Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 15
Evaluation of Skin Biophysical Parameters and Angiogenesis Using CD34 as a Biomarker in Older Diabetic Women Treated with Radiofrequency
Authors Sobkowska D, Gornowicz-Porowska J, Seraszek-Jaros A, Słomińska D , Adamski Z, Pawlaczyk M
Received 11 March 2022
Accepted for publication 15 June 2022
Published 14 July 2022 Volume 2022:15 Pages 1347—1355
DOI https://doi.org/10.2147/CCID.S365501
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Daria Sobkowska,1 Justyna Gornowicz-Porowska,1 Agnieszka Seraszek-Jaros,2 Daria Słomińska,3 Zygmunt Adamski,4 Mariola Pawlaczyk1
1Department and Division of Practical Cosmetology and Prevention of Skin Diseases, Poznan University of Medical Sciences, Poznań, 60-806, Poland; 2Department of Bioinformatics and Computational Biology, Poznan University of Medical Sciences, Poznań, 60-806, Poland; 3Department of Pharmacology, Poznan University of Medical Sciences, Poznan, 60-806, Poland; 4Department of Dermatology, Poznan University of Medical Sciences, Poznan, 60-355, Poland
Correspondence: Daria Sobkowska, Poznan University of Medical Sciences, 3 Rokietnicka Street, Poznań, 60-806, Poland, Tel +48 61 848-04-75, Email [email protected]
Background: The prevalence of type 2 diabetes mellitus (t2DM) has been steadily increasing. Patients with t2DM need to slow down the skin ageing processes and to obtain a rejuvenating effect. Treatments that do not damage the superficial layers of the epidermis could be a promising solution for those patients.
Purpose: The aim of this study was to evaluate the effects of radiofrequency therapy on the biophysical parameters and angiogenesis of facial skin, using CD34 as a biomarker in older diabetic women treated with metformin.
Patients and Methods: A total of 45 subjects with phototype 2 or 3 (Fitzpatrick scale) were investigated (25 t2DM – study group, 20 – healthy controls). A series of 6 treatments (once a week) with a Radio Frequency Skin Rejuvenation System device was used on facial skin. Measurements of skin hydration, transepidermal water loss (TEWL), melanin and erythema index, temperature, and pH, at baseline and after radiofrequency therapy were performed with the Courage + Khazaka MPA-9 device. Immunohistochemistry on paraffin-embedded sections was used to evaluate the intensity of CD34 expression.
Results: Radiofrequency treatment significantly improved facial skin hydration (p < 0.0001). Enhancement of the epidermal barrier observed, by reduced TEWL as a result of a series of treatments with radiofrequency on the facial skin (p < 0.0001), was observed. CD34 was more abundantly expressed after radiofrequency treatment. No side effects were observed.
Conclusion: Treatment with radiofrequency is an effective and non-invasive method of facial skin rejuvenation in older women with t2DM, with a relatively short post-procedure recovery time and low potential for severe adverse effects.
