Back to Journals » Infection and Drug Resistance » Volume 15
Evaluation of the Negative Predictive Value of Methicillin-Resistant Staphylococcus aureus Nasal Swab Screening in the Medical Intensive Care Units and Its Effect on Antibiotic Duration
Authors Tai CH, Liu WL, Pan SC, Ku SC , Lin FJ, Wu CC
Received 30 November 2021
Accepted for publication 11 March 2022
Published 24 March 2022 Volume 2022:15 Pages 1259—1266
DOI https://doi.org/10.2147/IDR.S351832
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Chih-Hsun Tai,1,2 Wei-Ling Liu,2 Sung-Ching Pan,3 Shih-Chi Ku,3 Fang-Ju Lin,1,2,4 Chien-Chih Wu1,2
1Department of Pharmacy, National Taiwan University Hospital, College of Medicine, National Taiwan University, Taipei, Taiwan; 2School of Pharmacy, College of Medicine, National Taiwan University, Taipei, Taiwan; 3Department of Internal Medicine, National Taiwan University Hospital, Taipei, Taiwan; 4Graduate Institute of Clinical Pharmacy, College of Medicine, National Taiwan University, Taipei, Taiwan
Correspondence: Chien-Chih Wu, Department of Pharmacy, National Taiwan University Hospital, College of Medicine, National Taiwan University, Taipei, Taiwan, Tel/Fax +886-2-23123456 ext. 63702 ; +886-2-23310930, Email [email protected] Fang-Ju Lin, Graduate Institute of Clinical Pharmacy, College of Medicine, National Taiwan University, Taipei, Taiwan, Tel/Fax +886-2-33668809 ; +886-2-23310930, Email [email protected]
Background: In addition to active surveillance of methicillin-resistant Staphylococcus aureus (MRSA) carrier, MRSA nasal screening can be valuable for antibiotic de-escalation. This study aimed to assess the correlations between the MRSA nasal swab and subsequent culture results in patients admitted to medical intensive care units (MICU). The impact of MRSA nasal swab on the antibiotic duration was also evaluated.
Materials and Methods: This retrospective study enrolled patients who received glycopeptides in the MICU of a medical center in 2019. Patients treated with glycopeptides for over 2 days before MICU admission were excluded. The associated data were collected through the electronic medical record system. The negative predictive value (NPV) of MRSA nasal swabs for MRSA infection was calculated, and their influence on empirical glycopeptide treatment duration was analyzed.
Results: Of the 338 patients who met the inclusion criteria, 277 underwent MRSA nasal screening. The NPV of MRSA-negative nasal swab for subsequent MRSA infection was 98.4%. The glycopeptide treatment duration of the patients with and without nasal screening was not significantly different (4.2 ± 2.8 vs 4.4 ± 3.0 days, p = 0.577). Of the 120 patients with MRSA-negative nasal swab and no subsequent MRSA infection, 75 continued empirical glycopeptides therapy. The additional treatment time was 3 days (interquartile range: 2– 6 days).
Conclusion: The MRSA nasal swabs have high NPV for MRSA infection in critically ill patients. However, it has no impact on the empirical glycopeptide treatment duration. The value of MRSA nasal swabs should be advocated to optimize antibiotic therapy.
