Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 7
Evaluation of the indications and arrhythmic patterns of 24 hour Holter electrocardiography among hypertensive and diabetic patients seen at OAUTHC, Ile-Ife Nigeria
Authors Adebayo R , Ikwu A, Balogun M , Akintomide A, Mene-Afejuku T , Adeyeye V, Bamikole O, Bisiriyu L, Ajayi O, Ogunyemi S, Oketona O
Received 26 May 2014
Accepted for publication 13 August 2014
Published 26 November 2014 Volume 2014:7 Pages 565—570
DOI https://doi.org/10.2147/DMSO.S68408
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ming-Hui Zou
Rasaaq A Adebayo,1 Amanze N Ikwu,1 Michael O Balogun,1 Anthony O Akintomide,1 Tuoyo O Mene-Afejuku,1 Victor O Adeyeye,1 Olaniyi J Bamikole,1 Luqman A Bisiriyu,2 Olufemi E Ajayi,1 Suraj A Ogunyemi,1 Omolola A Oketona1
1Cardiology Unit, Department of Medicine, Obafemi Awolowo University Teaching Hospitals Complex, 2Department of Demography and Social Statistics, Obafemi Awolowo University, Ile-Ife, Osun State, Nigeria
Background: There are very limited published studies in Nigeria on the use of 24 hour Holter electrocardiogram (Holter ECG) in the arrhythmic evaluation of hypertensive and diabetic patients.
Objective: To evaluate indications, arrhythmic pattern of Holter ECG, and heart rate variability (HRV) among patients with hypertensive heart disease (HHD) with or without heart failure and type 2 diabetes mellitus (T2DM) seen in our cardiac care unit.
Methods: Seventy-nine patients (32 males and 47 females) were studied consecutively over a year using Schiller type (MT-101) Holter ECG machine.
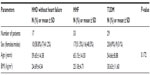
Results: Out of the 79 patients, 17 (21.5%) had HHD without heart failure, 33 (41.8%) had HHD with hypertensive heart failure (HHF), while 29 (36.7%) were T2DM patients. The mean (standard deviation) ages of HHD without heart failure, HHF and T2DM patients were 59.65 (±14.38), 65.15 (±14.30), and 54.66 (±8.88) respectively. The commonest indication for Holter ECG was palpitation (38%), followed by syncope (20.3%). Premature ventricular contraction was the commonest arrhythmic pattern among the 79 patients, especially among HHF patients. The HRV, using standard deviation of all normal-normal intervals was significantly reduced in T2DM patients (81.03±26.33, confidence interval [CI] =71.02–91.05) compared to the HHD without heart failure (119.65±29.86, CI =104.30–135.00) and HHF (107.03±62.50, CI =84.00–129.19). There was a negative correlation between the duration of T2DM and HRV (r=−0.613).
Conclusion: Palpitation was the commonest Holter ECG indication and premature ventricular contractions were the commonest arrhythmic pattern among our patients. HRV was reduced in T2DM patients compared with hypertensive patients.
Keywords: Holter electrocardiography, arrhythmias, hypertension, diabetes mellitus, Nigerians
Introduction
Hypertension and diabetes mellitus (DM) are common and a major public health problem in Nigeria.1,2 Twenty-four hour Holter electrocardiogram (Holter ECG) has been found useful in the detection of ischemic changes, cardiac arrhythmias, and heart rate variability (HRV) among hypertensive and diabetic patients.3,4 Type 2 diabetes mellitus (T2DM) and hypertensive left ventricular hypertrophy (LVH) are associated with increased risk of arrhythmias and mortality.5,6 Yet there are very limited published studies in Nigeria on the use of 24 hour Holter ECG in the arrhythmic evaluation of hypertensive and diabetic patients.7–9 In this study,7 it was observed that LVH increased arrhythmias, and multivalvular regurgitations predispose to greater supra-ventricular tachycardia and complex ventricular arrhythmias, especially in hypertensive heart failure (HHF).
