Back to Journals » International Medical Case Reports Journal » Volume 17
Evaluation of Oral Health-Related Quality of Life in Patient with Herpes-Associated Erythema Multiforme: A Unique Case Report
Received 29 December 2023
Accepted for publication 21 March 2024
Published 29 March 2024 Volume 2024:17 Pages 253—259
DOI https://doi.org/10.2147/IMCRJ.S456301
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Xudong Zhu
Fitrah Utari Bakti,1,* Tenny Setiani Dewi2,*
1Oral Medicine Residency, Faculty of Dentistry, Universitas Padjadjaran, Bandung, Indonesia; 2Oral Medicine Department, Faculty of Dentistry, Universitas Padjadjaran, Bandung, Indonesia
*These authors contributed equally to this work
Correspondence: Fitrah Utari Bakti, Oral Medicine Residency, Faculty of Dentistry, Universitas Padjadjaran, Jl. Sekeloa Selatan No. 1, Bandung, West Java, 40132, Indonesia, Email [email protected]
Introduction: Erythema multiforme (EM) is an acute mucocutaneous hypersensitivity reaction with various etiological factors, including herpes simplex virus type 1 (HSV-1) infection, known as herpes-associated erythema multiforme (HAEM). Oral health-related quality of life (OHRQoL) is a multidimensional concept of biopsychosocial aspects related to oral health. OHRQol contains information for patient-centered treatment plan development. The OHRQoL measurement instrument widely used by clinicians is the oral health impact profile-14 (OHIP-14). This case report aimed to evaluate OHRQoL in HAEM patient, which only manifests on the lips and oral cavity.
Case: A 25-year-old male patient came to the Department of Oral Medicine with the chief complaint of painful canker sores on the lips. Extra-oral examination revealed serosanguineous crusts on the lips that were painful and easily bleed. Intra-oral examination showed diffused and painful irregular erythematous lesions on the upper and lower labial mucosa. The anti-HSV1 IgG test was positive. The patient was diagnosed with HAEM.
Case Management: Pharmacological therapy included triamcinolone acetonide 0.1% in orabase, acyclovir tablets, multivitamins, and 0.9% NaCl. Non-pharmacological therapy included advice on maintaining good oral hygiene, avoiding spicy and sour foods, and breaking the bad habit of licking the lips.
Conclusion: The patient’s physical, psychological, and social conditions showed improvement and returned to normal after 7 days of treatment. In conclusion, oral health is an important factor that can improve the quality of life of HAEM patient.
Keywords: herpes-associated erythema multiforme, erythema multiforme, herpes simplex virus type 1, oral health-related quality of life, oral health impact profile-14
Introduction
Ferdinand von Hebra reported Erythema multiforme (EM) for the first time in 1866.1–3 EM is a self-limited acute mucocutaneous hypersensitivity reaction. The characteristics of EM are skin eruptions, with or without oral or other mucous membrane lesions.1,3–6 EM typically develops between the ages of 20 and 40, with a male-to-female predilection ratio of 3:2.1,5 In general, EM is classified as EM minor (involvement of ≤1 mucosal site) and EM major (involvement of ≥2 mucosal sites).1,5,6 Oral EM is a third type of EM that solely affects the oral mucosa and has no skin involvement.1,5 This case was reported less frequently and did not have a target lesion like the other two variants. EM rarely affects the oral cavity alone, making it a rare entity.1