Keywords: MRSA nasal swab, glycopeptides, antibiotic stewardship, critical care
Introduction
The Staphylococcus genus are gram-positive cocci and belong to the family Micrococcaceae.1 Staphylococci are typically categorized as coagulase-positive staphylococci (S. aureus) and coagulase-negative staphylococci (S. epidermidis).1,2 Both pathogens could lead to nosocomial infections, and biofilm formation further complicates clinical management.1,2 Biofilm formation evolves in three steps, starting with nonspecific adherence of individual cells to the materials, followed by growth and biofilm formation, and ending with detachment of surface bacteria.1 In S. epidermidis, biofilm formation is associated with the production of polysaccharide intercellular adhesion (PIA), and raise opsonic antibodies against PIA could be promising for the elimination of colonizing and biofilm-forming S. epidermidis.3,4 Frequently, S. aureus is resistant to methicillin (methicillin-resistant S. aureus [MRSA]) and almost all β-lactam drugs (in up to 50% of hospital isolates).1 MRSA are important bacteria in nosocomial infection and are listed by the World Health Organization as major bacteria in urgent need of new antibiotics.5 In associated treatment guidelines, the risk of MRSA infection—including having previous MRSA infection or colonization, receiving parenteral antibiotic therapy or having hospitalization within 90 days, receiving dialysis, and local rate of MRSA accounting for over 20% of the S. aureus strain—is mostly applied to determine the use of empirical anti-MRSA agents.6,7 Using risk assessment alone may result in overuse of anti-MRSA agents for critically ill patients. According to the internal analysis data, teicoplanin is primarily prescribed in the intensive care unit (ICU) of this hospital (with a monthly average defined daily dose per 1000 inhabitants per day of 257). Moreover, it is generally used as empirical therapy (80% of the patients stopped the treatment within 7 days). According to data from the Taiwan Nosocomial Infections Surveillance System in 2019, S. aureus only accounted for less than 3.5% of total isolates of the ICU of medical centers. Among all S. aureus isolates, MRSA accounted for 64.1%. Recent studies have indicated that MRSA nasal swabs have high negative predictive values (NPVs) in the specimen culture results of all body parts. The MRSA nasal screening can be utilized to reduce unwanted adverse drug reactions and cost by offering a high NPV and the opportunity to discontinue anti-MRSA agents.8–11 Furthermore, the latest community-acquired pneumonia diagnosis and treatment guidelines published by the Infectious Diseases Society of America also emphasize the value of MRSA nasal swabs on antibiotic de-escalation.12
Associated studies on MRSA nasal swabs in Taiwan has mostly examined MRSA colonization rate or the influence of decolonization on the subsequent MRSA infection rate.13–16 Only one study targeted patients admitted to the emergency room with skin infection and analyzed the specificity of MRSA nasal swab for community-acquired MRSA infection.17 As part of infection control policy, MRSA nasal swab screening is often conducted for patients at medical intensive care units (MICU) admission in our hospital. Patients with severe illnesses or MRSA risk factors typically receive empirical anti-MRSA therapy. Therefore, this study aimed to investigate the NPVs of MRSA nasal swabs in the MICU through retrospective analysis. The impact of MRSA nasal swab screening on the treatment duration of empirical glycopeptide therapy was also analyzed.
Materials and Methods
Study Design and Participants
This retrospective single-center study was approved by the institutional review board of National Taiwan University Hospital (202001097RIND). The informed consent was waived because of the retrospective nature of this study and all patient identification was removed. This study was conducted in accordance with the Declaration of Helsinki. The participants were patients who had MICU admission from January 1, 2019, to December 31, 2019. The inclusion criteria were as follows: (1) age ≥ 20 years-old and (2) receiving teicoplanin or vancomycin in the ICU (only those who had MRSA nasal screening within 7 days before or 2 days after they started using teicoplanin or vancomycin were included). Patients who had treated with teicoplanin or vancomycin for over 2 days before MICU admission were excluded. Positive MRSA culture result was defined as the specimen from blood, urine, sputum and catheter of a patient revealed MRSA within 7 days after nasal screening. When the impact of MRSA nasal screening on the treatment duration of glycopeptide was assessed, the patients who died during glycopeptide therapy and those who had other indications for glycopeptide therapy were further excluded. The patients with MRSA nasal screening comprised the experimental group, whereas those without screening formed the control group. The treatment duration of the two groups was analyzed.
Isolation and Identification of MRSA
Nasal screening samples were obtained by rotating sterile swabs (eSwab®, COPAN, Via Perotti, 10-Brescia, Italy) over the nasal vestibule. The swabs and other culture samples were then inoculated onto blood agar plates and incubated for 24 hours for colony identification via MALDI-TOF (Bruker). Identification of MRSA isolates were performed using the VITEK-2 automated antimicrobial susceptibility testing system (bioMérieux, Marcy l’Étoile, France).