In another study, palpitation was the commonest 24 hour Holter indication and premature ventricular contraction (PVC) was the commonest arrhythmic pattern in hypertensive heart disease (HHD) and chest pain patients.8 Adebola et al9 also reported that the commonest indications for Holter monitoring in their patients were unexplained palpitation, dizzy spells, chest pain and syncope. Rare indications include unexplained dyspnea on exertion and pacemaker implantation. More than half of the study subjects (54%) had documented episodes of severe tachycardia (heart rate ≥120 bpm) while 18 patients had severe bradycardia (heart rate ≤40 beats per minute). Twenty-one patients were confirmed to have ventricular ectopics while three patients had non-sustained ventricular tachycardia.9
We therefore evaluated the indications, arrhythmic pattern of Holter ECG, and HRV among patients with HHD without heart failure, HHF and T2DM seen in our cardiac care unit at Obafemi Awolowo University Teaching Hospitals Complex (OAUTHC), Ile-Ife, Osun State, Nigeria. This will further help define the pattern of Holter ECG in hypertensive and diabetic subjects in our environment, and the data from this study will also add to the national and global database.
Materials and methods
This is a descriptive cross-sectional study in which consecutive diabetic and hypertensive patients referred to our cardiac care unit for 24 hour Holter ECG over a 12 month period were studied using Schiller type (MT-101) Holter ECG machine.
Inclusion criteria:
- those who gave informed consent to participate in the study;
- adult patients with HHD with or without heart failure;
- adult patients with T2DM.
Exclusion criteria:
- those who were unwilling to participate in the study;
- hypertensive patients with myocardial infarction, renal failure, diabetic mellitus, or cerebrovascular diseases.
Glycated hemoglobin A1c was obtained in all T2DM patients. We separated the hypertensive patients into HHD without heart failure and HHD with heart failure. LVH and diastolic dysfunction are the early manifestations of cardiovascular target organ damage in patients with arterial hypertension and signify HHD, and can result in heart failure, arrhythmias, myocardial infarction, and sudden cardiac death.10 HHD with HHF patients were recruited from patients with documented systemic hypertension or on anti-hypertensive medication who satisfy the criteria for diagnosis of heart failure based on Framingham’s criteria11 or the European Society of Cardiology criteria.12 The clinical indications for Holter ECG were documented based on the recommendations of the American College of Cardiology (ACC)/American Heart Association (AHA) guidelines for ambulatory electrocardiography.13 The Holter ECGs were read by an experienced cardiologist and two senior registrars in cardiology. Ethical approval was obtained from the Ethics and Research Committee of OAUTHC and all participants gave informed consent.
The HRV was analyzed in the time domain in accordance with ACC/AHA Guidelines.13 The three parameters of the time domain HRV assessed were:
- standard deviation of all normal-normal (NN) intervals (SDNN);
- standard deviation of the averages of NN intervals in all 5 minute segments of the entire recording (SDANN); and
- the square root of the mean of the sum of the squares of differences between adjacent NN intervals (RMS-SD).
The normal values of HRV used for the study were in accordance with the ACC/AHA Guidelines for Ambulatory ECG,13 and are as follows: SDNN (141±39 milliseconds [ms]), SDANN (127±39 ms), and RMD-SD (37±15 ms).
The data were analyzed using both descriptive and inferential statistics. The descriptive statistics include frequency distribution, scatter plot, mean, and standard deviation (SD). Inferential statistics that were used in the study were analysis of variance (ANOVA) and Pearson’s correlation. ANOVA was used to determine whether differences exist between HRV using SDNN average of HHD without heart failure, HHF and T2DM patients. Pearson’s correlation was used to examine the relationship between duration of T2DM or hypertension and HRV. The duration of T2DM or hypertension was grouped into less than 5 years, 5–10 years, and greater than 10 years. For the 29 T2DM patients, ten patients were grouped into less than 5 years; nine patients were grouped into 5–10 years, while ten patients were grouped into greater than 10 years. 16, 12 and 22 patients out of the 50 hypertensive patients were also grouped into less than 5 years, 5–10 years and greater than 10 years respectively.