Keywords: diabetes, skin, radiofrequency, metformin
Introduction
Ageing skin is characterized by remarkable structural changes, involving the thinning of the dermis, collagen volume loss, vessel atrophy, and flattening of the dermal-epidermal junction. Skin ageing is induced by both intrinsic and extrinsic factors.1 Noordam et al showed that high serum glucose levels are associated with a higher perceived age in both, diabetic and non-diabetic subjects.2 Advanced glycation end-products (AGEs) occur slowly during ageing, but the process is accelerated in the presence of diabetes mellitus (DM). The glycation process promotes dermal damage and premature skin ageing.3 Diabetes and ageing present separate pathologies but retain a crucial overlap in senescence-linked molecular and cellular processes.4 Moreover, cellular senescence is associated with an imbalance between pro-inflammation and anti-inflammation, contributing to a low-grade pro-inflammatory state (“inflammaging”), which accelerates age-related diseases, including DM.5 Cutaneous alterations are common in diabetic patients.6 In older subjects, the nonenzymatic glycation is high, not only because of the possible hyperglycemia but also due to long-term exposure to normoglycemic conditions. The impairment of the skin barrier in t2DM is associated with a decrease in epidermal proliferation and AGEs. Serum AGEs and its epidermal receptors were increased in type 2 diabetic mice7 Protein glycation contributes to skin ageing as it deteriorates the existing collagen by crosslinking. The production of AGEs in skin cells promotes stiffness and loss of elasticity.8 Also, dermal equivalents containing collagen modified by glycation show a reduction of contraction as compared to control without glycation.9 The Raman study revealed that the ageing effects caused by glycation of proteins degrade type I collagen differently.10 Argyropoulos et al, investigated the molecular basis of aged-appearing skin, mainly connected with metalloproteinases appearance, which is largely responsible for the fragmentation of collagen fibers.11 AGEs are involved in the pathogenetic process of diabetic microangiopathy.12,13 CD34 is a marker of hematopoietic stem cells and a global marker of endothelial cells in human and mouse tissues.14 Therefore, it can be used to identify the vascular endothelial cells in the skin (membranous staining pattern) and visualize the dermal vascularisation.15
Advanced, non-invasive anti-ageing cosmetic treatments for the skin, as well as instrumental methods used to assess their efficacy, should be of special interest in diabetic patients,16 as they present with skin dryness, skin barrier damage, and increased transepidermal water loss (TEWL).17–19 One of the available methods is rejuvenating therapy with the use of radiofrequency (RF). The method allows controlled heating of the tissues, but the produced energy does not cause dermabrasion or ablation. Instead, it generates heat energy in the target tissue, which affects conformational changes in collagen fibers.20 The existence of thermal wounds zones between the areas of the unaffected zones can be a supply of reservoir cells to promote healing, so there is less downtime, and it is considered a safe modality.21 RF is widely used both, in cosmetology and dermatology.20–27 However, data about the usefulness of RF treatment in ageing diabetic skin remains limited, at best. Therefore, our study aimed to investigate the effects of RF therapy on the biophysical parameters of facial skin and its possible impact on angiogenesis in older women with t2DM.
Materials and Methods
The study was approved by the Local Bioethics Committee (Poznan University of Medical Sciences, no. 1320/18, Poland). The Declaration of Helsinki was followed. Written informed consent, including for publication of patient medical images, was obtained from all participants.
Study Population
The study was conducted at the Department of Cosmetology and Skin Diseases Prophylaxis, between 2019 and 2021. The study group included 45 non-smoking women (25 with t2DM and 20 controls without t2DM), aged 60–63 years, with signs of skin ageing such as wrinkling, dyspigmentation, sagging, and skin phototype of 2 or 3 according to Fitzpatrick scale.28 General practitioners confirmed the health status of the controls, while general practitioners and diabetologists confirmed the health status of the diabetic group. All t2DM women were treated with metformin hydrochloride (daily dose of 500–1000 mg). Detailed characteristics of the participants are presented in Table 1.
 |
Table 1 Characteristics of the Study Group |
The exclusion criteria were as follows: age <60, smoking, poor overall health condition, skin diseases, history of esthetic dermatology treatments in the last 2 years.
Measurements of Skin Biophysical Parameters
Skin biophysical parameters were measured at baseline and after the series of RF treatments on the same area of the face in the entire study population. The following parameters were evaluated: skin hydration, TEWL, melanin index (MI), erythema index (EI), temperature, and skin pH. The measurements were performed on the three anthropometric points on the facial skin: the forehead – 2 cm above Glabella in the midline, the left cheek – 2 cm below Orbitale on the left side of the face in the interpupillary line, and the chin – 1 cm above Gnathion in the midline.
