Back to Journals » Infection and Drug Resistance » Volume 15
Evaluation of Prevalence of Side-Effects Associated with Booster Dose of mRNA-Based COVID-19 Vaccine Among Healthcare Workers in Eastern Province, Saudi Arabia: A Descriptive Cross-Sectional Study
Authors Ali MD , Almadan LZ, Alghamdi RA, Alghamdi AS, Almarhoon SA, Hassan YAM , Ahmad A, Ghosn SA, Banu N, Eltrafi Z
Received 29 May 2022
Accepted for publication 13 July 2022
Published 9 August 2022 Volume 2022:15 Pages 4335—4346
DOI https://doi.org/10.2147/IDR.S374265
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Mohammad Daud Ali, Leena Zakariya Almadan, Ruba Ahmed Alghamdi, Alanood Saleh Alghamdi, Sarah Ali Almarhoon, Yousif AM Hassan, Ayaz Ahmad, Sherihan Ahmad Ghosn, Nuzhat Banu, Zainab Eltrafi
Department of Pharmacy, Mohammed Al-Mana College for Medical Sciences, Dammam, 34222, Saudi Arabia
Correspondence: Mohammad Daud Ali, Department of Pharmacy, Mohammed Al-Mana College for Medical Sciences, Abdulrazaq Bin Hammam Street, Al Safa, Dammam, 34222, Saudi Arabia, Email [email protected]
Background: The purpose of this study was to examine the mild and moderate side‐effects experienced by the healthcare workers (HCWs) in the Eastern Province of Saudi Arabia after receiving the booster dose of the Pfizer-BioNTech/BNT162b2 COVID‐19 vaccine.
Methods: We directed a descriptive cross-sectional study among adults living in the Eastern Province of Saudi Arabia. A survey link was distributed through WhatsApp, SMS, or e-mail to HCWs. Participants’ general and demographic information were also collected, as well as information about any local and systemic side‐effects reported following vaccination.
Results: The results of this study showed that 81.84% (401/490) of the HCWs who contributed to this study reported the minimum COVID‐19 post‐vaccination side‐effect. Body pain (89%) and pain at the site of injection (88.73%) were the most frequent frequently reported side‐effects, followed by headache (28.68%), joint or bone pain (27.18%), muscle pain (26.43%), nausea or vomiting (21.2%), fever (18.95%), skin rashes (10.22%). History of chronic diseases had a 0.44‐fold increased risk of side-effects compared to no history of chronic diseases HCWs (adjusted odds ratio (aOR) = 0.44; 95% CI = 0.224, 0.880), and significant association of occupation with side-effects was also 1.61-fold increased risk compared to nonmedical ((aOR) = 1.61; 95% CI = 1.037, 2.513).
Conclusion: According to this study, the Pfizer-BioNTech/BNT162b2 COVID-19 vaccine was safe when given to Saudi Arabian HCWs. All reported side‐effects were mild to moderate. The outcomes indicated that most participants had body pain and pain at the site of injection and fatigue is among the least reported side-effect post-booster dose. Healthcare was highly connected with more reporting of side‐effects.
Keywords: COVID‐19, vaccine, side‐effects, healthcare workers, Eastern Province, Saudi Arabia
Introduction
The pandemic impulse generated due to the infectious agent severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) resulted in millions of infections and around six million deaths globally.1,2 As late as May 9, 2022, there were 755,415 cases reported and 9103 deaths in KSA at the hand of SARS-CoV-2.2 Vaccination is regarded the at most survivor from the pandemic. However, keeping with the data collected as late as May 4, 2022, 11,562,157,794 inoculations are reported to be administered worldwide.3 The earliest COVID-19 vaccine manufactured and approved was Pfizer-BioNTech/BNT162b2 which has the goal to hold off the pandemic. The experimental studies were completed in a short duration and were given Food and Drug Administration (FDA) approval just after a few months following its I or II clinical trials that were held in May 2020. The EUA (emergency use authorization) for this vaccine was granted in the last month of the year 2020. It received 23 August 2021 the full FDA approval, after passing all safety and efficacy requirements.4 Three approved companies whose doses are presently available in Saudi Arabia include Pfizer-BioNTech/BNT162b2, Moderna, and Oxford/AstraZeneca COVID‐19 vaccines.5,6 Among the currently approved COVID-19 vaccines, Pfizer-BioNTech/BNT162b2 and Moderna mRNA-1273 are based on mRNA technology.7–10
With the detection of novel viral variants, like “Omicron”, “Delta”, and “Coronavirus Mu variant”, there is a worry that the decline in immunity over time will diminish the potency; therefore, the third dose called booster may be required to boost immunity in fighting against new strains.8 After getting FDA approval, the CDC (Centre for Disease Control and Prevention) recommended a third dose for all aged 65 and above, and later the eligibility was expanded to other groups. The WHO-World Health Organization is also emphasizing an additional dose of the vaccine, to emphasize vaccine efforts to decrease the death rates and turn down the severity of disease, to protect the general maintenance of health.11 In Saudi Arabia, an additional dose was suggested for all who crossed the age of 16 and had completed their 2 doses. The Ministry of Health (MOH) Saudi Arabia created cognizance of the third dose – booster dose – through a campaign called “Maintain Your Level with Booster Shot” so as to achieve maximum immunity in the community.5