Data Collection
Patient data were collected through the electronic medical system of National Taiwan University Hospital, and their demographic data were documented. The collected data were as follows: sex; age; height; kidney function; undergoing renal replacement therapy or not; Acute Physiology and Chronic Health Evaluation II score (APACHE II); risk factors of MRSA infection (previous MRSA infection/received parenteral antibiotics/prior hospitalization for over 2 days/had dialysis within 90 days or had positive influenza test/used anti-influenza drugs in the preceding 5 days); MRSA nasal screening results; specimen culture results within 1 month; and indications, dosage, and treatment duration of teicoplanin or vancomycin.
Calculation of Predictive Value, Sensitivity and Specificity
The sensitivity, specificity, positive predictive value (PPV), and NPV of the MRSA nasal swabs were investigated in the patients who underwent screening to assess whether the swabs can predict MRSA in the subsequent culture specimens. The calculation was based on following equations:
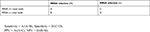 |
|
Sensitivity = A/(A+B), Specificity = D/(C+D).
PPV = A/(A+C), NPV = D/(B+D).
Statistical Analysis
For the assessment of the impact of MRSA nasal swabs on the treatment duration of teicoplanin and vancomycin, the categorical variables were analyzed with the chi-square test or Fisher’s exact test. The continuous variables were analyzed with the independent t test. Moreover, multivariable analysis was performed to examine if the patients’ demographic data and characteristics influence the treatment duration of teicoplanin and vancomycin. The categorical variables were displayed by number and percentage (n, %), and the continuous variables were displayed by mean ± standard deviation (SD). A p value of <0.05 is viewed as statistically significant. The statistical analysis was conducted with SPSS 20 (IBM Corp., Armonk, NY, USA).
Results
This study included 338 patients who received teicoplanin or vancomycin treatment, and 23 patients (7%) had positive MRSA culture (Figure 1). Among them, 277 patients (82.0%) underwent MRSA nasal swab screening and 61 (18.0%) did not. Overall, 214 (63.3%) of the patients were male (Table 1). The average age was 64.9 ± 15.7 years, and the average body mass index (BMI) was 23 ± 4.6 kg/m2. Regarding risk factors for MRSA infection, 12 patients (3.6%) had MRSA infection in the preceding 90 days, 294 (87.0%) had previous parenteral antibiotic therapy, and 289 (85.6%) had prior hospitalization for over 2 days. The number of people undergoing dialysis was 85 (25.1%), and 25 patients (7.4%) tested positive during influenza screening or used anti-influenza drugs in the preceding 5 days. In addition, 280 patients (82.8%) used teicoplanin, and the remaining 58 (17.2%) used vancomycin. Most of the patient who used teicoplanin had creatinine clearance of ≤ 60 mL/min. Most of the patients were treated empirically for pneumonia (58%), followed by intra-abdominal infections (14.2%).
 |
Table 1 Demographic Data of Study Population |
 |
Figure 1 Flow chart. Abbreviations: MRSA, methicillin resistant Staphylococcus aureus; MICU, medical intensive care unit. |
Among the 277 patients who underwent MRSA nasal screening, 21 tested positive, and 15 of them had MRSA in the subsequent culture results. Among the 256 patients who tested negative in the screening, only four had MRSA in the specimen culture results. The sensitivity, specificity, PPV, and NPV of the MRSA nasal swabs for MRSA infection were 78.9%, 97.7%, 71.4%, and 98.4%, respectively (Table 2). The NPV of MRSA nasal swab for different types of infections were ranging from 93.8% to 100%. In addition, among those who tested negative in the screening with no MRSA in the subsequent culture results within 7 days, only one had MRSA in the skin pus culture 27 days after the screening.