Results
Demography and clinical indications
Demography and clinical characteristics of the studied patients were as shown in Table 1. Out of the 79 patients reviewed, 32 were males (40.5%), while 47 were females (59.5%). Seventeen (21.5%) patients had HHD without heart failure, 33 (41.8%) had HHF, while 29 (36.7%) were T2DM patients. The mean SD ages (years) of HHD without heart failure, HHF, and T2DM patients were 59.65 (±14.38), 65.15 (±14.30), and 54.66 (±8.88), respectively. The mean (SD) body mass index (BMI in kg/m2) of HHD without heart failure, HHF, and T2DM were 24.69 (±4.34), 25.18 (±4.71), and 30.63 (±11.6), respectively. The mean (SD) hemoglobin A1c (%) of T2DM patients are 7.33% (±1.18%). The mean (SD) of the duration of T2DM and hypertension (years) are 6.95±4.40 and 9.08±6.58, respectively.
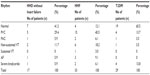
Clinical indications for Holter ECG are shown in Table 2 and the commonest indication for Holter ECG among our study group was palpitation (38%). PVCs were the commonest type of arrhythmia accounting for 69.4% among patients with arrhythmia, followed by premature atrial contractions (8.2%) as shown in Table 3. PVCs were observed in 45.5% of HHF patients, 29.4% of HHD without heart failure and 13.7% of T2DM patients respectively. The mean total PVCs/24 hours was numerically more in HHF (5,936) as compared to HHD without failure (1,946) and T2DM (1,088). Eighteen point two percent of HHF patients had non-sustained ventricular tachycardia and one HHF patient had sustained ventricular tachycardia.
  | Table 2 Clinical indications |
Applying Lown’s grading of PVC14 among patients with PVC, Lown’s grade 2 was commonest in the HHD without heart failure (35.3%) and T2DM (10.3%) patients, while Lown’s grade 4 was commonest among HHF patients (51.5%). Nine point one percent of HHF patients had fibrillation (AF). Seven patients had severe bradycardia (heart rate less than 40 beats per minute).
HRV
The mean (SD) average heart rate of HHD without heart failure, HHF, and T2DM patients were 68.47 (±10.35), 81.12 (±18.73), and 80.52 beats per minute (±12.68) respectively. The HRV using SDNN average (ms) was significantly reduced in T2DM patients (81.03±26.33, confidence interval [CI] =71.02–91.05) compared to the HHD without heart failure (119.65±29.86, CI =104.30–135.00) and HHF (107.03±62.50, CI =84.00–129.19) patients; (P<0.015). Similar findings were also noted when RMD-SD average (ms) was used to assess the HRV, with significant reduction in T2DM patients (36.48±21.10, CI =28.46–44.51) compared to HHD without heart failure (103.82±78.91, CI =63.25–144.39) and HHF (99.45±88.14, CI =60.51–94.05) patients. When SDANN average (ms) was used, the HRV of HHD without heart failure, HHF and T2DM patients were variously reduced in the three studied groups, being 81.18 ms ±21.58 CI =70.08–92.27, 67.64 ms ±37.10 CI =54.48–80.79 and 65.79 ms ±18.92 CI =58.60–72.99, respectively, (P>0.078). Using SDNN, there was a statistically significant variation between day and night HRV in T2DM patients (P<0.001) and also in HHF patients (P<0.001), while the day and night HRV in HHD (without heart failure) patients was not statistically significant (P>0.051). There was a negative correlation between the duration of T2DM and HRV; (r=−0.613, P<0.001), as shown in Figure 1. The duration of hypertension and HRV was also negatively correlated but was not statistically significant; (r=−0.51, P>0.731), as shown in Figure 2.
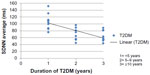  | Figure 1 Relationship between HRV and duration of T2DM. |
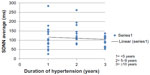  | Figure 2 Relationship between HRV and duration of hypertension. |
Discussion
Arrhythmias, both atrial and ventricular are common co-morbidities in hypertension and DM.3,13 The underlying mechanisms of arrhythmogenicity in hypertension and DM include LVH, myocardial ischemia, impaired left ventricular function, and left atrial enlargement, among other factors.6,16
The age in the demography of our study group revealed that HHF patients were older than HHD (without heart failure) and T2DM. Obesity (grade 1) was prevalent in the T2DM patients in our study group.