Vaccine hesitancy (VH), the biggest challenge to overcome and managing the pandemic, is elucidated as refusal or delaying its acceptance despite its availability.12 A research in the Kingdom of Saudi Arabia (KSA) has reported that the driving factor in hesitancy in accepting the inoculations of the vaccine was the concern over its efficacy and safety. It was revealed in a review that the inclined awareness about safe vaccines and being honest in reporting adverse effects could help in tackling the delay faced in vaccine acceptance and improving the uptake of immunization.13
Several studies from various other areas of the world reported fewer side effects after administrating the first or second dose, such as pain at the site of injection, swelling, tenderness, redness, warmth, itch, and swollen armpit glands, headache, fever, fatigue, night sweats, chills and shivering, joint pain, muscle pain, nausea, vomiting, and loss of appetite.14–19 Despite the reality that the clinical studies assured the vaccine was safe and effective, it had limitations, such as a few participants recruited in the clinical studies and a healthier than real-world sample. This may neglect some less frequent deleterious effects that may happen. The vaccine developed in a short span and using new technology triggered a worry among people regarding its safety. Post-marketing evaluation is necessary to increase the public confidence in the vaccine and motivate them to accept the vaccine.10,20–23
Keeping the limitations of clinical studies in mind, further epidemiological studies are important to evaluate the short-term COVID-19 side effects and to increase public trust in the safety and efficacy of the vaccine. Health professionals are recruited in the present study with the hope that the unwanted effects being reported by health practitioners will be accurate in comparison to the non-health professionals. Therefore, this research objective is to identify the short-duration side effects after receiving the Pfizer-BioNTech/BNT162b2 COVID-19 vaccine in health practitioners in the Eastern Province of the Kingdom of Saudi Arabia.
Materials and Methods
Study Settings
A descriptive cross-sectional study, conducted in the Eastern Province of Saudi Arabia, used a self-administered online survey to look for adverse reactions reported by healthcare workers (HCWs) who have received the booster dose of Pfizer-BioNTech/BNT162b2 vaccine. All vaccinated respondents who had taken their booster dose of Pfizer-BioNTech/BNT162b2 vaccine were requested for online surveys wherever the respondents stayed requested to self‐report some mild‐to‐moderate indications that they developed post-vaccination. The target people of the current study HCWs (aged 18 to more than 55 years) were working in the Eastern Province, Saudi Arabia. The entitled respondents were to have taken their booster dose of the COVID‐19 vaccine prior to participating and filling in the survey questionnaire. Clinical (physicians, nurses, medical technicians, and pharmacists) and nonclinical (administrative and medical record professionals, and nonmedical technicians) HCWs serving at private and public hospitals in Eastern Province during the study time were the target populations of the current study. Respondents who had not taken booster doses as well as not HCWs were excluded from the study. The survey team identified the respondents (HCWs) from their professional and personal contact. Information was collected about those who were entitled and eager to participate in the survey. We followed STROBE guidelines for cross-sectional studies.24
Sample Size
In this study, we calculated the minimum sample size according to Lwanga and Lemeshow.25 Due to the absence of previous data on post-vaccination side-effects of COVID-19 booster dose among HCWs in Saudi Arabia, the <0.50 presumed quantity was used,26 through a 5% margin of error and 95% confidence level. A total of 384 participants were deemed necessary for this study based on the minimum number. However, as this study was based on an online survey disseminated via social media, it was decided to decrease the sampling bias in our method. We increased it to include 577 participants, which is near about 1.5-fold of the required size. During the study period, 580 eligible respondents participated in the study.
