Back to Journals » Clinical Ophthalmology » Volume 9
Evaluation of the effect of intravitreal ranibizumab injections in patients with neovascular age related macular degeneration on retinal nerve fiber layer thickness using optical coherence tomography
Authors El-Ashry M, Lascaratos G, Dhillon B
Received 10 January 2015
Accepted for publication 20 March 2015
Published 13 July 2015 Volume 2015:9 Pages 1269—1274
DOI https://doi.org/10.2147/OPTH.S80704
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Mohamed F El-Ashry,1–3 Gerassimos Lascaratos,1 Baljean Dhillon1,4
1Princess Alexandra Eye Pavilion, Edinburgh, UK; 2Ophthalmology Department, Tanta University Hospital, Tanta, Egypt; 3North Cumbria University Hospital, Carlisle, UK; 4Centre for Clinical Brain Sciences, School of Clinical Sciences, University of Edinburgh, Edinburgh, UK
Purpose: To evaluate the effect of repeated intravitreal ranibizumab injections for neovascular age related macular degeneration (nAMD) on the retinal nerve fiber layer (RNFL) thickness using optical coherence tomography.
Design: A prospective observational cohort study of patients with nAMD.
Methods: Thirty eyes of 30 patients with nAMD were selected. All patients received three ranibizumab injections and underwent scans using the fast RNFL thickness protocol (Stratus optical coherence tomography) before starting the first injection and 1 month after the third injection. The RNFL thickness measurements prior to the injections and after the third injection were used for the analysis. We also evaluated the effect of the lens status as well as the type of choroidal neovascular membrane on RNFL thickness measurements pre- and post-injection. Pre- and post-injection average and individual quadrant RNFL thickness were measured and statistically analyzed.
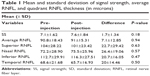
Results: The mean (± standard deviation) pre-injection RNFL thickness was 90.8±18. The mean (± standard deviation) post-injection RNFL thickness was 91.03±15. The pre- and post-injection values of the mean RNFL thickness were not statistically significant. Likewise, the pre- and post-injection values for RNFL thickness in the different quadrants were not statistically significant. There was no statistical significance for the lens status or the type of choroidal neovascular membrane on the RNFL thickness.
Conclusion: Repeated ranibizumab injections in nAMD appear to have no harmful effect on the RNFL thickness in the short term, in spite of the proven neurotrophic effect of vascular endothelial growth factor. Nevertheless, the safety profile of ranibizumab injections in nAMD needs to be further evaluated in a large multicenter trial with special emphasis on the long-term effects on the retina and optic nerve.
Keywords: optical coherence tomography, anti-VEGF, intraocular pressure, ranibizumab, retinal nerve fiber layer
Introduction
Neovascular age related macular degeneration (nAMD), characterized by new formation of choroidal vessels into the sub-retinal space,1 is responsible for nearly 90% of severe vision loss among elderly people in the developed world.2 Vascular endothelial growth factor (VEGF)-A is a key contributory factor in the pathophysiology underlying nAMD.3 Several studies have shown that the rise in ocular VEGF levels is linked to the initiation of neovascularization, which is the first step in the pathogenesis of nAMD in both humans3 and animals.4,5
Ranibizumab (anti-VEGF), a recombinant humanized monoclonal antibody antigen-binding fragment, has been reported to neutralize all known active forms of VEGF-A and to block vessel permeability and angiogenesis and hence prevent vision loss and improve visual acuity in patients with nAMD.6
Major trials have proven the beneficial effect of ranibizumab (anti-VEGF) injections in nAMD.7,8 However, VEGF has a potential role in the maintenance and survival of retinal neural cells, since it was shown to increase vascular permeability and vascular endothelial cell proliferation, as well as promote cell survival.9 Being expressed by astrocytes in the retinal ganglion cell layer and by cells of the inner nuclear layer, Muller cells, and the retinal pigment epithelium, VEGF-A imparts a neuroprotective role on the retina and optic nerve. In addition, it plays a central role in the trophic maintenance of the choriocapillaris and in protecting retinal neurons from apoptosis.9 Repeated ranibizumab injections might therefore induce a detrimental effect on the retinal nerve fiber layer (RNFL) thickness. The purpose of this study was to evaluate the effect of repeated intravitreal ranibizumab injections for nAMD on the RNFL thickness using Stratus optical coherence tomography (OCT).
