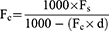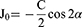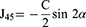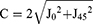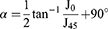Back to Journals » Clinical Ophthalmology » Volume 16
Evaluation of Ocular Residual Astigmatism in Eyes with Myopia and Myopic Astigmatism and Its Interaction with Other Forms of Astigmatism
Authors Elshahat A, Hamed AM, El Habbak AH, Tabl MA
Received 19 October 2022
Accepted for publication 29 November 2022
Published 15 December 2022 Volume 2022:16 Pages 4179—4190
DOI https://doi.org/10.2147/OPTH.S393477
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Ahmed Elshahat, Abdelmonem M Hamed, Ashraf H El Habbak, Marwa Abdelshafy Tabl
Department of Ophthalmology, Benha Faculty of Medicine, Benha University, Benha, Qalyopia, Egypt
Correspondence: Abdelmonem M Hamed, Email [email protected]
Purpose: To evaluate the prevalence, magnitude, and direction of ocular residual astigmatism (ORA) in eyes with myopia and myopic astigmatism, and its interaction with refractive, anterior corneal, posterior corneal, and true net power astigmatism.
Patients and Methods: Refractive surgery candidates with myopia and myopic astigmatism were studied. Refractive astigmatism (RA) was measured using the Nidek® AR-310A autorefractometer. Anterior corneal astigmatism (ACA), posterior corneal astigmatism (PCA), and true net power astigmatism (TNP) were measured using the Wavelight® Oculyzer II. Astigmatism was converted from polar to vector notation. ORA was calculated by vector subtraction of ACA from RA vertexed to corneal plane. Compensation factor (CF) was calculated as the ratio of ORA that compensates ACA for both J0 and J45.
Results: 154 eyes of 88 patients (mean age 31.7± 7.1 years) were included. With-the-rule (WTR) astigmatism was the most common for both RA (55.6%) and ACA (74%), while against-the-rule (ATR) was the most common for PCA (87.7%) and ORA (74.0%). The axes of RA and ACA were within 10° of each other in 46.8% of the eyes, and within 30° of each other in 76.0%. The mean difference in value between the axis of RA and ACA was 25.6°. 71.4% of eyes in the study had an ORA ≥ 0.5D, 44.1% had ORA ≥ 0.75D and 26% had ORA ≥ 1D. There was a statistically significant difference between ACA and each of RA and TNP. Using TNP to calculate ORA instead of ACA reduced its magnitude. RA is positively correlated to ACA and more strongly to TNP. The most common pattern of compensation between ORA and ACA was under-compensation for J0 (49%) and same-axis-augmentation for J45 (35%).
Conclusion: ORA, PCA, and the interaction between ORA and ACA can affect results during refractive planning.
Keywords: refractive astigmatism, anterior corneal astigmatism, posterior corneal astigmatism, refractive surgery
Introduction
Astigmatism is a common form of refractive error.1–3 Manifest astigmatism, measured by subjective refraction, or objectively by retinoscopy or autorefractometry, is representative of the astigmatism exhibited by the entire eye and visual pathway as a complete optical and perceptual system. It can be broken down into individual components arising from different parts of the visual system, including the cornea, the crystalline lens, the retina, as well as the visual cortex.4–6 The anterior cornea has historically been considered the primary source of ocular astigmatism since the difference of refractive index between air and is the greatest in the optical system of the eye, but the presence of intraocular astigmatism, generated by both posterior corneal astigmatism and crystalline lens, has been known since the end of the 19th century, when Javal coined his famous law. Tscherning in the early 20th century indeed managed to design a device to perform measurements of posterior corneal astigmatism, and published the results in the eyes of three patients. However, the technical difficulty involved in determining posterior corneal astigmatism left this topic virtually untouched until about a decade ago, when it was revived by Koch, with respect to intraocular lens toricity calculation. However, even today with technologies with slit-light source with the Scheimpflug principle, or optical coherence tomography, the difficulty of its precise determination persists. That is the reason why the most frequently measured components are only manifest refractive astigmatism and anterior corneal topographic astigmatism, measured by keratometry or corneal topography. However, due to the influence of intraocular astigmatism, there is frequently a difference in magnitude and axis between refractive and topographic astigmatism. The difference between both has been termed Ocular Residual Astigmatism ORA,7 a term used to describe the astigmatism of the eye arising from sources other than the anterior corneal surface, as calculated by vectoral subtraction of corneal topographic astigmatism from total manifest astigmatism.8–11