Tool and Data Collection
An appraisal of literature, including PubMed, Google Scholar, and other databases, was conducted with the goal of identifying short-term and potential side‐effects that might occur after the Pfizer-BioNTech/BNT162b2 vaccination. The questionnaire was designed using Google Forms and was written in English and Arabic. The final versions were reviewed by four different experts in the field to check for face and content validity. It was initially distributed to a pilot sample consisting of 10 participants to check for any technical misunderstandings or contraindications. The final data analysis did not include the pilot survey response. The survey was made as short in length as possible. It was clarified that the information would be used only for medical research purposes, as the participants were not identified and had the right to withdraw at any stage. An online survey using choice randomization displays answer options at random to the participants to avoid selection bias. Multiple sections were included in the questionnaire. A brief description of the purpose of the study is contained in the first section, along with contact details for the investigators, and an agreement for participants to consent to participate. The second section of the questionnaire was designed for the purpose of gathering general information about the respondents comprising age, sex, educational qualification, occupation, chronic diseases such as hypertension, diabetes mellitus, asthma, and others, in addition to whether they had, previous infection history with SARS-CoV-2 infection. The third section of the study focused on the COVID-19 vaccine, specifically side‐effects experienced after Pfizer-BioNTech’s/BNT162b2 COVID-19 vaccine, their timing, and duration. Participants were able to leave the box unchecked if no symptoms were reported. There is a subsection with a list of the most frequently side‐effects reported in other studies,21,27–29 redness at the site of the injection, pain at the site of the injection, body pain, fatigue, other, fever, chills, delayed menstruation, headaches, skin rashes, swelling at the site of the injection, nausea, and vomiting. Moreover, a section was provided for reporting other possible unpublished side‐effects. The study participants may have experienced. Further-more, participants were asked to report visits to doctors and any hospitalizations after vaccination, as well as any medications taken after vaccination. The target group for this study are HCWs working in the Eastern Province of Saudi Arabia. The survey team identified the respondents for the survey. The survey’s link was disseminated to the respondents via WhatsApp, SMS, or e-mail and in individual to set a meeting. Survey/data collection has been performed from 7 March 2022 to 20 April 2022. Since all participants were asked to conduct the survey, members of the survey team identified and verified the responses of those who did not participate. Survey responses were provided by eligible participants based on their willingness to participate in the research.
Data for only those who had taken a booster dose of the Pfizer-BioNTech/BNT162b2 vaccine, respondent’s adherence with the inclusion and exclusion criteria and submitted a complete questionnaire. Of 577 participants, 74 (12.82%) individuals were excluded due to incomplete survey. Thirteen (2.25%) participants received the Moderna COVID-19 vaccine and were excluded from this study. In total, 490 (84.92%) respondents were included in the final analysis.
Ethical Approval
For conducting this study, we obtained ethical approval from the standing ethical review committee at Mohammed Al-Mana College for Medical Sciences (Reference number: SR/RP/80). We obtained the consent of all study participants before participation in the study. The study omitted responses with no informed consent, incomplete responses, those who failed to take a booster dose, respondents other than HCWs and those who taken a COVID-19 vaccine booster dose other than the Pfizer-BioNTech/BNT162b2 vaccine.
Statistical Analysis
Statistical analysis was conducted using the data spread from Google Forms (Mountain View, California, USA) and Microsoft Excel (Version 2016), and then exported into Statistical Package for Social Sciences (SPSS) version 26.0 (IBM, Inc., Armonk, NY, USA). To depict the distribution of categorical variables, we used descriptive statistics such as occurrence, percentage, and mean ± standard deviation (SD), and to represent quantitative variables, we used median (interquartile range, IQR), respectively. Shapiro–Wilk tests were performed before the analysis to determine the normality of all quantitative variables. We cast off Pearson’s chi-square (χ2) for examining the connotation amongst COVID-19 post-vaccination side‐effects and the independent variables (demographics and background features). Mann–Whitney U-tests and Kruskal–Wallis tests were used to evaluate the number of COVID-19 post-vaccination adverse effects reported by the respondents as an ordinal dependent variable corresponding to the independent variables.
Additionally, a multivariate logistic regression investigation was conducted to determine the factors related to a complaint about side‐effects subsequent to administration of the Pfizer–BioNTech/BNT162b2 COVID-19 vaccine, which was coded as a replica reliant on variable (yes = 1 and no = 0). A multivariate ordinal logistic regression exploration was conducted to determine whether certain factors are associated with side‐effects following vaccination. A multivariate logistic regression model was created based on all variables that were associated with p ≤ 0.25 in univariate investigations.30 Odds ratios were calculated and intervals of confidence were calculated for all analyses. P-values were amended for multiple evaluations using the consecutive Bonferroni method (Bonferroni-Holm). Statistical significance was defined as p-values less than 0.05.31
Results
Respondent’s General Characteristics
Table 1 shows the general demographic characteristics of 490 vaccinated HCWs with the Booster dose of Pfizer-BioNTech/BNT162b2 vaccine participated in the study.
 |
Table 1 General Demographic and Health Characteristics of the Participants (n = 490) |
The mean± SD age of the contributors was 33.83±6.91; more than half (56.74%, n = 278) of them were females, and 43.26% were males. Nurses were accounted for the majority of HCW group at 25.51% (n = 125), followed by Pharmacist (Medical) (18.37%, n = 90). Among all the HCW 29.18% (n = 143) have experienced between 1 and 2 years. Participants who had been previously diagnosed with COVID‐19 made up 32.86% (n = 161), most of whom were females (56.52%, 91/161). Only 8.37% (n = 41) of participants had reported suffering from a chronic disease.