EM can be induced by drugs or microorganism infection, especially HSV-1 infection which has been identified in 70% of cases of EM, called herpes-associated erythema multiforme (HAEM).1,3,4,7,8 HAEM develops after previous episodes of clinical or subclinical HSV.9 This condition may be preceded by prodromal symptoms, such as fever, malaise, headache, sore throat, rhinorrhea, and cough. The most frequently affected sites were the lips (36%), buccal mucosa (31%), tongue (22%), and labial mucosa (19%). Intraoral findings range from mild erythema and erosions to large painful ulcerations. These conditions can cause patients to have difficulty eating, drinking, and swallowing. The diagnosis is established based on history, clinical findings, and the history of HSV infection. A positive IgG level proves that HSV is the etiological agent. Psychological stress also increases HSV-1 titer. Psychological condition examination can be evaluated using the Depression Anxiety Stress Scale-21 (DASS-21) questionnaire. EM is considered a separate condition from Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN).6,10 Topical steroids can be used to treat the lesions. Cases related to HSV must be accompanied by treatment with antivirals, and prophylaxis to prevent recurrence.5
Oral health-related quality of life (OHRQoL) is a multidimensional concept involving biopsychosocial aspects of dental and oral health. This assessment can identify the patient’s perception of dental and oral health and its impact on the patient’s life.11–15 The Oral Health Impact Profile (OHIP) is the most widely used instrument by researchers and clinicians to assess OHRQoL. OHIP was originally developed by Slade and Spencer in Australia, containing 49 items (OHIP-49). The items are distributed taking into account the seven dimensions (functional limitations, physical pain, psychological discomfort, physical disability, psychological disability, social limitations, and inhibitions) which are elaborated from the theoretical model by Locker. A shortened version of this instrument was developed, known as the Oral Health Impact Profile-14 (OHIP-14). The OHRQoL contains useful information to create the right treatment plan for the patient.12,13 This case report aims to evaluate OHRQoL in HAEM patients who have manifestations only on the lips and oral cavity.
Case Presentation
Male patient, 25 years old, Sundanese, presented at the Dental Hospital of the Faculty of Dentistry Universitas Padjadjaran with the chief complaint of mouth sores, which are painful on the upper and lower lips and exacerbated when eating and talking. Initially, four days ago, canker sores started in the oral cavity, then appeared on the lips two days later. The patient tried to self-medicate by applying petroleum jelly which he used to relieve his symptoms, but it did not improve. The patient replaced the drug with triamcinolone acetonide 0.1% in orabase ointment purchased at the pharmacy and applied it once a day. Canker sores were getting better but did not cure.
The patient had history a of fever for about a week before the canker sores appeared and there were no lesions on other parts of the body. He stated that the workload was quite heavy and he had not consumed a balanced nutritional diet for about one and a half months. He had no medical history, history of food allergies, or history of taking medication. He had no history of alcohol consumption or smoking, but he had a frequent habit of licking his lips. He also had a history of chickenpox when he was a child.
The patient had no fever with all vital signs within normal limits on general examination. Extra-oral examination showed no abnormalities in the lymph nodes. There were serosanguineous crusts that felt painful and bleed easily on the lips. Intra-oral examination revealed erythematous lesions, irregular in shape, and had diffuse borders, accompanied by pain in the upper and lower labial mucosa. Hyperkeratotic white plaque that could not be scraped off, irregular in shape, has diffuse borders, without pain in the region of tooth 38 left buccal mucosa. Yellowish-white plaques were seen on 1/3 of the posterior surface of the dorsal tongue, which could be scraped off without leaving an erythematous area, and there were indentations in the form of dental impressions without pain on the lateral right and left sides of the tongue. A painless hard nodule about 2×1 x 0.5 cm in size was seen in the midline of the hard palate. Several teeth were found in caries, radix, and edentulous conditions in all regions. The oral hygiene was poor (Figure 1).