The in vivo non‐invasive skin bioengineering techniques were used with the Courage + Khazaka MPA-9 device (Courage + Khazaka Electronic, Köln, Germany) with the following probes: Corneometer CM825, Tewameter CM300, Mexameter MX18, Thermometer ST 500, and Skin-pH-Meter PH 908. All measurements were performed in controlled conditions at a temperature of 22–25°C and average relative humidity of 52–58%. Before the measurements, the volunteers were asked to stay in the test room for at least 15 minutes, so the skin could acclimatize to room conditions. To minimize study errors, the participants were instructed not to apply any cosmetics in the tested area before the study and avoid ultraviolet radiation and solar exposition.
Immunohistochemical Analysis
A 4 mm skin punch biopsy was taken from the area subjected to RF treatment from 10 patients with t2DM, at baseline and after RF. Paraffin-embedded 4-μm skin sections were mounted on poly-L-lysine-coated glass slides. Immunohistochemical staining (IHC) using immunoperoxidase technique with monoclonal antibodies against human CD34 (Perlan Technologies), and Real EnVision detection kit (Dako, Denmark) was applied to detect dermal micro-vessels. CD34 staining was performed after enzymatic digestion with proteinase K (Dako, Denmark). Then, the slides were incubated with an appropriate dilution of antibody (1:100) in Antibody Diluent (Dako, Denmark) at room temperature for 1 hour, and, after visualization, washed with PBS, counterstained with hematoxylin, dehydrated with an alcohol gradient, treated with xylene, and coverslipped. The slides were examined by the EuroStar III Plus microscope operated by Bluelight LED technology (Euroimmun, Germany) and digitally photographed. The intensity of positive immunostaining signals (expression intensities) on the slides was reported according to the arbitrarily assigned semiquantitative five-point scale (from “-” to “+++”) at identical objective magnifications (×20; ×40).
Radiofrequency
RF treatments were carried out with the use of a Radio Frequency Skin Rejuvenation System device (Hebe, Poland), and the following parameters: 1 MHz frequency generator, 230 V, 50 Hz voltage, and 40–42 W power. The duration of a treatment session started at 20 minutes, and was extended by 1 minute upon every visit, up to 26 minutes at the last visit. Every woman was subjected to a series of 6 treatments, once a week. After the treatment, the use of a soothing, moisturizing cream (for day and night), without any other facial cosmetics, as well as drinking a minimum of 2 liters of water a day, were recommended.
Statistical Analysis
Statistical analysis was conducted using Software Statistica PL 10.0 (StatSoft Inc., Tulsa, OK, USA). The Shapiro–Wilk test was used to analyze the results. A comparative analysis was performed with the use of a non-parametric Mann–Whitney U-test. Other analyses were performed using Student t-test for independent variables. The p value of <0.05 was considered as statistically significant.
Results
The results of skin rejuvenation in a representative t2DM woman at baseline and after RF are presented in Figures 1–3.
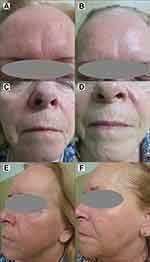 |
Figure 1 A 63-year old female patient with diabetes mellitus type 2: (A, C and E) at baseline; (B, D and F) after a series of radiofrequency treatments. Note: Images property of Daria Sobkowska. |
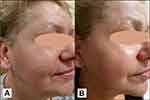 |
Figure 2 A 61-year-old female patient with diabetes mellitus type 2: (A) at baseline; (B) after a series of radiofrequency treatments. Note: Images property of Daria Sobkowska. |
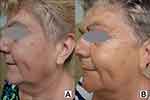 |
Figure 3 A 63-year-old female patient with diabetes mellitus type 2: (A) at baseline; (B) after a series of radiofrequency treatments. Note: Images property of Daria Sobkowska. |
Adverse Events
No adverse events were observed during the study. Skin erythema of the face persisted for approximately 20 minutes after the RF procedure, which is a normal reaction, and resolved.