Details of COVID‐19 Vaccine Side‐Effects
As a result of this study, 81.84% (401/490) of the HCWs who took part described at least one post-vaccination side effect of COVID-19. There were 3 (1–4) reported side effects (median (IQR). Amongst the 401 HCWs who experienced post‐vaccination side‐effects of COVID‐19, Figure 1 displays that body pain (89%) and pain at the site of injection (88.73%) were the utmost frequent reported side‐effects, followed-by headache (28.68%), joint or bone pain (27.18%), muscle pain (26.43%), nausea or vomiting (21.2%), fever (18.95%), skin rashes (10.22%). On the other hand, the smallest often reported unwanted effects were redness at the site of injection (8.23), chills (7.98%), fatigue (7.73%), and swelling at the site of injection. However, 11.93% of the female respondents also reported delayed menstruation.
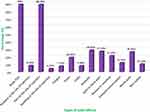 |
Figure 1 The frequency of COVID‐19 vaccine side‐effects reported among the participants (n = 401). |
Participants who developed COVID-19 post-vaccination side effects were grouped according to their gender (Table 2). The results displayed that female HCWs had a meaningfully higher occurrence of side-effects of body pain (90.12% vs 87.34%; χ2 = 18.37; p<0.001), pain at the site of the injection (89.30% vs 87.97%; χ2 = 17.08; p = 0.001), swelling at the site of the injection (5.35% vs 2.53%; χ2 = 4.76; p = 0.03), headache (89.30% vs27.85%; χ2 = 6.33; p = 0.01), muscle pain (28.40% vs 23.42%; χ2 = 9.66; p<0.001) and skin rashes (11.11% vs 8.86%; χ2 = 6.33; p = 0.01) compared to male HCWs. On the other hand, the male HCWs had an ominously higher frequency of whole‐body pain/fatigue (67.8%, 61/90) related to their female peers (50.0%, 80/160) (χ2 = 4.12; p = 0.04). Nonetheless, these significant differences were not retained when the Bonferroni–Holm adjustment was applied.
 |
Table 2 Distribution of COVID‐19 Vaccine Side‐effects Reported Among the Healthcare Worker Participants According to Gender (n = 401) |
Associations of the Described COVID‐19 Vaccine Side‐Effects
In Table 3, we explore how COVID-19 vaccine side effects are associated with participant demographics and health background characteristics. HCWs developed side-effects at comparable frequencies (p = 0.001) among females (87.41%, 243/278) and males (74.52%, 158/221). There was substantial variance in the frequency of side‐effects consistent with participants’ age (<0.001) except >55 years age group. Interestingly, medical HCWs had a significantly lower frequency of unwanted effects than nonmedical HCWs (61.67% vs 80.73%; p = 0.001). Similarly, more than 5 years of experience significant side-effects (86.08%; p = 0.04) compared to another experience group of HCWs. There is no significant relationship between the history of comorbidities of COVID‐19 infection and side-effects experienced with the booster dose of vaccination. After Bonferroni–Holm correction, only two variables, sex, and occupation (medical) displayed a substantial link with the reporting of side‐effects.
 |
Table 3 Associations of the Reported COVID‐19 Vaccine Side‐effects with the Participants’ Background Characteristics (n = 490) |
In Table 4, we display the changes in the number of COVID-19 post-vaccination adverse effects stated by the deliberate HCWs according to self-governing variables. Medical HCWs have far fewer side effects (median = 4; IQR = 2, 3) as compared to nonmedical HCWs (median = 1; IQR = 1, 2) (U = 4148; p = 0.05) based on the Mann–Whitney U-test. Nevertheless, the Kruskal–Wallis test did not show any significant differences in the number of side effects between age groups (H = 2; p = 0.36). As well, there were no significant differences in the number of side effects across variables (p > 0.05).
 |
Table 4 Number of Side‐effects Reported by the Participants Following the Booster Dose of COVID‐19 Vaccine According to the Participants’ Background Characteristics (n = 401) |
A Multivariate Analysis of the Factors Associated with Reported Side Effects of the COVID-19 Vaccine
In Table 5, we present the results of multivariate logistic regression analysis for the factors accompanying reporting side-effects following the booster dose of the COVID‐19 vaccine among the HCWs. All variables that displayed relations with p ≤ 0.25 in the univariate analysis are available in Table 3. Hosmer–Lemeshow test, cast-off for the inferential goodness-of-fit test, indicated the model fitted the data well (χ2 = 13.584; p = 0.317). The results revealed that a history of chronic diseases had a 0.44‐fold increased risk of side-effects compared to no history of chronic diseases health care workers (adjusted odds ratio (aOR) = 0.44; 95% CI = 0.224, 0.880), and a significant association of occupation with side-effects was also 1.61-fold increased risk compared to nonmedical ((aOR) = 1.61; 95% CI = 1.037, 2.513). Though, the substantial connotation of the year of experience with side-effects was not maintained in the multivariate analysis (p = 0.418).
