidence-based information to prove it.
The aim of this clinical study was to demonstrate the efficacy and safety of a new combined protocol with injection of a biorevitalizing solution, NCTF 135HA, in combination with one or two HA-based dermal fillers, either ART FILLER Fine lines and/or ART FILLER Universal (based on the aging grade at baseline). The separate effect of biorevitalizing solutions and dermal fillers has been widely demonstrated in the literature. Biorevitalization looks at improving the intrinsic quality of the cutaneous envelope. However to our knowledge, no published clinical studies have demonstrated the efficacy and safety of the combination of dermal fillers and a biorevitalizing product.
Clinically, the most immediate effects observed after facial biorevitalization are the reduction of wrinkles, the improvement of skin hydration level, skin firmness, uniformity and radiance of the complexion.14,15 Likewise, an increase in density and thickness of the dermis is reported with biorevitalization solutions and hyaluronic acid fillers.8,16
As dermal fillers are used to fill large static defaults, facial aging treatment with dermal fillers alone cannot reach the improvement of skin quality such as biorevitalizing solutions products because they contain numerous nutritive compounds, as well as nonreticulated hyaluronic acid to temporarily “fill” the tissues with water. The interest of this study was to show the effect of this combination treatment with HA fillers and a biorevitalization product.
This study showed a significant improvement of skin wrinkles score for cheek folds and skin radiance score at the same time and this improvement remained significant even 120 days after injections. These proven improvements confirm the pertinence of a combined protocol in order to offer global therapeutic in a short- and long-term period.
Interestingly, the combined protocol showed the distance lifting effect of vector A (cheek injection) and vector B (mandibular injection) on perioral zone. Proven in our clinical study, this distance effect remained significant 120 days after injections. Regarding the safety, most reported reactions remained of light intensity and corresponded to usual and expected adverse effects reported with such treatments (ecchymosis, swelling, erythema, hematoma, edema).8,14
Conclusion
The benefit of this combined protocol was the improvement of skin quality of the face at the same time with filling effect of the fillers on the facial wrinkles. The risk– benefit ratio of such treatment can therefore be considered as favorable. Moreover, this protocol suggests the interest to adapt injection protocol to the skin needs of the patient. This noninvasive therapeutic strategy brings patient satisfaction through an adaptative and a more global approach to facial aging.
Abbreviations
NLF, nasolabial folds; AF, ART FILLER; FL, fine lines; Uni, universal.
Acknowledgments
Special thanks to Dr Karim Nadra and Dr Hanane Issa for their valuable supports on launching and executing the study. The authors also appreciate Mme Audrey Binabout for the monitoring of this study.
Disclosure
FF and SC are the employee of FILLMED Laboratories in Paris. The authors report no conflicts of interest in this work. This study was financially supported by FILLMED Laboratories.
References
1. Grand-Vincent A. Place de la mésothérapie dans le rajeunissement facial: mise à jour (Place of mesotherapy in facial rejuvenation: update). J Med Esth Et Chir Derm. 2008;XXXV(137):31–37.
2. Robin S, Fanian F, Courderot-Masuyer C, et al. Efficacy of a biorevitalizing-filler solution on all skin aspects: 10 years approach through in vitro studies and clinical trials. J Cosmet Dermatol Sci Appl. 2021;11(01):18–37. doi:10.4236/jcdsa.2021.111003
3. Grand-Vincent A, Boisnic S, Salomon C, Prinderre P, Piccerelle P. Clinical assessment of a mesotherapy formulation for skin rejuvenation in healthy volunteers. J Cosmet Dermatol Sci Appl. 2017;07(04):291–305. doi:10.4236/jcdsa.2017.74026
4. Kablik J, Monheit GD, Yu L, Chang G, Gershkovich J. Comparative physical properties of hyaluronic acid dermal fillers. Dermatol Surg. 2009;35(Sup1):302–312. doi:10.1111/j.1524-4725.2008.01046.x
5. Johl SS, Burgett RA. Dermal filler agents: a practical review. Curr Opin Ophthalmol. 2006;17(5):471–479. doi:10.1097/01.icu.0000243021.20499.4b
6. Bukhari SNA, Roswandi NL, Waqas M, et al. Hyaluronic acid, a promising skin rejuvenating biomedicine: a review of recent updates and pre-clinical and clinical investigations on cosmetic and nutricosmetic effects. Int J Biol Macromol. 2018;120:1682–1695. doi:10.1016/j.ijbiomac.2018.09.188
7. Price RD, Berry MG, Navsaria HA. Hyaluronic acid: the scientific and clinical evidence. J Plast Reconstr Aesthet Surg. 2007;60(10):1110–1119. doi:10.1016/j.bjps.2007.03.005
8. Tedeschi A, Lacarrubba F, Micali G. Mesotherapy with an intradermal hyaluronic acid formulation for skin rejuvenation: an intrapatient, placebo-controlled, long-term trial using high-frequency ultrasound. Aesthetic Plast Surg. 2015;39(1):129–133. doi:10.1007/s00266-014-0432-1
9. Taieb M, Gay C, Sebban S, Secnazi P. Hyaluronic acid plus mannitol treatment for improved skin hydration and elasticity: hyaluronic acid plus mannitol for deep hydration. J Cosmet Dermatol. 2012;11(2):87–92. doi:10.1111/j.1473-2165.2012.00608.x
10. Wiest L, Kerscher M. Native hyaluronic acid in dermatology – results of an expert meeting. JDDG. 2008;6(3):176–180. doi:10.1111/j.1610-0387.2008.06639.x
11. Trevidic P, Andre P, Benadiba L, et al. Objective 18-month comparison of the tolerability of 2 dermal fillers formulated with tri-hyal technology. Plast Reconstr Surg Glob Open. 2020;8(12):e3274. doi:10.1097/GOX.0000000000003274
12. Bazin R, Doublet E. Skin Aging Atlas. In: Caucasian Type. Vol. 1. MED’COM; 2007.
13. Braccini F, Dohan Ehrenfest DM. Intérêt des thérapies combinées en médecine esthétique pour le traitement du vieillissement du visage: toxine botulique, fillers et mésothérapie [Advantages of combined therapies in cosmetic medicine for the treatment of face aging: botulinum toxin, fillers and mesotherapy]. Rev Laryngol Otol Rhinol. 2010;131(2):89–95. French.
14. Sparavigna A, Tenconi B, De Ponti I. Antiaging, photoprotective, and brightening activity in biorevitalization: a new solution for aging skin. Clin Cosmet Investig Dermatol. 2015;57. doi:10.2147/CCID.S77742
15. Savoia A, Landi S, Baldi A. A new minimally invasive mesotherapy technique for facial rejuvenation. Dermatol Ther. 2013;3(1):83–93. doi:10.1007/s13555-012-0018-2
16. Lacarrubba F, Tedeschi A, Nardone B, Micali G. Mesotherapy for skin rejuvenation: assessment of the subepidermal low-echogenic band by ultrasound evaluation with cross-sectional B-mode scanning. Dermatol Ther. 2008;21:S1–S5. doi:10.1111/j.1529-8019.2008.00234.x
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.