Biophysical Parameters of the Skin
Measurements of the biophysical parameters at baseline and after RF are presented in Tables 2 (for the forehead), 3 (for the left cheek), and 4 (for the chin). At baseline, skin hydration was higher and TEWL was lower in t2DM patients and controls for all of the investigated areas of the face. RF therapy significantly improved skin hydration and reduced TEWL in all areas for t2DM women and controls, with no differences between the groups. RF reduced EI on the left cheek (p=0.0135) and the chin (p=0.0299) in both groups, but on the forehead only in controls (p=0.0001). MI measurements and temperature of the skin were nearly the same for all areas of the face, at baseline and after RF. A significant reduction of skin pH was observed in both t2DM women and controls, but only for the left cheek (p=0.0284). The value was reduced to acidic pH.
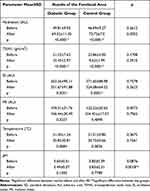 |
Table 2 The Results of Biophysical Parameters Measurements on the Skin of the Forehead Area Before, and After the Radiofrequency Intervention |
Immunohistochemical Staining
Comparison of CD34 expression at baseline and after RF revealed significant differences. Skin samples after RF showed higher expression of CD34 (“++”) as compared to baseline (“+”). Biopsy from patients with t2DM presented visible serration patterns of CD34 just below the dermo-epidermal junction (DEJ). The number of the micro-vessels was higher in the dermal infiltrate and at DEJ after RF compared with baseline. CD34 expression in skin biopsy of a representative t2DM patient before and after RF is shown in Figure 4.
Discussion
Diabetes Mellitus and the Skin
The number of people with t2DM has quadrupled in the last three decades, and DM has become the ninth major cause of death globally.29 DM incidence has been known to increase with age so the number of people suffering from t2DM is expected to steadily increase as the life expectancy figures are on the rise.30 Altered carbohydrate metabolism in the human body considerably affects the condition of the skin, an important barrier for the external environment.7,31 Diabetic skin becomes dry, sensitive, itchy, congested, extensively flaky, and aged-appearing.2–4,7,9–11
Metformin reduces AGEs-induced reactive oxygen species (ROS) generation in high glucose conditions and is widely used as the first-line anti-diabetic medication.32,33 Studies confirmed the protective effects of metformin in the in vitro model of ageing 3T3 fibroblasts under high glucose conditions.33,34 Exposure to metformin induces cell proliferation, collagen I and III production, protects from apoptosis, and reduces pro-inflammatory cytokines.32,33,36,37 As reported by Pennacchi et al, glycation processes affect the dermal matrix and the epidermal compartments, leading to poor stratification of the epidermal layers and vacuolization keratinocyte cytoplasm, which are typical for aged skin.35 In our study, women with t2DM were treated with metformin hydrochloride. The results of corneometry and TEWL were very similar in diabetic women ≥60 years and their control peers, which may be indicative of the positive effect of metformin on the skin barrier in patients with t2DM.17,31
Efficacy of Radiofrequency for Diabetic Patients
After a series of 6 RF treatments, a significant reduction of TEWL and increased skin hydration were observed in both groups for all of the investigated areas of the face. Louis et al38 investigated normal human skin substitutes subjected to RF and ultrasound treatment, and found significant induction of genes related to epidermal differentiation processes, ie, keratin. Histology assessment showed a higher expression of cytokeratin 10 and 14, which may be evidence of a possible reactivation of the proliferative skin state as a rejuvenation strategy, as well as explain the beneficial effect of RF on the skin barrier.
Diabetic skin requires special daily care with gentle cleansers and moisturizers.39 Together with proper skincare, RF can be used to strengthen the skin barrier in older women, including those with t2DM. Devices using electromagnetic radiation are easily available and offer non-invasive and safe therapeutic options for skin rejuvenation. In our study, immediate short-term erythema, which is a normal skin reaction after such a procedure, was observed in both groups.