Materials and methods
This is a prospective observational cohort study of patients with nAMD. The study was conducted in accordance with the Declaration of Helsinki recommendations. Informed consent was obtained from all participants. Thirty white Caucasian patients (30 eyes) diagnosed with nAMD participated in the study that was carried out in a tertiary referral center in the UK. All patients underwent full ophthalmological examinations including slit lamp examination and fundus biomicroscopy. Fundus fluorescein angiography was performed to identify the type of choroidal neovascular membrane (CNV). Patients who showed evidence of glaucoma, ocular hypertension, other retinal pathology or systemic diseases, such as diabetes, were excluded. All patients received initially three monthly intravitreal ranibizumab injections and then were reviewed on a monthly basis and given further injections if needed based on the OCT-guided PrONTO trial (Prospective OCT Imaging of Patients with nAMD Treated With Intraocular Lucentis). Intraocular pressure (IOP) was checked immediately after each injection and patients were given anti-glaucoma drops, as required.
OCT scanning
Cross-sectional imaging of the peripapillary area was performed with the Stratus OCT (version 3, Carl Zeiss Meditec AG, Jena, Germany). RNFL thickness was assessed by the fast RNFL program, a commonly used protocol, which compares results to a reference normative database. The protocol generates A-scans along a 360-degree circular path located 1.74 mm from the center of the optic disc, producing a total of three separate scans during each test. It generates RNFL thickness measurement for each of the twelve 30-degree sectors, computing an average for each of the four quadrants (superior, inferior, nasal, temporal) and an overall average.
Internal fixation was used during scanning, and all eyes had circular scans of 3.4 mm diameter centered around the optic disc. The patients underwent scans prior to the first injection and 1 month after the third injection at the same time as their macular scans for nAMD were performed. All the scans were performed by the same investigator. Pre- and post-injection scans were analyzed and scans with signal strength (SS) of 7 and above were considered acceptable except in cases of cataract causing degraded image quality on OCT, SS values of 6 and above were considered acceptable. Pre- and post-injection average and individual quadrant RNFL thickness measurements at different quadrants were measured and statistically analyzed using SPSS10. We also evaluated the effect of the lens status as well as the type of CNV on RNFL thickness measurements. Statistical comparisons were done using the paired Student’s t-test. A P-value of less than or equal to 0.05 was considered statistically significant.
Results
Thirty participants (30 eyes) were included in the analysis: 24 (80%) women and six (20%) men, with age range between 59 and 93 years (mean 77.6). 27 eyes were phakic and three were pseudophakic. All patients were diagnosed with CNV (ten classic and 19 occult) except one who was diagnosed with retinal angiomatous proliferation. The mean (± standard deviation) pre-injection RNFL thickness was 90.8±18. The post-injection mean (± standard deviation) RNFL thickness was 91.03±15. Pre- and post-injection average and quadrant RNFL thickness values are shown in Figure 1. Statistical analyses of average and quadrant RNFL thickness differences are summarized in Table 1. The pre- and post-injection values of the mean RNFL thickness were not statistically significant. Likewise, the pre- and post-injection values for RNFL thickness in the different quadrants were not statistically significant. Differential analysis of the changes in the RNFL thickness has been performed and showed no statistically significant changes due to variation in the type of the CNV membrane (classic, occult), as shown in Table 2.
  | Figure 1 Pre- and post-injection quadrant and average RNFL thickness. |
Post-injection high IOP was recorded in six patients, but only two had a reading above 30 mmHg controlled with topical anti-glaucoma medications. IOP changes after each injection compared with the initial reading were not statistically significant (data not shown).
Discussion
Endogenous VEGF has neurotrophic and neuroprotective effects on neuronal cells with an important role in the maintenance and function of the adult retina and neural cells.10 Suppression of endogenous VEGF by the use of intravitreal ranibizumab could potentially have a detrimental effect on the neuronal cells and hence the rationale for assessing the effect of anti-VEGF on the RNFL thickness in nAMD patients.