 |
Table 5 Multivariate Analysis of Factors Associated with Reporting of Side‐effects Following COVID‐19 Vaccine Among the Participants (n = 401) |
Moreover, Table 6 provides the multivariate ordinal logistic regression analysis results for the factors prompting the number of COVID-19 vaccine side-effects described by the studied health care workers. Table 4 presents a univariate analysis that showed differences with a probability of p ≤ 0.21; all variables that showed a difference were included in the model. The model had a good fit and was statistically significant (χ2 = 19.254, p < 0.001). In the current study, the rate of stated side-effects was meaningfully predisposed by HCW gender and history of COVID-19 infection. The results indicated that holding other variables unchanged, a history of COVID-19 infection was fewer expected to report a greater number of side-effects than no history of COVID-19 infection (aOR = 17.410; 95% CI = 0.575, 1.974; p < 0.001). Likewise, female respondents were less likely to report a greater number of side-effects than male contributors (aOR = 5.164; 95% CI = 0.116, 1.576; p = 0.023).
 |
Table 6 Results of Ordinal Logistic Regression for the Factors Associated with the Number of Reported Side‐effects Following the COVID‐19 Vaccine (n = 401) |
Period and controlling of the described COVID‐19 vaccine side‐effects among the health care workers who developed COVID‐19 post-booster dose vaccination side‐effects, 77.81% (312/401) of the testified side‐effects happened on the day of getting the vaccine (Day 0). In contrast, 16.21% and 5.49% of the stated side‐effects happened on Day 1 and Day 2 post-vaccination (the second and third post‐vaccination days of booster dose), correspondingly. However, only 0.50% HCWs reported side effects appeared after day 2 of a post-booster dose of vaccination. The period of informed side‐effects was 1–2 days for 58.35% (n = 234) and 3–5 days for 25.94% (n = 104), while the side‐effects lasted for more than 5 days for only 7.73% (n = 31) of the subcategory of members who reported unwanted effects. Furthermore, 3.0% (n = 12) of those who experienced side‐effects go to a doctor, and medicine for a post-booster dose of vaccination side‐effects was taken by 83.79% (n = 336). Only 1.5% HCWs (n=6) were admitted to the hospital for less than 24 hours; however, only 0.50% (n = 2) were admitted to the hospital for 24–36 hours (Table 7).
 |
Table 7 The Onset, Duration, and Management of COVID‐19 Vaccine Side‐effects Were Reported Among the Participants in the Study (n = 401) |
Discussion
Presenting transparently the possible side effects post-vaccine administration is an approach to keeping trust in the vaccines and maximizing the benefits of vaccines safely. Subsequently, populations’ fears against vaccines could be dissipated. In this context, the current research sheds light on the prevalence of Side-Effects related to mRNA-based COVID-19 Vaccine booster dose among HCWs in the Eastern Province of Saudi Arabia.