The use of mono- or bipolar RF for the treatment of facial skin caused the thickening of skin layers.40 Kokolakis et al41 used this technology for deep ablation, obtaining the growth of fibrous connective tissue, with the subsequent new creation of collagen fibers. Other authors also reported the beneficial rejuvenating effect of RF on the ageing facial skin,42–44 ie reduced laxity, an increased skin elasticity, and smoothing of the wrinkles. In our study, we did not assess the clinical signs of ageing, but concentrated on the evaluation of the biophysical parameters of facial skin in diabetic older women as compared to non-diabetic controls.
Moreover, age-related skin inflammation results in compromised epidermal barrier, impaired moisture retention, erythema, and pigment alteration.45 Our findings indicate that RF may especially reduce erythema, as the objective sign of different phases of skin inflammation. In our study, the probands developed lesser erythema on the left cheek (p=0.0135) and chin (p=0.0299), which significantly affects beautifying treatments.
It is a well-known fact that barrier abnormalities in the ageing skin may be normalized by acidifying the epidermis exogenously with proper therapies.46 Therefore, the slight reduction in the baseline skin surface pH after RF in our study could be the result of improved integrity of the stratum corneum.
Effect of Radiofrequency on Angiogenesis
Using semi-quantitative IHC to investigate protein expression and localization within tissues, our study demonstrated that CD34, a marker of vascular endothelial cells, was abundantly expressed after RF. The increased number of micro-vessels after RF suggests a possible link between angiogenesis and the RF treatment. These results present morphological evidence for the angiogenic activity of RF, indicating that local microvasculature may in fact undergo the process of treatment-dependent angiogenesis.
Reduced turnover rate and cell renewal slowdown in the epidermis are observed with advancing age,47 resulting in dry, rough, uneven, and dull skin in older people. Epidermal stem cells are responsible for the constant renewal of the epidermis. CD34 is one of the markers which they express and, as such, may be used to characterize epidermal stem cell properties.48 Our results indicated that CD34 expression was upregulated after RF, which may suggest the stimulation of undifferentiated cells in epidermal development. Notably, the expression pattern of CD34 after RF is compatible with the localization of the epidermal stem cells – they are dispersed in the inner layer of the epidermis. Based on our findings, it seems safe to conclude that RF activates epidermal renewal by stimulating cell proliferation, compensating for the usual deterioration associated with ageing. That, in turn, leads to higher epidermal turnover rate and, consequently, much improved skin complexion, as demonstrated in our study by improved biophysical parameters of the skin after RF.
As the process of ageing has a degenerative effect on the skin, increasing skin vulnerability to damage, especially in diabetic patients, all available methods should be implemented to restore normal biophysical and mechanical properties of the skin.
Limitation
Our study is not without limitations, chief among them a relatively small sample size and short period of observation at only one center, as well as lack of comparison with other methods of skin rejuvenation.
Conclusion
RF is an effective, non-invasive, and safe method of enhance facial skin hydration, reduce TEWL and skin pH in older women affected with t2DM.
Abbreviations
AGEs, advanced glycation end-products; DEJ, dermo-epidermal junction; EI, erythema index; HSP47, heat shock protein 47; IHC, immunohistochemical staining; MI, melanin index; NFKB, nuclear factor kappa-light-chain-enhancer of activated B cells; RF, radiofrequency; ROS, reactive oxygen species; t2DM, type 2 diabetes mellitus; TEWL, transepidermal water loss.
Acknowledgments
The microscopy system (EuroStar III Plus microscope operated by Bluelight LED technology; Euroimmun, Germany) was loaned to us courtesy of the Cutaneous Histopathology and Immunopathology Section, Department of Dermatology, Poznan University of Medical Sciences, Poland (Head of the Section: Monika Bowszyc-Dmochowska).
Disclosure
The authors have no conflicts of interest in relation to this work to report.