In 2010, Horsley et al evaluated the effect of long-term treatment with anti-VEGF agents on the RNFL thickness using patients who received more than ten injections and found no significant change.11 Our findings are consistent with their results, however, in our study, the scope of comparison was wider, as further analysis of the changes in the RNFL thickness in the different quadrants was adopted and showed no statistically significant change in any quadrant. An additional unique feature of our study is the fact that we have also taken into consideration the effect of lens status and the type of membrane (Tables 2 and 3).
In another recent study, Seth et al investigated the potential change in the optic disc structure due to the combined effect of short-term IOP rise, frequent IOP fluctuation, as well as the anti-VEGF properties of pegaptanib, and found no significant change.12 Moreover, Luke et al showed no significant electroretinographic changes on isolated bovine retinas after exposure to a concentration of 0.2 mg/mL of ranibizumab.13 Similarly, Kim et al reported no severe adverse effects on monkeys’ retinas from the combination of intravitreal ranibizumab and verteporfin photodynamic therapy compared with photodynamic therapy alone.14
In agreement with our study, Sobaci et al found that repeated intravitreal injections of ranibizumab or bevacizumab does not seem to have adverse effects on RNFL thickness in wet AMD patients in contrast to Martinez-de-la-Casathe et al who concluded that intravitreal ranibizumab injections used to treat AMD caused a significant change in RNFL thickness after 12 months of follow-up.15,16
The anti-VEGF properties of bevacizumab have also been studied in vivo and in vitro and equally no significant difference was noted in retinal ganglion cell numbers following either single or repeated injections.17 Bevacizumab was also well tolerated by ganglion and photoreceptor cells even at concentrations fivefold higher than those used clinically, which further supports the safety profile of ranibizumab on the RNFL.18
Intravitreal injections are known to be associated with a transient IOP elevation due to volume increase with the injection of the drug. The IOP usually returns to baseline within 30–60 minutes post-injection. However, short-term IOP fluctuation has been considered a potential risk factor for the progression of glaucomatous damage to the optic nerve head.19 Therefore, the cumulative effect of the initial injections could potentially damage the RNFL. This effect might be important in cases with advanced glaucoma, in which even the slightest change in IOP could have a detrimental effect on the optic nerve.
OCT has become an increasingly important tool for detecting damage to the RNFL, which may precede the onset of detectable visual field defects in patients with glaucoma. Papillary RNFL thickness scans have been suggested as the preferred metric for measuring the extent of glaucomatous damage.20 SS, which is a proxy for the quality of the scan, may have a significant impact on the measurements of the RNFL on the Stratus OCT. It is claimed that scans with lower SS tend to underestimate the thickness of the RNFL. This variability is especially pronounced in patients with advanced optic nerve damage, even in scans without error messages. Therefore, it is important to obtain scans of similar SS to facilitate the longitudinal follow-up of RNFL changes.21 It was concluded in another study that SS of at least 7 was required to reduce variability, regardless of the RNFL thickness.22 In this study scans were repeated until satisfactory level of SS (7 and above) was achieved in all cases except in cases with cataract (due to the underlying effect of degraded image quality) in which SS with values of 6 and above were accepted.
Poor quality scans induced by cataracts might have affected the accuracy of RNFL thickness, as measured by the Stratus OCT with software version 3.0. It may be possible that the algorithms in Stratus OCT version 3.0 used to identify the RNFL are affected by degraded image quality causing imprecision in the measurements.23 OCT has routinely been used in the assessment of the RNFL thickness as a monitoring and screening tool for glaucoma and has been shown to have fairly good reproducibility on repeated measurements.24–26
The effect of anti-VEGF on the size and thickness of different types of CNV has been evaluated in other studies.27,28 Quantitative measurements underlined stable CNV diameters for all subtypes but revealed significant reduction of thickness especially for classic CNV components. In occult CNV, the thickness of CNV showed a significant reduction only in the ranibizumab group. It is not clear whether the peripapillary RNFL thickness would be concurrently affected with anti-VEGF injections parallel with the changes in size and thickness of the CNV.27,28 Based on the study by Martinez-de-la-Casa et al the effect of anti-VEGF on the RNFL thickness might not be significant shortly after three repeated anti-VEGF injections but possibly become evident in the long-term.16 In our study there was no significant changes in the RNFL thickness in association with the different types of CNV.