 |
Table 2 NPV, PPV, Sensitivity and Specificity of MRSA Nasal Screening by Types of Infections |
The median time required to report the MRSA nasal screening results was 3 days (range: 2–5 days; interquartile range: 2–3 days). The median number of MRSA nasal screenings of the patients within 1 month of ICU admission was one time (range: 0–3 times; interquartile range: 1–1 time). The treatment duration of teicoplanin or vancomycin between patients with and without nasal swab screening did not differ significantly (4.2 ± 2.8 vs 4.4 ± 3.0 days, p = 0.577) (Table 3). The patients with no MRSA nasal swab screening were considerably more likely to have undergone dialysis in the preceding 90 days (59.4% versus 22.1%, p < 0.001). The two groups also differed significantly according to the two kidney function distribution populations (creatinine clearance >60 mL/min or ≤30 mL/min). Multivariable analysis revealed that the patients’ characteristics including gender, age, BMI, APACHE II, and risk factors for MRSA infections had no impact on the treatment duration of teicoplanin or vancomycin. Among the 120 patients who tested negative in the MRSA nasal screening with no subsequent MRSA infection within 7 days, 75 did not stop using teicoplanin or vancomycin following the negative MRSA screening results. The total excessive usage time was 300 days, and the median was 3 days (interquartile range: 2–6 days).
 |
Table 3 Patients with or without MRSA Nasal Screening in Medical Intensive Care Unitsa |
Discussion
This study verified that MRSA nasal swabs have extremely high specificity and NPV for subsequent MRSA infection (97.7% and 98.7%) under 7% prevalence rate of MRSA in this population. These test results can effectively predict whether patients who tested negative in the initial nasal swab will have MRSA culture in the subsequent 7 days or not. Thus, they can be used for antibiotic de-escalation to stop unnecessary anti-MRSA therapy. Related research in different ICU has shown the correlations between MRSA-negative nasal swab and negative MRSA culture results.9,18–21 The swabs were not only applicable to respiratory tract specimens but also had extremely high NPVs in the bacterial culture results of specimens in other sites (eg, blood, catheters, and urinary catheters), which was compatible with our result.11,19 When applying MRSA nasal swab for antibiotic de-escalation, the prevalence rate of MRSA in the population should take into consideration. The NPV of MRSA nasal swab would decrease when the prevalence rate increase. Sarikonda et al. reported the NPV of MRSA nasal screen was around 85%, which was lower than that of this study due to higher MRSA prevalence rate (~16%).18 The patients in our study were treated with teicoplanin or vancomycin empirically in the ICU (ie, the patient group deemed by doctors to have high MRSA infection risk). Thus, the NPV of MRSA nasal swabs for MRSA infection is also applicable to this high-risk group. This result is consistent with the latest community-acquired pneumonia diagnosis and treatment guidelines published by the Infectious Diseases Society of America. The guidelines recommend ceasing empirical anti-MRSA therapy upon receiving negative MRSA nasal screening results, particularly for patients with community-acquired pneumonia that is not severe.12 However, the sensitivity and PPV of the MRSA nasal swabs are unfavorable, and they are unreliable for the prediction of MRSA infection.18
This study indicated that the MRSA-negative nasal swabs do not influence the treatment duration of empirical teicoplanin or vancomycin of patients in MICU. A retrospective study reported that nasal MRSA polymerase chain reaction screening can significantly reduce the duration of empirical anti-MRSA therapy by approximately 2 days for patients with suspected MRSA pneumonia.8 However, the MRSA nasal screening in this study employed culture-based method, which generally takes 3 days (the polymerase chain reaction test requires only 1~2 hours).8 The turnaround time of nasal swab results were close to that of specimen culture results, which may result in similar treatment duration. In spite of the higher cost of PCR-based screening (~35 USD/time), it should be more cost effectiveness than culture-based method by shortening costly antibiotic use such as teicoplanin (~60 USD/day). Further study to demonstrate the better cost effectiveness of PCR-based screening than that of culture-based screening was needed to increase its clinical application.