References
1. Wong QYA, Chew FT. Defining skin aging and its risk factors: a systematic review and meta-analysis. Sci Rep. 2021;11(1):22075. doi:10.1038/s41598-021-01573-z
2. Noordam R, Gunn DA, Tomlin CC, et al. High serum glucose levels are associated with a higher perceived age. Age. 2013;35(1):189–195. doi:10.1007/s11357-011-9339-9
3. Ibuki A, Kuriyama S, Toyosaki Y, et al. Aging-like physiological changes in the skin of Japanese obese diabetic patients. SAGE Open Med. 2018;6(6):2050312118756662. doi:10.1177/2050312118756662
4. Wilkinson HE, Hardman MJ. Wound senescence: a functional link between diabetes and ageing? Exp Dermatol. 2021;30(1):68–73. doi:10.1111/exd.14082
5. Lee YI, Choi S, Roh WS, Lee JH, Kim TG. Cellular senescence and inflammaging in the skin microenvironment. Int J Mol Sci. 2021;22(8):3849. doi:10.3390/ijms22083849
6. Duff M, Demidova O, Blackburn S, Shubrook J. Cutaneous manifestations of diabetes mellitus. Clin Diabetes. 2015;33(1):40–48. doi:10.2337/diaclin.33.1.40
7. Kim JH, Yoon NY, Kim DH, et al. Impaired permeability and antimicrobial barriers in type 2 diabetes skin are linked to increased serum levels of advanced glycation end-product. Exp Dermatol. 2018;27(8):815–823. doi:10.1111/exd.13466
8. Lee EJ, Kim JY, Oh SH. Advanced glycation end products (AGEs) promote melanogenesis through receptor for AGEs. Sci Rep. 2016;6(1):27848. doi:10.1038/srep27848
9. Pageon H, Técher MP, Asselineau D. Reconstructed skin modified by glycation of the dermal equivalent as a model for skin aging and its potential use to evaluate anti-glycation molecules. Exp Gerontol. 2008;43(6):584–588. doi:10.1016/j.exger.2008.04.004
10. Pereira L, Tellez-Soto CA, dos Santos L, Favero PP, Martin AA. Confocal Raman spectroscopic analysis of the changes of type I collagen resulting from amide I glycation. Biomed J Sci Tech Res. 2017;1(3):629–632.
11. Argyropoulos AJ, Robichaud P, Balimunkwe RM, et al. Alterations of dermal connective tissue collagen in diabetes: molecular basis of aged-appearing skin. PLoS One. 2016;11(4):e0153806. doi:10.1371/journal.pone.0153806
12. Okamoto T, Tanaka S, Stan AC, et al. Advanced glycation end products induce angiogenesis in vivo. Microvasc Res. 2002;63(2):186–195. doi:10.1006/mvre.2001.2371
13. Chen L, Cui Y, Li B, et al. Advanced glycation end products induce immature angiogenesis in in vivo and ex vivo mouse models. Am J Physiol Heart Circ Physiol. 2020;318(3):519–533. doi:10.1152/ajpheart.00473.2019
14. Krause DS, Fackler MJ, Civin CI, May WS. CD34: structure, biology, and clinical utility. Blood. 1996;87(1):1–13. doi:10.1182/blood.V87.1.1.1
15. Hussein MR. Evaluation of angiogenesis in normal and lichen planus skin by CD34 protein immunohistochemistry: preliminary findings. Cell Biol Int. 2007;31(10):1292–1295.
16. Kołodziejczak A, Wieczorek A, Rotsztejn H. The assessment of the effects of the combination of microdermabrasion and cavitation peeling in the therapy of seborrhoeic skin with visible symptoms of acne punctata. J Cosmet Laser Ther. 2019;21(5):286–290. doi:10.1080/14764172.2018.1525751
17. Sakai S, Tagami H. Dry skin in diabetes mellitus and in experimental models of diabetes. In: Farage M, Miller K, Maibach H, editors. Textbook of Aging Skin. Berlin, Heidelberg: Springer; 2015:1–12.