The outcomes of the current study revealed a high incidence of side effects among the respondents where 81.84% of them have stated the minimum of one side-effect post-COVID-19 vaccine booster dose. This is consistent with data from other studies examining the safety of booster dose.32 However, compared to a previous study for first and second doses in which short-term side‐effects of Pfizer-BioNTech/BNT162b2 in individuals age span between 18 and 70 years old were investigated, fewer overall side‐effects (40%, n = 208) were recorded.33
Our findings show that body pain (89%) and injection site pain (88.73%) were typically indicated side‐effects by the HCW respondents, similar to the data publicized by Phase 3 clinical trial with the Pfizer-BioNTech/BNT162b2 vaccine revealed that pain at the injected site was the prevalent complication (71–83%). However, in contrast to findings of the stated clinical trial where fatigue was the second reported side-effects (34–47%), our data show fatigue was among the least frequently reported side effects (7.73%).34 We found higher values for delayed menstruation indicated by female participants (11.93%) than those reported by Edelman et al in the USA (4.3%).35
Our results corroborate with previous studies where a gender‐related variance in describing adverse events after numerous vaccines. It was detected a vigorous inflammatory and immune responses to vaccines in females as opposed to males, which could elaborate the gender‐based variances response toward vaccinations.36 Additionally, behavioral, genetic, and hormonal elements could trigger the divergence in adverse events after vaccination between genders.37,38
Remarkably, the contemporary paper revealed that medical profession participants showed an important connotation with the reporting of side‐effects, which concurs well with the findings of a study done by Darraj et al in Saudi Arabia.39 Medical HCWs were more likely than nonmedical HCWs to detail a higher figure of side effects. These could be related to the medical and scientific background of medical HCWs about vaccine safety, which would improve their capacity to recognize and distinguish manifestations.39
Contrary to several earlier reports indicating that people with prior exposure to COVID-19 infection detailed more side effects, our results do not seem to confirm their observation.20,34,40 In fact, no significant relations with history of COVID‐19 infection and side effects experienced with the booster dose of vaccination were noted in our study. However, a noteworthy link between increased reporting side effects and participants having a history of chronic diseases was noted and also confirms previous findings in Germany.41
In this study, 77.81% of the reported side‐effects happened on vaccination day (0 day), which is in agreement with the results stated in other comparable studies.33,42 In the present investigation around 83.79% of the respondents were on medications (chiefly analgesics) to lessen the side effects. Analgesics are commonly used by Saudi Arabians both currently working in healthcare and those not in healthcare to ease the COVID-19 vaccine’s side effects.43 Moreover, just 3% of the respondents necessitated seeing a physician owing to side‐effects from the vaccines, and only 2% of the respondents were hospitalized. This will fully endorse the vaccines’ safety.
As a result of the present study, we can add more social confidence related to the safety of the COVID-19 Pfizer-BioNTech/ BNT162b2 vaccine, allowing to speed vaccination coverage. The transparent reporting of side effects by health care workers is especially central for accepting vaccinations for the general public. This is because people are more likely to be vaccinated when directed by a healthcare professional.44
However, our study is not without limitations. First, a self-administered online survey was used to assemble the data, which could result in a reporting bias and quite a small sample size. We chose an online study because we wanted to guarantee participants’ safety, due to the COVID-19 pandemic, SARS-CoV-2 viruses, and the recommendation to continue social distancing as a precaution measure in Saudi Arabia. In addition, conducting community-based surveys would be difficult during this pandemic. Thus, we collected our data online through a self-reported survey, and it was distributed based on the authors’ networks. Another limitation of the current study is that it observed the vaccine’s short-term side-effects (from day 0). Vaccine effects on our cohort are still unknown in both the short- and long-term. Likewise, side effects subsequent to the booster dose of the vaccine were investigated, whereas the side-effects post the first and second dose of the vaccine were not studied. Additionally, the participants were only Pfizer-BioNTech/BNT162b2 recipients. Therefore, more multicentred investigations with bigger sample sizes are necessary to confirm the different COVID-19 vaccines safety profiles permitted in the country as well as, forthcoming studies to assess other rare events such as thromboembolic profiles and myocarditis and to confirm the immunogenicity of the booster doses are also important.45
Conclusions
The current research comes up with relevant information regarding the side effects that can occur post-COVID-19 vaccine administration amongst HCW in the Eastern Province of Saudi Arabia. The outcomes indicated that most respondents had body pain and site injection pain, whereas fatigue is among the least reported side-effects post-booster dose. The female respondents also report delayed menstruation, but it would be necessary to collect extensive data from a large number of vaccinated populations to assess any significant association between delayed menstruation and the COVID-19 vaccine. Additionally, occupations related to healthcare were highly connected with more reporting of side‐effects. Self-medication practice to alleviate side-effects due to the vaccination is clearly manifested in the. Overall, the outcomes pointed to entirely tolerable mild to moderate reported COVID‐19 post‐booster dose vaccination side‐effects. Consequently, the existing study’s findings reinforce the vaccine’s safety and offer main standard facts to rise healthcare workers’ and the general community’s awareness of the predictable side‐effects subsequent COVID‐19 vaccines. This could convince the reluctant, refusal persons and pessimists to receive the booster dose COVID‐19 vaccine.
Funding
There is no funding to report.
Disclosure
The authors have no conflicts of interest to declare in this work.
References
1. World Health Organization. Global coronavirus statistics. United Nations. Available from: https://covid19.who.int/region/amro/country/us.
2. Coronavirus cases. Reported cases and deaths by country or territory. Available from: https://www.worldometers.info/coronavirus/#countries.
3. Global Situation. WHO coronavirus (COVID-19) dashboard global situation. Available from: https://covid19.who.int/?mapFilter=cases.
4. FDA; 2021 [updated March 11, 2022]. FDA Approves First COVID-19 Vaccine. Available from: https://www.fda.gov/news-events/press-announcements/fda-approves-first-covid-19-vaccine.
5. Ministry of Health. COVID‐19 & vaccine FAQs. Riyadh: Ministry of Health; 2021. Available from: https://www.moh.gov.sa/en/Ministry/HotTopics/Pages/COVID‐19‐Vaccine.aspx.