A prospective observational study reported that up to 83% of the anti-gram-positive antibiotics usage is inappropriate, and 78.5% of the inappropriate use is attributable to the absence of de-escalation.22 However, antibiotic de-escalation has been demonstrated to not increase mortality rates for hospital-acquired pneumonia. Instead, it can reduce the hospitalization duration and acute kidney injury.23 The MRSA nasal swab is indeed a useful de-escalation tool. In many hospitals, routine MRSA nasal screening is conducted for patients at admission, and some institutions even perform weekly screening after ICU admission.24 However, in some hospitals in the Asia-Pacific region, MRSA nasal screening is not routinely conducted because of limited medical resources.25 The MRSA nasal swab can be used to identify carrier for contact isolation, and its additional application is also promoted in recent research. Active screening can be used as the basis for future antibiotic de-escalation when empirical glycopeptide is prescribed to patients.26,27
Because Staphylococci could lead to severe disease and are highly resistant to antibiotics, preventive strategy such as vaccine has been investigated. Vaccine could prevent or decrease the severity of Staphylococci infection through blocking the effect of toxins, blocking the functional surface adhesins or other relevant proteins, or stimulating phagocytosis.28 Experimental vaccines have been developed and against constituents as diverse as the capsule or specific surface determinants such as PIA and S.epidermidis surface protein C (SesC).28 Most of these vaccines conferred some protection in experimental models. For example, Mirzaei et al. have demonstrated that the conjugation of PIA with a specialized superficial protein of S.epidermidis as a carrier enhances the function of raised antibodies both in in-vivo and in-vitro experiments.4 The arisen antibodies had the adequate effectiveness in biofilm inhibition so that about 90% of bacterial killing in phagocytosis and survival likelihood occurred following the intravenous challenges by S.epidermidis.4 Although the data are promising in animal model, the vaccine efficacy remains controversial in clinical trial. A conjugated capsular vaccine conferred promising but transient protection in patients on hemodialysis; however, the other trial using a vaccine targeted on the iron surface determinant B to prevent deep sternal wound infections did not provide protection and paradoxically increased the mortality rate in S. aureus–infected patients.29,30 It seems that vaccine targeting on different site may have distinct effect. Further studies are needed to prove the efficacy of anti-staphylococcal vaccine.
This study has several limitations. First, this was a retrospective single-center study. Thus, the NPV of our study may not be generalizable to other ICUs with different MRSA prevalence rates. Second, the time of MRSA nasal screening and administering anti-MRSA agents could not be fully controlled. Not all patients received the anti-MRSA agents on the same day as the MRSA nasal screening. The screening results or the calculation of excessive treatment duration of anti-MRSA agents may have been affected accordingly. Moreover, the judgment of MRSA infection in this study was based on the diagnosis of clinicians and the culture results. The MRSA colonization and infection were not truly distinguished. Therefore, the true subsequent MRSA infection rate may even lower.
Conclusions
The MRSA nasal swabs have high NPV of MRSA infection for different types of infections in critically ill patients. However, it has no impact on the empirical glycopeptide treatment duration. The value of MRSA nasal swabs should be advocated to optimize antibiotic therapy.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Que YA, Moreillon P. Staphylococcus aureus (Including Staphylococcal Toxic Shock Syndrome). In: Bennett JE, Dolin R, Blaser MJ, editors. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases.
2. Rupp ME, Fey PD. Staphylococcus epidermidis and other coagulase-negative Staphylococci. In: Bennett JE, Dolin R, Blaser MJ, editors. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases.