The commonest indication for Holter ECG among our study group was palpitation (38%). Katibi et al7 reported palpitation as the commonest indication (25%). PVC was the commonest arrhythmic pattern among our study group (69.4%). PVCs were more prevalent in HHF patients (45.5%). Katibi et al7 also reported PVC as the commonest arrhythmic pattern (47.6%) in his study. The higher proportion in our study may be attributable to the fact that, our study group already has a cardiovascular disease risk factor or a cardiac disease. However, Schillaci et al17 in Italy studied 126 never treated subjects with essential hypertension and reported PVCs in 71% of the subjects. The higher proportion in his study may be due to the fact that his study groups are – “never treated hypertensive patients”. Schillaci et al17 also reported that complex ventricular arrhythmias (Lown’s class ≥2) were predicted by age greater than or equal to 60 years.
LVH and complex ventricular arrhythmias are significant predictors of sudden cardiac death.16–19 Cygankiewicz et al20 reported that combining electrocardiographic stratification with assessment of myocardial substrate may provide insight into interplay between factors contributing to sudden cardiac death in heart failure. In our study, Lown’s grade 4 was found more prevalent in HHF patients compared with HHD (without heart failure) and T2DM patients. Advanced HHF irreversibly damages myocardial fibers which may serve as arrhythmogenic foci for various forms of arrhythmias.21,22 Ajayi et al8 in a study of 24 hour Holter arrhythmic patterns of 37 HHF patients with or without valvular heart disease, reported that LVH increases arrhythmias but multivalvular regurgitation predisposes to greater supraventricular tachycardia and complex ventricular arrhythmias. Nine point one percent of HHF patients had AF. Familoni et al23 in a clinical study of pattern and factors affecting outcome in Nigerian patients with advanced heart failure, reported that AF was associated with increased mortality rates among patients with advanced heart failure.
HRV is a temporal variation between sequences of consecutive heart beats.24 The state of activity of the cardiac autonomic nervous system is evaluated by HRV. Diabetic autonomic neuropathy is a serious and common complication of DM and dysfunction of the autonomic nervous system is associated with increased risk of mortality in patients with diabetes.25,26 At the time of diagnosis, a reduced HRV is evident in T2DM patients which reflects the asymptomatic process over the years before diagnosis.27,28
T2DM is characterized by widespread degeneration of the small nerve fibers of sympathetic and parasympathetic branches of the autonomic nervous system, which may affect autonomic regulation of the sinus node, thereby reducing HRV.29 The degree of autonomic dysfunction associated with DM is related to the severity and duration of the disease.29 Diabetic patients with autonomic neuropathy also display impairment of the absolute values of the frequency domain analysis (both low-frequency component and high-frequency component). Studies in DM patients with or without chronic autonomic neuropathy indicate that both time- and frequency-domain measures of spontaneous baroreceptor-cardiac reflex sensitivity could allow early detection of chronic autonomic neuropathy in diabetic patients.30,31
HRV is also reduced in hypertensive patients when compared with normotensive individuals, indicating a decrease in baroreceptor reflex.32 Using SDNN and RMD-SD, the HRV in T2DM patients was significantly reduced in our study. Boer-Martins et al in Brazil33 reported decreased HRV in T2DM (mean age 54.7 years), and BMI (33.7±4 kg/m2) patients with resistant HTN, when compared with non-T2DM group. Nolan et al34 in UK-HEART study of HRV and mortality in chronic heart failure patients (mean age 62±9.6 years), reported decreased HRV in chronic heart failure patients in some of the time domain parameters. In our study, the HRV of HHF patients using SDANN was also decreased. Our study showed a negative correlation between the duration of T2DM and HRV. Fujimoto et al35 in Japan reported that all spectral variables of HRV were inversely related to the duration of DM in all diabetic patients. Khoharo et al36 reported that chronic autonomic neuropathy as evidenced by decreased HRV was common in diabetic patients of greater than 5 years duration when compared with diabetic patients of less than 5 years.
The limitation of the study is the inability of the present Holter system software MT-101 to calculate the frequency domain parameters of HRV.