In our study the differences in the measurements pre- and post-injection were not statistically significant, possibly due to the small number of patients. However, there were two outliers in our statistics with up to 40 μm reduction in the RNFL thickness after the third injection. Although this variance was not statistically significant, the effect could be detrimental in cases of end stage glaucoma with advanced optic cupping and vulnerable RNFL.
In summary, we evaluated the effect of ranibizumab injections on the RNFL thickness and our findings, despite originating from a small cohort of patients, suggest that there was no significant harmful effect to the RNFL induced by the injections. We compared RNFL thickness, both average as well as in various peripapillary quadrants, before and after three successive monthly ranibizumab injections. Comparing the effect of these injections on the RNFL with specific emphasis on the different quadrants is a new parameter that has not been studied previously and would add strength to this study.
Although our subgroup analysis did focus on the nature of the membrane (classic versus occult) and in a similar way on the status of the lens the study was not powered for such analyses, thus potentially introducing a type II error. Admittedly, our limited subgroups’ numbers, and relatively short follow-up period precludes drawing firm conclusions regarding the value of such parameters. Trials with a larger number of patients would be recommended to highlight if there was any significance of either the lens status or the type of CNV membrane as a variable that might have an implication on the effect of anti-VEGF on the RNFL thickness. Similarly, another trial including a larger number of glaucoma patients would be recommended so that the effect of ranibizumab on the RNFL layer could be evaluated in such vulnerable eyes, especially in the context of the combined effect of IOP fluctuations and the absence of the neuroprotective and neurotrophic effects of VEGF.
Disclosure
The authors have no conflicts of interest to disclose.
References
Green WR, Enger C. Age-related macular degeneration histopathologic studies: the 1992 Lorenz E. Zimmerman Lecture. Ophthalmology. 1993; 100(10):1519–1535. | ||
Ferris FL, Fine SL, Hyman L. Age-Related Macular Degeneration and Blindness due to Neovascular Maculopathy. Arch Ophthalmol. 1984; 102(11):1640–1642. | ||
Aiello LP, Pierce EA, Foley ED, et al. Suppression of retinal neovascularization in vivo by inhibition of vascular endothelial growth factor (VEGF) using soluble VEGF-receptor chimeric proteins. Proc Natl Acad Sci U S A. 1995;92(23):10457–10461. | ||
Scott-Burden T, Vanhoutte PM. The endothelium as a regulator of vascular smooth muscle proliferation. Circulation. 1993;87(5):V51–V55. | ||
Schwesinger C, Yee C, Rohan RM. Intrachoroidal neovascularization in transgenic mice overexpressing vascular endothelial growth factor in the retinal pigment epithelium. Am J Pathol. 2001;158(3):1161–1172. | ||
McAvoy C, Chakravarthy U. VEGF inhibition: latest developments. Expert Review of Ophthalmology. 2007;2:621–632. | ||
Rosenfeld PJ, Brown DM, Heier JS, et al, MARINA study group. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–1431. | ||
Brown DM, Michels M, Kaiser PK, et al. Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: two-year results of the ANCHOR study. Ophthalmology. 2009;116(1):57–65. | ||
Storkebaum E, Lambrechts D, Carmeliet P. VEGF: once regarded as a specific angiogenic factor, now implicated in neuroprotection. Bioessays. 2004;26(9):943–954. | ||
Saint-Geniez M, Maharaj AS, Walshe TE, et al. Endogenous VEGF Is Required for Visual Function: Evidence for a Survival Role on Müller Cells and Photoreceptors. PLoS One. 2008;3(11):e3554. | ||