3. Mirzaei B, Moosavi SF, Babaei R, Siadat SD, Vaziri F, Shahrooei M. Purification and evaluation of Polysaccharide Intercellular Adhesion (PIA) antigen from Staphylococcus epidermidis. Curr Microbiol. 2016;73(5):611–617. doi:10.1007/s00284-016-1098-5
4. Mirzaei B, Mousavi SF, Babaei R, et al. Synthesis of conjugated PIA-rSesC and immunological evaluation against biofilm-forming Staphylococcus epidermidis. J Med Microbiol. 2019;68(5):791–802. doi:10.1099/jmm.0.000910
5. Tacconelli E, Carrara E, Savoldi A, et al. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis. 2018;18(3):318–327. doi:10.1016/S1473-3099(17)30753-3
6. Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63(5):e61–e111. doi:10.1093/cid/ciw353
7. Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the infectious diseases society of America. Clin Infect Dis. 2014;59:147–159. doi:10.1093/cid/ciu444
8. Baby N, Faust AC, Smith T, Sheperd LA, Knoll L, Goodman EL. Nasal Methicillin-Resistant Staphylococcus aureus (MRSA) PCR testing reduces the duration of MRSA-targeted therapy in patients with suspected MRSA pneumonia. Antimicrob Agents Chemother. 2017;61:e02432–16.
9. Chotiprasitsakul D, Tamma PD, Gadala A, Cosgrove SE. The role of negative methicillin-resistant Staphylococcus aureus nasal surveillance swabs in predicting the need for empiric vancomycin therapy in intensive care unit patients. Infect Control Hosp Epidemiol. 2018;39:290–296. doi:10.1017/ice.2017.308
10. Giancola SE, Nguyen AT, Le B, et al. Clinical utility of a nasal swab methicillin-resistant Staphylococcus aureus polymerase chain reaction test in intensive and intermediate care unit patients with pneumonia. Diagn Microbiol Infect Dis. 2016;86(3):307–310. doi:10.1016/j.diagmicrobio.2016.08.011
11. Mergenhagen KA, Starr KE, Wattengel BA, Lesse AJ, Sumon Z, Sellick JA. Determining the utility of methicillin-resistant Staphylococcus aureus nares screening in antimicrobial stewardship. Clin Infect Dis. 2020;71(5):1142–1148. doi:10.1093/cid/ciz974
12. Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200(7):e45–e67. doi:10.1164/rccm.201908-1581ST
13. Kang YC, Tai WC, Yu CC, Kang JH, Huang YC. Methicillin-resistant Staphylococcus aureus nasal carriage among patients receiving hemodialysis in Taiwan: prevalence rate, molecular characterization and de-colonization. BMC Infect Dis. 2012;12(1):284. doi:10.1186/1471-2334-12-284
14. Lee YJ, Chen JZ, Lin HC, et al. Impact of active screening for methicillin-resistant Staphylococcus aureus (MRSA) and decolonization on MRSA infections, mortality and medical cost: a quasi-experimental study in surgical intensive care unit. Crit Care. 2015;19(1):143. doi:10.1186/s13054-015-0876-y
15. Tsai MS, Chen CJ, Lin TY, Huang YC. Nasal methicillin-resistant Staphylococcus aureus colonization among otherwise healthy children aged between 2 months and 5 years in northern Taiwan, 2005-2010. J Microbiol Immunol Infect. 2018;51(6):756–762. doi:10.1016/j.jmii.2017.07.014
16. Hsu YY, Wu D, Hung CC, et al. Methicillin-resistant Staphylococcus aureus nasal colonization among HIV-infected patients in Taiwan: prevalence, molecular characteristics and associated factors with nasal carriage. BMC Infect Dis. 2020;20(1):254. doi:10.1186/s12879-020-04979-8
17. Hsu MS, Liao CH, Fang CT. Role of nasal swab culture in guiding antimicrobial therapy for acute cellulitis in the era of community-acquired methicillin-resistant Staphylococcus aureus: a prospective study of 89 patients. J Microbiol Immunol Infect. 2019;52(3):494–497. doi:10.1016/j.jmii.2019.04.001