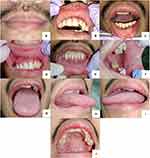 |
Figure 1 Clinical features of the patient’s lips and oral cavity at the initial visit (A–J). |
Examination of psychological conditions was evaluated using the DASS-21 questionnaire and showed normal depression level (score 0), normal anxiety level (score 6), and normal stress level (score 6). Based on history and clinical examination, the working diagnosis was suspected HAEM, accompanied by the coated tongue, frictional keratosis, crenated tongue, torus palatinus, reversible pulpitis of tooth 18, irreversible pulpitis of tooth 47, chronic apical periodontitis et causa radix of tooth 15, and edentulous teeth 28, 37, 36, and 46. The differential diagnosis of suspected HAEM lesions on the lips was exfoliative cheilitis. However, exfoliative cheilitis did not have herpes virus involvement. The patient was indicated for serological testing (IgG anti-HSV-1) to confirm the diagnosis. Oral health-related quality of life was measured, and the results of the OHIP-14 examination at the first visit were 35 (moderate OHRQol) (Table 1).
 |
Table 1 Evaluation of OHRQol Based on OHIP-14 |
The non-pharmacological therapy included instruction to maintain oral hygiene by brushing the teeth and tongue using a soft-bristled toothbrush two times a day and using non-detergent toothpaste. Education was given such as increasing the intake of water by at least two liters per day, consuming a balanced nutritional diet, avoiding acidic, spicy, hard, and monosodium glutamate-containing foods, and stopping the bad habit of licking and peeling the skin of the lips. The pharmacological therapy included topical and systemic medications. The topical medications included instructions to compress the lips with gauze moistened with 0.9% NaCl solution at least three times a day and to apply a thin layer of triamcinolone acetonide 0.1% in orabase to the lips three times a day. The systemic medications included instruction to take a multivitamin once a day.
The progress of improvement was visible in the first follow-up, two days after the initial visit. The pain in the lips was reduced, but the canker sores have not healed. Extra-oral examination revealed serosanguinous crusts on the lips which were still painful and bled easily. The serological test result (IgG anti-HSV-1) was positive with a ratio of: 6.32 (positive: ratio > 1.1). The definitive diagnosis was established based on the history, clinical examination, and serological tests as HAEM. The non-pharmacological and pharmacological therapy was continued, and systemic medication was added in the form of instructions to consume acyclovir 200 mg tablets five times a day for one week.
Significant improvement was visible in the second follow-up, five days after the previous visit, showing excellent healing in all of the patient’s oral lesions (Figure 2). The OHIP-14 result at the last visit was 4 (good OHRQoL) (Table 1). The patient’s physical, psychological, and social conditions showed improvement and returned to normal after 7 days of treatment. Patient was referred to continue dental and oral care in the periodontics, dental conservation, oral surgery, and prosthodontics departments. The patient has approved and written informed consent for the case details to be published included publication of the images, and the institution has also approved for publication. This case had complied with the Declaration of Helsinki.
 |
Figure 2 Clinical features of the patient’s lips and oral cavity in the last follow-up (A–J). |
Discussion
HAEM is considered an immune complex disease that occurs 7–10 days after herpes simplex infection. The prodromal symptoms experienced by this patient a week before the lesions appeared confirmed the presence of a viral infection. The pathogenesis mechanism of HAEM is not precisely understood. The causative agent is thought to induce a T-cell-mediated immune reaction, resulting in a cytotoxic immunological attack on non-self-antigen-expressing keratinocytes, causing local inflammation such as vesicles, erosions, blisters, or ulceration of the tissue.1,5