6. Two more COVID-19 vaccines approved for use in Saudi Arabia. Available from: https://www.arabnews.com/node/1794826/saudi-arabia.
7. Background document on the mRNA vaccine BNT162b2 (Pfizer-BioNTech) against COVID-19. Available from: https://apps.who.int/iris/rest/bitstreams/1327316/retrieve.
8. Interim statement on booster doses for COVID-19 vaccination. Available from: https://www.who.int/news/item/22-12-2021-interim-statement-on-booster-doses-for-covid-19-vaccination—update-22-december-2021.
9. News. Available from: https://sfda.gov.sa/en/cnews.
10. Barda N, Dagan N, Ben-Shlomo Y, et al. Safety of the BNT162b2 mRNA Covid-19 vaccine in a nationwide setting. N Engl J Med. 2021;385(12):1078–1090. doi:10.1056/NEJMoa2110475
11. AHelping latinos recover and thrive. Available from: https://www.who.int/news/item/22-12-2021-interim-statement-on-booster-doses-for-covid-19-vaccination—update-22-december-2021.
12. Riad A, Pokorná A, Attia S, Klugarová J, Koščík M, Klugar M. Prevalence of COVID-19 vaccine side effects among healthcare workers in the Czech Republic. J Clin Med. 2021;10(7):1428. doi:10.3390/jcm10071428
13. Alrajeh AM, Daghash H, Buanz SF, Altharman HA, Belal S. COVID-19 vaccine hesitancy among the adult population in Saudi Arabia. Cureus. 2021;13(12):e20197. doi:10.7759/cureus.20197
14. Kaur RJ, Dutta S, Bhardwaj P, et al. Adverse events reported from COVID-19 vaccine trials: a systematic review. Indian J Clin Biochem. 2021;36(4):427–439. doi:10.1007/s12291-021-00968-z
15. Abu-Halaweh S, Alqassieh R, Suleiman A, et al. Qualitative assessment of early adverse effects of pfizer-BioNTech and sinopharm COVID-19 vaccines by telephone interviews. Vaccines. 2021;9(9):950. doi:10.3390/vaccines9090950
16. Klugar M, Riad A, Mekhemar M, et al. Side effects of mRNA-based and viral vector-based COVID-19 vaccines among German healthcare workers. Biology. 2021;10(8):752. doi:10.3390/biology10080752
17. Menni C, Klaser K, May A, et al. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: a prospective observational study. Lancet Infect Dis. 2021;21(7):939–949. doi:10.1016/S1473-3099(21)00224-3
18. Saeed BQ, Al‐Shahrabi R, Alhaj SS, Alkokhardi ZM, Adrees AO. Side effects and perceptions following Sinopharm COVID‐19 vaccination. Int J Infect Dis. 2021;111:219–226. doi:10.1016/j.ijid.2021.08.013
19. Thomas SJ, Moreira ED, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid‐19 vaccine through 6 months. N Engl J Med. 2021;385:1761–1773. doi:10.1056/NEJMoa2110345
20. Mulligan MJ, Lyke KE, Kitchin N, et al. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature. 2020;586(7830):589–593. doi:10.1038/s41586-020-2639-4
21. Ossato A, Tessari R, Trabucchi C, Zuppini T, Realdon N, Marchesini F. Comparison of medium-term adverse reactions induced by the first and second dose of mRNA BNT162b2 (Comirnaty, Pfizer-BioNTech) vaccine: a post-marketing Italian study conducted between 1 January and 28 February 2021. Eur J Hosp Pharm. 2021;
22. Arce JSS, Warren SS, Meriggi NF, et al. COVID-19 vaccine acceptance and hesitancy in low- and middle-income countries. Nat Med. 2021;27(8):1385–1394. doi:10.1038/s41591-021-01454-y
23. Banerji A, Wickner PG, Saff R, et al. mRNA vaccines to prevent COVID-19 disease and reported allergic reactions: current evidence and suggested approach. J Allergy Clin Immunol Pract. 2021;9(4):1423–1437. doi:10.1016/j.jaip.2020.12.04
24. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Available from: https://www.equator-network.org/reporting-guidelines/strobe/.
25. Lwanga SK, Lemeshow S; World Health Organization. Sample Size Determination in Health Studies: A Practical Manual. Geneva, Switzerland:World Health Organization; 1991. Available from: https://apps.who.int/iris/handle/10665/40062.
26. Israel, G.D. Determining sample size; technical report. Gainesville, FL, USA: University of Florida; 1992. Available from: https://www.tarleton.edu/academicassessment/documents/samplesize.pdf.