Conclusion
Palpitation was the commonest Holter ECG indication and PVCs were the commonest arrhythmic pattern among our patients. HRV was reduced in T2DM patients compared with hypertensive patients.
Disclosure
The authors have no conflicts of interest to disclose.
References
Ogah OS, Okpechi I, Chukwunonye II, et al. Blood Pressure, Prevalence of Hypertension and Hypertension Related Complications in Nigerian Africans: A Review. World J Cardiol. 2012;4(12):327–340. | |
Akinkugbe OO. Non-communicable Disease in Nigeria. Final Report of National Survey, Federal Ministry of Health and Social Services, Lagos.1997:64–90. | |
Takase B, Kurita A, Noritake M, et al. Heart Rate Variability in Patients with Diabetes Mellitus, Ischemic Heart Disease and Congestive Heart Failure. J Electrocardiol.1992;25(2):79–88. | |
Gasilin VS, Stetsenko AE, Kruglov VA, Boikova OI, Chernysheva GV. [Possibilities of Holter ECG Monitoring in Detecting Arrhythmias and Myocardial Ischemia in Persons with Ischemic Heart Disease Risk Factors]. Kardiologiia. 1989;29(3):10–13. Russian. | |
Movahed MR, Hashemzadeh M, Jamal M. Increased Prevalence of Ventricular Fibrillation in Patients with Type 2 Diabetes Mellitus. Heart Vessels. 2007;22(4):251–253. | |
Galinier M, Balanescus S, Fourcade J, et al. [Prognostic Value of Ventricular Arrhythmia in Hypertensive Patients]. Arch Mal Coeur Vaiss. 1997;90(8):1049–1053. French. | |
Katibi IA, Beshir S, Mudashiru Z. Ambulatory 24-hour Holter Electrocardiography among Nigerians: Our Experience at a Referral Cardiac Centre in Lagos, Nigeria. Nigerian Medical Journal. 2006;47(2):25–27. | |
Ajayi OE, Ajayi AA. Valvular Regurgitations may Increase the Risk of Arrhythmias in Hypertensive Heart Failure in Nigerians. J Cardiovasc Med (Hagerstown). 2013;14(6):453–460. | |
Adebola AP, Daniel FA, Lasisi GT, Ogunleye O. 24-Hour Holter Monitoring at the Lagos State University Teaching Hospital-a report of 85 cases. Nigerian Journal of Clinical Medicine. 2009;2(2). | |
Diamond JA, Phillips RA. Hypertensive Heart Disease. Hypertens Res. 2005;28(3):191–202. | |
McKee PA, Castelli WP, McNamara, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med. 1971;285(26):1441–1446. | |
McMurray JJ, Adamopoulos S, Anker SD, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012;33(14):1787–1847. | |
Crawford MH, Bernstein SJ, Deedwania PC, et al. ACC/AHA Guidelines for Ambulatory Electrocardiography. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the Guidelines for Ambulatory Electrocardiography). Developed in collaboration with the North American Society for Pacing and Electrophysiology. J Am Coll Cardiol. 1999;34(3):912–948. | |
Lown B, Wolf M. Approaches to Sudden Death from Coronary Heart Disease. Circulation. 1971;44(1):130–142. | |
Anastos K, Charney P, Charon R A, et al. Hypertension in Women: What is Really Known? The Women Caucus, Working Group on Women’s Health of the Society of General Internal Medicine. Ann Intern Med. 1991;115(4):287–293. | |
Powers AC. Diabetes Mellitus. In: Kasper DL, Fausi AS, Braunwald E, et al, editors. Harrison’s Principles of Internal Medicine. 16th ed. New York: McGraw-Hill; 2005;2:2166–2167. | |
Schillaci G, Verdechia P, Borgioni C, et al. Association Between Persistent Pressure Overload and Ventricular Arrhythmias in Essential Hypertension. Hypertension. 1996;28(2):284–289. | |