Inflammation of the oral cavity with lesions resembling typical EM lesions was first described by Kenneth in 1968.1 The EM lesions in this patient appeared on the lips and labial mucosa only. Several studies have shown that 40% of EM patients have lesions limited to the oral and lip mucosa, without skin involvement. Farthing et al stated that EM due to herpes accounts for 20–50% of cases.1,8 Investigations to establish the diagnosis of herpes infection are the Tzanck smear, serology, viral culture, and polymerase chain reaction (PCR). This patient underwent an anti-HSV-1 IgG serological examination using the ELISA technique, and the result was positive with a ratio of: 6.32 (positive: ratio > 1.1). A result that shows a value fourfold the normal value indicates an ongoing infection or a current infection. Antibodies appear 4–7 days after infection and reach a peak in 2–4 weeks. Anti-HSV IgG antibody titers usually rise 1–2 weeks after primary infection, peaking 6–8 weeks after infection.10 Biopsy of HAEM lesions is not necessary if the clinical picture is clear because the histopathological findings are not pathognomonic. Histopathologic features may include intercellular and intracellular edema of the epithelial layer, exocytosis, focal microvesicle formation, vasodilation, diffuse inflammatory infiltration, and connective tissue edema. Direct immunofluorescence will differentiate between EM and bullous autoimmune diseases, such as bullous pemphigoid.16
There is no specific treatment for EM. The choice of treatment depends on the severity of the clinical condition and the underlying or coexisting cause.3,4,6 Treatment of mild cases of EM lesions can be done locally with topical steroids, analgesics, or antiseptic creams.3 In cases of HAEM, where the specific cause is the herpes virus, the first line of treatment is antiviral therapy. Antiviral therapy can cause herpes lesions and EM lesions to go into remission.3 Early administration of oral acyclovir can reduce the severity and duration of EM eruptions.17 Marzano et al reported that the use of acyclovir has been shown to reduce the signs and symptoms of HSV oral lesions.8 Acyclovir is given five times daily as a treatment of the causative agent. Triamcinolone acetonide 0.1% in orabase is used as symptomatic treatment. Supportive treatment includes giving multivitamins, consuming a balanced nutritional diet, drinking enough water, and stopping the bad habit of licking and exfoliating the skin of the lips. HAEM resolved without sequelae within two weeks.4
The results of the OHRQoL assessment provide important information to the clinician regarding the patient’s perspective on the impact of dental and oral conditions on the patient’s life. This information can be used by the clinician to move towards patient demands and expectations, and to place the patient in a central role in the treatment plan.12 OHIP-14 consists of 14 points that assess seven dimensions. Patients respond at each point according to the frequency they experience with a score range of 0–4 (0 = never, 1 = very rarely, 2 = sometimes, 3 = often, and 4 = very often). The total OHIP-14 score is calculated by adding up all the scores from each point. A higher OHIP-14 score indicates a worse OHRQoL (OHIP-14 score 0–18 = good OHRQol, OHIP-14 score 19–37 = moderate OHRQol, and OHIP-14 score 38–56 = poor OHRQol). This instrument is more specific for assessing the impact on oral conditions in everyday life.12,13
The OHRQoL assessment in this patient showed better results at the last follow-up compared to the first visit with the OHIP-14 score at the first visit being 35 (moderate OHRQol), and the last follow-up was 4 (good OHRQoL) (Table 1). Patient experienced improvements in all dimensions, namely improvements in the dimensions of functional limitations, physical pain, psychological discomfort, physical disability, psychological disability, social limitations, and inhibitions. Improvements in quality of life have not yet achieved maximum results, namely in the assessment of psychological discomfort and disability, even though HAEM lesions have been declared cured, the patient feels that he still needs treatment to improve the condition of his teeth and other mouths. Multidisciplinary and comprehensive care is expected to achieve a better patient quality of life. The limitation of this case report is that a biopsy of the lesion and statistical analysis were not carried out to complete this case report. However, the patient was satisfied with the treatment after the treatment was completed. We recommend future prospective studies regarding the assessment of OHRQoL using the OHIP-14 in EM patients in a large sample size from various medical centers. This is expected to produce data that can be analyzed statistically and become evidence-based for developing dental and oral health clinical service policies in the future.12,13,15
Conclusion
EM with oral involvement is rare, it may pose a diagnostic challenge. Therefore, it is crucial to recognize ulcerative lesions involving the oral cavity to ensure an accurate diagnosis, early treatment, and follow-up. Clinicians should be vigilant and consider EM as a potential diagnosis in such cases. Good oral health is an important factor that can improve the quality of life of HAEM patients through comprehensive multidisciplinary care and good communication between dentists and patients.