Horsley MB, Mandava N, Maycotte MA, Kahook MY. Retinal nerve fiber layer thickness in patients receiving chronic anti-vascular endothelial growth factor therapy. Am J Ophthalmology. 2010;150(4):558–561. | ||
Seth RK, Salim S, Shields MB, Adelman RA. Assessment of optic nerve cup-to-disk ratio changes in patients receiving multiple intravitreal injections of antivascular endothelial growth factor agents. Retina. 2009;29(7):956–959. | ||
Lüke M, Januschowski K, Lüke J, et al. The effects of ranibizumab (Lucentis) on retinal function in isolated perfused vertebrate retina. Br J Ophthalmol. 2009;93(10):1396–1400. | ||
Kim IK, Husain D, Michaud N, et al. Effect of intravitreal injection of ranibizumab in combination with verteporfin PDT on normal primate retina and choroid. Invest Ophthalmol Vis Sci. 2006;47(1):1357–1363. | ||
Sobaci G, Güngör R, Ozge G. Effects of multiple intravitreal anti-VEGF injections on retinal nerve fiber layer and intraocular pressure: a comparative clinical study. Int J Ophthalmol. 2013;6(2):211–215. | ||
Martinez-de-la-Casa JM, Ruiz-Calvo A, Saenz-Frances F, et al. Retinal nerve fiber layer thickness changes in patients with age-related macular degeneration treated with intravitreal ranibizumab. Invest Ophthalmol Vis Sci. 2012;53(10):6214–6218. | ||
Cheng CK, Peng PH, Tien LT, Cai YJ, Chen CF, Lee YJ. Bevacizumab is not toxic to retinal ganglion cells after repeated intravitreal injection. Retina. 2009;29(3):306–312. | ||
Kaempf S, Johnen S, Salz AK, Weinberger AW, Walter P, Thumann G. Effects of Bevacizumab (Avastin) on Retinal Cells in Organotypic Culture. Invest Ophthalmol Vis Sci. 2008;49(7):73164–73171. | ||
Nouri-Mahdavi K, Hoffman D, Coleman AL, et al. Advanced Glaucoma Intervention Study. Predictive factors for glaucomatous visual field progression in the Advanced Glaucoma Intervention Study. Ophthalmology. 2004;111(9):1627–1635. | ||
Leung CK, Chan WM, Yung WH, et al. Comparison of macular and peripapillary measurements for the detection of glaucoma: an optical coherence tomography study. Ophthalmology. 2005;112(3):391–400. | ||
Wu Z, Vazeen M, Varma R, et al. Factors associated with variability in retinal nerve fiber layer thickness measurements obtained by optical coherence tomography. Ophthalmology. 2007;114(8):1505–1512. | ||
Samarawickrama C, Mitchell P. Influence of Signal Strength on OCT Measurements. J Glaucoma. 2009;18(6):499–500. | ||
El-Ashry M, Appaswamy S, Deokule S, Pagliarini S. The effect of phacoemulsification cataract surgery on the measurement of retinal nerve fiber layer thickness using optical coherence tomography. Curr Eye Res. 2006;31(5):409–413. | ||
Schuman JS, Pedut-Kloizman T, Hertzmark E, et al. Reproducibility of Nerve Fiber Layer Thickness Measurements Using Optical Coherence Tomography. Ophthalmology. 1996;103(11):1889–1898. | ||
Blumenthal EZ, Williams JM, Weinreb RN, Girkin CA, Berry CC, Zangwill LM. Reproducibility of nerve fiber layer thickness measurements by use of optical coherence. Ophthalmology. 2000;107(12):2278–2282. | ||
Carpineto P, Ciancaglini M, Zuppardi E, Falconio G, Doronzo E, Mastropasqua L. Reliability of nerve fiber layer thickness measurements using optical coherence tomography in normal and glaucomatous eyes. Ophthalmology. 2003;110(1):190–195. | ||
Framme C, Panagakis G, Birngruber R. Effects on choroidal neovascularization after anti-VEGF Upload using intravitreal ranibizumab, as determined by spectral domain-optical coherence tomography. Invest Ophthalmol Vis Sci. 2010;51(3):1671–1676. | ||
You JY, Chung H, Kim HC. Evaluation of changes in choroidal neovascularization secondary to age-related macular degeneration after anti-VEGF therapy using spectral domain optical coherence tomography. Curr Eye Res. 2012;37(5):438–445. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.