Saadeh AM, Jones JV. Predictors of Sudden Cardiac Death in Never Previously Treated Patients with Essential Hypertension: Long Term Follow-up. J Hum Hypertens. 2001;15(10):677–680. | |
Dzudie A, Milo O, Edwards C, et al. Prognostic Significance of Electrocardiographic Abnormalities for Mortality Risk in Acute Heart Failure: Insight from sub-Saharan Africa Survey for Heart Failure (THESUS-HF). J Card Fail. 2014;20(1):45–52. | |
Cygankiewicz I, Zareba W, de Luna AB. Prognostic Value of Holter Monitoring in Congestive Heart Failure. Cardiol J. 2008;15(4):313–323. | |
Pohwidz SM, Corr PB. Biochemical and Electrophysiological Alterations Underlying Ventricular Arrhythmias in the Failing Heart. Eur Heart J. 1994;15 Suppl D:145–154. | |
Araoye MA, Olowoyeye JO. The Clinical Spectrum of Hypertensive Heart Failure: A Point-score System for Solving an Old Problem. East Afr Med J. 1984;61(4):306–315. | |
Familoni OB, Olunuga TO, Olufemi BW. A Clinical Study of Pattern and Factors Affecting Outcome in Nigerian Patients with Advanced Heart Failure. Cardiovasc J Afr. 2007;18(5):308–311. | |
Karim N, Hassan JA, Ali SS. Heart Rate Variability-A Review. Journal of Basic and Applied Sciences. 2011;7(1):71–77. | |
Vinik AI, Maser RE, Mitchell BD, Freeman R. Diabetic Autonomic Neuropathy. Diabetes Care. 2003;26(5):1553–1579. | |
Gerritsen J, Dekker JM, TenVoorde BJ, et al. Impaired Autonomic Function is Associated with Increased Mortality Especially in Subjects with Diabetes, Hypertension, or a History of Cardiovascular Disease. Diabetes Care. 2001;24(10):1793–1798. | |
Schonauer M, Thomas A, Morbach S, Niebauer J, Schonauer U, Thiele H. Cardiac Autonomic Diabetic Neuropathy. Diab Vasc Dis Res. 2008;5(4):336–344. | |
Pfeifer MA, Weinberg CR, Cook DL, et al. Autonomic Neural Dysfunction in Recently Diagnosed Diabetic Subjects. Diabetic Care.1984;7(5):447–453. | |
Ahamed Seyd PT, Thajudin Ahamed VI, Jacob J, Joseph PK. Time and frequency domain analysis of HRV and their correlations in DM. World Academy of Science, Engineering and Technology. 2008;2:3–26. | |
Frattola A, Parati G, Gamba P, et al. Time and frequency domain estimates of spontaneous baroreflex sensitivity provide early detection of autonomic dysfunction in DM. Diabetologia. 1997;40(12):1470–1475. | |
Weston PJ, Panerai RB, McCullough A, et al. Assessment of baroreceptor-cardiac reflex sensitivity using time domain analysis in patients with IDDM and the relation of left ventricular mass index. Diabetologia. 1996;39(11):1385–1391. | |
Menezes Aa S Jr, Moreira HG, Daher MT. Analysis of Heart Rate Variability in Hypertensive Patients Before and After Treatment with Angiotensin II Converting Enzyme Inhibitors. Arg Bras Cardiol. 2004; 83(2):169–172. | |
Boer-Martins L, Figueiredo VN, Demacq C, et al. Relationship of Autonomic Imbalance and Circadian Disruption with Obesity and Type 2 Diabetes in Resistant Hypertension Patients. Cardiovasc Diabetol. 2011;10:24. | |
Nolan J, Batin PD, Andrews R, et al. Prospective Study of Heart Rate Variability and Mortality in Chronic Heart Failure. Circulation. 1998;98(15):1510–1516. | |
Fujimoto Y, Fukuki M, Hoshio A, et al. Decreased Heart Rate Variability in Patients with Diabetes Mellitus and Ischemic Heart Disease. Jpn Circ J. 1996;60(12):925–932. | |
Khoharo HK, Halepoto AW. QTc- interval, Heart Rate Variability and Postural Hypotension as an Indicator of Cardiac Autonomic Neuropathy in Type II Diabetes Mellitus. J Pak Med Assoc. 2012;62(4):328–331. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.