Acknowledgment
The authors would like to express our sincere gratitude to the patient for granting his consent and to all those who kindly assisted us at the Universitas Padjadjaran Dental Hospital. The authors also gratefully acknowledge funding support from the directorate of research and community service at Universitas Padjadjaran.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Buch SA, Babu SG, Castelino RL, Rao S, Rao K, Pillai DS. A rare case of oral erythema multiforme: a case report with a literature review. J Dent Indon. 2017;24(2). doi:10.14693/jdi.v24i2.1065
2. Hao M, Zang P, Miller M, Cutler L, Worswick S. Herpes associated erythema multiforme: a retrospective study. Am J Emergency Med. 2020;38(12):
3. Magri F, Chello C, Pranteda G, Pranteda G. Erythema Multiforme: Differences Between HSV-1 and HSV-2 and Management of the disease—A Case Report and Mini Review. In: Dermatologic Therapy. Blackwell Publishing Inc; 2019.
4. Shahriar R, Khan MM, Uddin KN, Islam MN; Informations Md Raziur Rahman A, Professor A. Herpes associated erythema multiforme: a case report. BIRDEM Med J. 2017;7:72–75. doi:10.3329/birdem.v7i1.31277
5. Glick M, Greenberg MS, Lockhart PB, Challacombe SJ. Burket’s Oral Medicine.
6. Lerch M, Mainetti C, Terziroli Beretta-Piccoli B, Harr T. Current Perspectives on Erythema Multiforme. In: Clinical Reviews in Allergy and Immunology. Vol. 54. Humana Press Inc; 2018:177–184.
7. Thongprasom K. Liječenje trudnice od erythema multiforme povezanog s herpesom (HAEM): prikaz slučaja. Acta Stomatol Croat. 2016;50(3):265–268. doi:10.15644/asc50/3/10
8. AlFar MY, AlRousan M, Almajali Z, et al. The use of corticosteroids in management of Herpes associated Erythema Multiforme. J Pak Med Assoc. 2015;65(12):1351–1353.
9. Gu Y, Sun J, Zhang J. HSV-associated erythema multiforme in a patient after hematopoietic stem cell transplantation. Dermatol Ther. 2019;32(5). doi:10.1111/dth.13066
10. Hasanah NT, Hidayat W. Stress as trigger factor of HSV-1 reactivation causing recurrent intraoral herpes mimicking HAEM: a case report. Int Med Case Rep J. 2022;15:699–706. doi:10.2147/IMCRJ.S388708
11. Karimi M, Health BJ. Health-related quality of life, and quality of life: what is the difference? Pharmacoeconomics. 2016;34(7):645–649. doi:10.1007/s40273-016-0389-9
12. Campos LA, Peltomäki T, Marôco J, Campos JA. Use of oral health impact profile-14 (OHIP-14) in different contexts. what is being measured? Int J Environ Res Public Health. 2021;18(24):13412. doi:10.3390/ijerph182413412
13. Ratnawidya W, Rahmayanti F, Irmagita Soegiyant A, Mandasari M. Indonesian short version of the oral health impact profile (OHIP-14). J Int Dent Med Res. 2018;11:1065–1071.
14. Crimi S, Fiorillo L, Bianchi A, et al. Herpes Virus, Oral Clinical Signs and Qol: Systematic Review of Recent Data. In: Viruses. MDPI AG; 2019.
15. Parsaei A, Mehdipour A, Ghadimi H, et al. Oral health-related quality of life in rheumatoid arthritis: a comparative analysis. BMC Rheumatol. 2022;6(1). doi:10.1186/s41927-022-00292-w
16. Isaias PHC, Bezerra TMM, Chaves FN, Alves da Silva RAD, Mota MRL, Pereira KMA. Management of pediatric erythema multiforme restricted to the mouth and perioral region: a case report. Rev Portug De Estomatol. 2023;64(3):128–132.
17. Trayes Kathryn P, Love G, Studdiford JS. Erythema multiforme: recognition and management. Am Fam Physician. 2019;100(2):82–88.
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