The role of the posterior cornea as a refractive surface of the eye for many years was considered not clinically significant, due to its low magnitude, related to the small difference in refractive index between the cornea (n = 1.376) and the aqueous humor (n = 1.336). 12 Additionally, the anterior and posterior corneal surfaces were considered almost parallel in shape. All keratometers (manual, automated), and Placido-disc-based topographers, measure only the anterior curvature and use a fixed curvature ratio between the anterior and posterior corneal surfaces to calculate the total corneal curvature and power.10 Consequently, it has been wrongly assumed that ORA comes only from the astigmatism induced by the crystalline lens. If this were the case, ORA should disappear after cataract extraction. On the contrary, a study by Sano et al found that not only is ORA still observed in pseudophakic eyes, but the magnitude of ORA in pseudophakic eyes is significantly smaller when subtracting total corneal astigmatism from refractive astigmatism, thus accounting for both anterior and posterior corneal surfaces, as compared to using only anterior corneal astigmatism.13 This highlights the role of the posterior corneal surface as an effective ocular refractive surface. In addition, it has been shown that the cornea does not exhibit the same thickness along the horizontal and vertical meridians, but it is thicker in the vertical than the horizontal periphery, and it is thicker superiorly than inferiorly, meaning that anterior and posterior surfaces of the cornea do not always have a parallel relationship that is constant in all meridians.12,14 Similar regional disparities could be found in the posterior corneal elevation data measured by the Pentacam®.15 Koch et al found that in eyes with posterior corneal astigmatism increase with the increase in anterior corneal WTR astigmatism, reaching values as high as 1 D. However, it stays relatively constant with increasing anterior corneal ATR astigmatism.13 This means that the relationship between anterior and posterior corneal astigmatism can no longer be regarded to be constant. Based on these arguments, relying only on anterior corneal astigmatism while completely neglecting posterior corneal astigmatism, or assuming a constant relationship between the anterior and posterior corneal surfaces could result in the miscalculation of total corneal astigmatism when implanting toric intraocular lenses.4,10–12,16–18
In this study, we aimed to evaluate the prevalence, magnitude, and direction of ocular residual astigmatism in eyes with myopia and myopic astigmatism. We studied the relationship and interaction between ORA and other forms of astigmatism, including RA, ACA, PCA, and TNP astigmatism, to better understand the discrepancy between refractive and topographic astigmatism and help guide the clinician to make informed decisions on the magnitude and axis of astigmatism in different situations.
Subjects and Methods
This is a single-center observational cross-sectional study, conducted on refractive surgery candidates with myopia and myopic astigmatism. The study adhered to the tenets of the Declaration of Helsinki and was approved by the research ethics committee of Benha Faculty of Medicine, Benha University, Egypt.
The study included 154 eyes of 88 subjects, of which 41% were males (n = 36) and 59% were females (n = 52). Subjects had a mean age of 31.72±7.19 years, with an age range of 20–57 years. All subjects underwent a full ophthalmological examination including unaided distance visual acuity, autorefractometry, best-corrected distance visual acuity, corneal tomography, slit lamp biomicroscopy, and fundoscopy. The exclusion criteria were patients with a history or signs of preexisting corneal disease, eg, corneal dystrophies, herpetic eye disease, corneal scarring, or severe dry-eye syndrome, as well as patients with corneal contact-lens warpage, as evidenced by history of contact lens wear within 14 days before examination. Also, patients who underwent previous refractive or intraocular surgery or patients with any other ocular pathologies were excluded from this study, as well as patients unwilling to give informed consent.