18. Sarikonda KV, Micek ST, Doherty JA, Reichley RM, Warren D, Kollef MH. Methicillin-resistant Staphylococcus aureus nasal colonization is a poor predictor of intensive care unit-acquired methicillin-resistant Staphylococcus aureus infections requiring antibiotic treatment. Crit Care Med. 2010;38(10):1991–1995. doi:10.1097/CCM.0b013e3181eeda3f
19. Smith MN, Erdman MJ, Ferreira JA, Aldridge P, Jankowski CA. Clinical utility of methicillin-resistant Staphylococcus aureus nasal polymerase chain reaction assay in critically ill patients with nosocomial pneumonia. J Crit Care. 2017;38:168–171. doi:10.1016/j.jcrc.2016.11.008
20. Pangli H, Papp A. The relation between positive screening results and MRSA infections in burn patients. Burns. 2019;45(7):1585–1592. doi:10.1016/j.burns.2019.02.023
21. Stodghill J, Finnigan A, Newcomb AB, Lita E, Liu C, Teicher E. Predictive Value of the methicillin-resistant Staphylococcus aureus nasal swab for methicillin-resistant staphylococcus aureus ventilator-associated pneumonia in the trauma patient. Surg Infect (Larchmt). 2021;22:889–893. doi:10.1089/sur.2020.477
22. Özger HS, Fakıoğlu DM, Erbay K, Albayrak A, Hızel K. Inapropriate use of antibiotics effective against gram positive microorganisms despite restrictive antibiotic policies in ICUs: a prospective observational study. BMC Infect Dis. 2020;20(1):289. doi:10.1186/s12879-020-05005-7
23. Cowley MC, Ritchie DJ, Hampton N, Kollef MH, Micek ST. Outcomes associated with de-escalating therapy for methicillin-resistant staphylococcus aureus in culture-negative nosocomial pneumonia. Chest. 2019;155(1):53–59. doi:10.1016/j.chest.2018.10.014
24. Parente DM, Cunha CB, Mylonakis E, Timbrook TT. The clinical utility of Methicillin-Resistant Staphylococcus aureus (MRSA) nasal screening to rule out MRSA pneumonia: a diagnostic meta-analysis with antimicrobial stewardship implications. Clin Infect Dis. 2018;67(1):1–7. doi:10.1093/cid/ciy024
25. Cho SY, Chung DR. Infection prevention strategy in hospitals in the era of community-associated methicillin-resistant Staphylococcus aureus in the Asia-Pacific Region: a review. Clin Infect Dis. 2017;64:S82–S90. doi:10.1093/cid/cix133
26. Woolever NL, Schomberg RJ, Cai S, Dierkhising RA, Dababneh AS, Kujak RC. Pharmacist-driven MRSA nasal PCR screening and the duration of empirical vancomycin therapy for suspected MRSA respiratory tract infections. Mayo Clin Proc Innov Qual Outcomes. 2020;4(5):550–556. doi:10.1016/j.mayocpiqo.2020.05.002
27. Dadzie P, Dietrich T, Ashurst J. Impact of a pharmacist-driven methicillin-resistant Staphylococcus aureus polymerase chain reaction nasal swab protocol on the de-escalation of empiric vancomycin in patients with pneumonia in a rural healthcare setting. Cureus. 2019;11(12):e6378. doi:10.7759/cureus.6378
28. Mirzaei B, Babaei R, Valinejad S. Staphylococcal vaccine antigens related to biofilm formation. Hum Vaccin Immunother. 2021;17(1):293–303. doi:10.1080/21645515.2020.1767449
29. Shinefield H, Black S, Fattom A, et al. Use of a Staphylococcus aureus conjugate vaccine in patients receiving hemodialysis. N Engl J Med. 2002;346:491–496. doi:10.1056/NEJMoa011297
30. Fowler VG, Allen KB, Moreira ED, et al. Effect of an investigational vaccine for preventing Staphylococcus aureus infections after cardiothoracic surgery: a randomized trial. JAMA. 2013;309(13):1368–1378. doi:10.1001/jama.2013.3010
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