27. Cuschieri S, Borg M, Agius S, Souness J, Brincat A, Grech V. Adverse reactions to Pfizer-BioNTech vaccination of healthcare workers at Malta’s state hospital. Int J Clin Pract. 2021;75:e14605. doi:10.1111/ijcp.14605
28. Hagin D, Freund T, Navon M, et al. Immunogenicity of Pfizer-BioNTech COVID-19 vaccine in patients with inborn errors of immunity. J Allergy Clin Immunol. 2021;148:739–749. doi:10.1016/j.jaci.2021.05.029
29. Nittner-Marszalska M, Rosiek-Biegus M, Kope´c A, et al. Pfizer-BioNTech COVID-19 vaccine tolerance in allergic versus non-allergic individuals. Vaccines. 2021;9:553. doi:10.3390/vaccines9060553
30. Bendel RB, Afifi AA. Comparison of stopping rules in forward “stepwise” regression. J Am Stat Assoc. 1977;72:46–53.
31. Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6:65–70.
32. Munro APS, Janani L, Cornelius V. Safety and immunogenicity of seven COVID-19 vaccines as a third dose (booster) following two doses of ChAdOx1 nCov-19 or BNT162b2 in the UK (COV-BOOST): a blinded, multicentre, randomised, controlled, Phase 2 trial. Lancet. 2021;398(10318):2258–2276. doi:10.1016/S0140-6736(21)02717-3
33. Alhazmi A, Alamer E, Daws D, et al. Evaluation of side‐effects associated with COVID-19 vaccines in Saudi Arabia. Vaccines. 2021;9(6):674. doi:10.3390/vaccines9060674
34. Skowronski DM, De Serres G. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2021;384:1576–1577. doi:10.1056/NEJMc2036242
35. Edelman A, Boniface ER, Benhar E, et al. Association between menstrual cycle length and coronavirus disease 2019 (COVID-19) vaccination: a US cohort. Obstet Gynecol. 2022;139(4):481–489. doi:10.1097/AOG.0000000000004695
36. McCartney PR. Sex-based vaccine response in the context of COVID-19. J Obstet Gynecol Neonatal Nurs. 2020;49(5):405–408. doi:10.1016/j.jogn.2020.08.001
37. Vassallo A, Shajahan S, Harris K, et al. Sex and gender in COVID-19 vaccine research: substantial evidence gaps remain. Front Glob Womens Health. 2021;2:761511. doi:10.3389/fgwh.2021.761511
38. Harris T, Nair J, Fediurek J, Deeks SL. Assessment of sex-specific differences in adverse events following immunization reporting in Ontario, 2012–2015. Vaccine. 2017;35(19):2600–2604. doi:10.1016/j.vaccine.2017.03.035
39. Darraj MA, Al-Mekhlafi HM. Prospective evaluation of side-effects following the first dose of Oxford/AstraZeneca COVID-19 vaccine among healthcare workers in Saudi Arabia. Vaccines. 2022;10(2):223. doi:10.3390/vaccines10020223
40. Lv M, Luo X, Shen Q, et al. Safety, immunogenicity, and efficacy of COVID-19 vaccines in children and adolescents: a systematic review. Vaccines. 2021;9(10):1102. doi:10.3390/vaccines9101102
41. Loosen SH, Bohlken J, Weber K, et al. Factors associated with non-severe adverse reactions after vaccination against SARS-CoV-2: a Cohort Study of 908,869 outpatient vaccinations in Germany. Vaccines. 2022;10(4):566. doi:10.3390/vaccines10040566
42. Alghamdi AA, Alkazemi A, Alissa A, Alghamdi I, Alwarafi G, Waggas HA. Adverse events following AstraZeneca COVID-19 vaccine in Saudi Arabia: a cross-sectional study among healthcare and nonhealthcare workers. Intervirology. 2022;65(2):104–109. doi:10.1159/000519456
43. Adam M, Gameraddin M, Alelyani M, et al. Evaluation of post‐vaccination symptoms of two common COVID‐19 vaccines used in Abha, Aseer Region, Kingdom of Saudi Arabia. Patient Prefer Adherence. 2021;15:1963–1970. doi:10.2147/PPA.S330689
44. Kałucka S, Kusideł E, Głowacka A, Oczoś P, Grzegorczyk-Karolak I. Pre-vaccination stress, post-vaccination adverse reactions, and attitudes towards vaccination after receiving the COVID-19 vaccine among health care workers. Vaccines. 2022;10(3):401. doi:10.3390/vaccines10030401
45. Dionne A, Sperotto F, Chamberlain S, et al. Association of myocarditis with BNT162b2 messenger RNA COVID-19 vaccine in a case series of children. JAMA Cardiol. 2021;6(12):1446–1450. doi:10.1001/jamacardio.2021.3471
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