18. Lai CCK, NMd N, Kamaruddin NA, Jamil A, Safian N. Comparison of transepidermal water loss and skin hydration in diabetics and non diabetics. Clin Exp Dermatol. 2021;46(1):58–64. doi:10.1111/ced.14363
19. Piérard GE, Seité S, Hermanns-Lê T, Delvenne P, Scheen A, Piérard-Franchimont C. The skin landscape in diabetes mellitus. Focus on dermocosmetic management. Clin Cosmet Investig Dermatol. 2013;6:127–135. doi:10.2147/CCID.S43141
20. Rodrigues de Araújo A, Campos Soares VP, Souza da Silva F, da Silva Moreira T. Radiofrequency for the treatment of skin laxity: mithortruth. An Bras Dermatol. 2015;90(5):707–721. doi:10.1590/abd1806-4841.20153605
21. Kabiri S, Pourazizi M, Abtahi-Naeini B. Can fractionated microneedle radiofrequency be an effective procedure for treatment of Fox–Fordyce disease? A medical hypothesis. Adv Biomed Res. 2018;7:71.
22. Seo KY, Yoon MS, Kim DH, Lee HJ. Skin rejuvenation by microneedle fractional radiofrequency treatment in Asian skin; clinical and histological analysis. Lasers Surg Med. 2012;44(631):6. doi:10.1002/lsm.22071
23. Cho SI, Chung BY, Choi MG, et al. Evaluation of the clinical efficacy of fractional radiofrequency microneedle treatment in acne scars and large facial pores. Dermatol Surg. 2012;38(7):1017–1024. doi:10.1111/j.1524-4725.2012.02402.x
24. Fatemi Naeini F, Abtahi Naeini B, Pourazizi M, Nilforoushzadeh MA, Mirmohammadkhani M. Fractionated microneedle radiofrequency for treatment of primary axillary hyperhidrosis: a sham control study. Australas J Dermatol. 2015;56(4):84. doi:10.1111/ajd.12260
25. AbtahiNaeini B, Naeini FF, Saffaei A, et al. Treatment of primary axillary hyperhidrosis by fractional microneedle radiofrequency: is it still effective after long term follow up? Indian J Dermatol. 2016;61(2):234. doi:10.4103/0019-5154.177789
26. Fatemi Naeini F, Behfar S, Abtahi Naeini B, Keyvan S, Pourazizi M. Promising option for treatment of striae alba: fractionated microneedle radiofrequency in combination with fractional carbon dioxide laser. Dermatol Res Pract. 2016;2016:2896345. doi:10.1155/2016/2896345
27. Ekelem C, Thomas L, Van Hal M, et al. Radiofrequency therapy and noncosmetic cutaneous conditions. Dermatol Surg. 2019;45(7):908–930. doi:10.1097/DSS.0000000000001925
28. Fitzpatrick TB. Soleil et peau [Sun and skin]. J Méd Esthét. 1975;2:33–34.
29. Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14(2):88–98. doi:10.1038/nrendo.2017.151
30. Yakaryılmaz FD, Öztürk ZA. Treatment of type 2 diabetes mellitus in the elderly. World J Diabetes. 2017;8(6):278–285. doi:10.4239/wjd.v8.i6.278
31. Sekijima H, Goto K, Hiramoto K, Komori R, Ooi K. Characterization of dry skin associating with type 2 diabetes mellitus using a KK-A y/TaJclmouse model. Cutan Ocul Toxicol. 2018;37(4):391–395. doi:10.1080/15569527.2018.1490746
32. He L. Metformin and systemic metabolism. Trends Pharmacol Sci. 2020;41(11):868–881. doi:10.1016/j.tips.2020.09.001
33. Soydas T, YaprakSarac E, Cinar S, et al. The protective effects of metformin in an in vitro model of aging 3T3 fibroblast under the high glucose conditions. J Physiol Biochem. 2018;74(2):273–281.
34. Soydas T, Sayitoglu M, Sarac EY, et al. Metformin reverses the effects of high glucose on human dermal fibroblasts of aged skin via downregulating RELA/p65 expression. J Physiol Biochem. 2021;77(3):443–450. doi:10.1007/s13105-021-00823-y
35. Pennacchi PC, de Almeida ME, Gomes OL, et al. Glycated reconstructed human skin as a platform to study the pathogenesis of skin aging. Tissue Eng Part A. 2015;21(17–18):2417–2425. doi:10.1089/ten.tea.2015.0009
36. Chung MM, Nicol CJ, Cheng YC, et al. Metformin activation of AMPK suppresses AGE-induced inflammatory response in hNSCs. Exp Cell Res. 2017;352(1):75–83. doi:10.1016/j.yexcr.2017.01.017
37. Zhou Z, Tang Y, Jin X, et al. Metformin inhibits advanced glycation end products-induced inflammatory response in murine macrophages partly through AMPK activation and RAGE/NFκB pathway suppression. J Diabetes Res. 2016;2016:4847812. doi:10.1155/2016/4847812
38. Louis F, Fujii N, Katsuyama M, Okumoto S, Matsusaki M. Effects of radiofrequency and ultrasound on the turnover rate of skin aging components (skin extracellular matrix and epidermis) via HSP47-induced stimulation. Biochem Biophys Res Commun. 2020;525(1):73–79. doi:10.1016/j.bbrc.2020.02.020
39. Kirsner RS, Yosipovitch G, Hu S, et al. Diabetic skin changes can benefit from moisturizer and cleanser use: a review. J Drugs Dermatol. 2019;18(12):1211–1217.
40. Kruglikov IL. Influence of the dermis thickness on the results of the skin treatment with monopolar and bipolar radiofrequency. Currents Biomed Res Int. 2016;2016:1953203.
41. Kokolakis G, von Eichel L, Ulrich M, Lademann J, Zuberbier T, Hofmann MA. Kinetics and tissue repair process following fractional bipolar radiofrequency treatment. J Cosmet Laser Ther. 2019;21(2):71–75. doi:10.1080/14764172.2018.1461232
42. Kassim AT, Goldberg DJ. Assessment of the safety and efficacy of a bipolar multi-frequency radiofrequency device in the treatment of skin laxity. J Cosmet Laser Ther. 2013;15(2):114–117. doi:10.3109/14764172.2013.764438
43. Willey A, Kilmer S, Newman J, et al. Elastometry and clinical results after bipolar radiofrequency treatment of skin. Dermatol Surg. 2010;36(6):877–884. doi:10.1111/j.1524-4725.2010.01563.x
44. de Oliveira TC, Rocha SF, Ramos DG, Ramos CG, Carvalho MV, Ramos MG. Effects of multipolar radiofrequency and pulsed electromagnetic field treatment for Face and neck rejuvenation. Dermatol Res Pract. 2017;2017:4146391. doi:10.1155/2017/4146391
45. Landriscina A, Rosen J, Friedman AJ. Nanotechnology, inflammation and the skin barrier: innovative approaches for skin health and cosmesis. Cosmetics. 2015;2(2):177–186. doi:10.3390/cosmetics2020177
46. Choi EH. Aging of the skin barrier. Clin. Dermatol. 2019;37(4):336–345. doi:10.1016/j.clindermatol.2019.04.009
47. Russell-Goldman E, Murphy GF. The pathobiology of skin aging: new insights into an old dilemma. Am J Pathol. 2020;190(7):1356–1369. doi:10.1016/j.ajpath.2020.03.007
48. Kretzschmar K, Watt FM. Markers of epidermal stem cell subpopulations in adult mammalian skin. Cold Spring Harb Perspect Med. 2014;4(10):a013631. doi:10.1101/cshperspect.a013631
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