Autorefractometry was done using the Nidek® AR-310A autorefractometer (Nidek, Aichi, Japan). The printout produced shows the multiple measurements taken by the machine for objective refraction, described as sphere, cylinder, and axis, as well as the confidence index of each measurement, and the median measurement highlighted in-between brackets. Corneal tomography was done using the Wavelight® Oculyzer II (Alcon, Geneva, Switzerland). After a measurement has been taken, the operator checks the Quality Specification (QS) field on the screen, which should display “OK”. If the field is marked red or yellow indicating a possible error, the measurement is repeated. The topometric display shows keratometer values for Cornea Front and Cornea Back, calculated by analyzing the front and back surfaces of the cornea on the 3 mm ring and determining the 2 principal meridians (flat meridian = K1 and steep meridian = K2). The dioptric power of K1 and K2 is then subtracted from each other to give the magnitude of astigmatism and its axis. Total corneal dioptric power and astigmatism values are also calculated and displayed in the corresponding fields of the True Net Power section. Firstly, the difference between the refractive index of air (n = 1) and the true refractive index of the corneal tissue (n = 1.376) is used to calculate the refractive power of the anterior corneal surface. Secondly, the difference between the refractive index of the corneal tissue (n = 1.376) and that of the aqueous humor (n = 1.336) is used to calculate the refractive power of the posterior corneal surface. The system then adds the refractive power of the back surface of the cornea to that of the front surface of the cornea to give the true net power astigmatism (TNP) map and calculate the TNP keratometry values accordingly.
In order to apply statistical analysis to various forms of astigmatism in this study, the following workflow was adopted:
Step 1: Data Input
A Microsoft Excel spreadsheet was constructed for data collection. The first set of input data included demographic details, ie, age and gender. Next, the median autorefraction result for each eye was inputted from the Nidek® AR-310A autorefractometer printout slip into the Excel sheet. The cylinder and axis from this printout constituted refractive astigmatism (RA) in polar form. Anterior corneal astigmatism (ACA), posterior corneal astigmatism (PCA), and true net (TNP) are then inputted from the corresponding fields on the Wavelight® Oculyzer II topometric display into the spreadsheet, in polar form. In this study, polar representations of astigmatism were always expressed in the plus cylinder notation. We described astigmatism as with-the-rule (WTR) if its axis of in plus cylinder was at 90° ± 30°. Conversely, astigmatism was described as against-the-rule (ATR) if its axis in plus cylinder at 0°/180° ± 30°, and oblique if otherwise.
Step 2: Transformation of RA to the Corneal Plane
In order to be able to compare RA to other forms of corneal astigmatism, and to accurately calculate ORA, RA needed to be vertexed from the spectacle plane to the corneal plane, to eliminate the effect of back vertex distance. This was done by converting autorefraction values at the spectacle plane into the two principal lens powers in a power cross format, which were then vertexed to the corneal plane according to the following equation, where Fc is the dioptric lens power at the corneal plane, Fs is the dioptric lens power at the spectacle plane and d is the back vertex distance.19,20
The back vertex distance d was set to 12 mm, which matches that from the autorefractometer settings. The calculation was carried out in Microsoft Excel using the following function, applied to each of the principal lens powers in the power cross. The difference between the resulting 2 principal lens powers then represents RA at the corneal plane.
Fc=(1000*Fs)/(1000-(Fs*12))
Step 3: Conversion of Astigmatism from Polar to Vector Form
Different forms of astigmatism measured for each eye (RA, ACA, PCA, TNP) are converted from their polar notation of cylinder and axis to the corresponding vector notation of J0 and J45 using the following equations by Thibos and Horner21 and corresponding Microsoft Excel functions as demonstrated by Miller22 where J0 is the component of astigmatism that can be represented by the power of a Jackson’s cross cylinder at 0°/180°, J45 is the component of astigmatism that can be represented by the power of a Jackson’s cross cylinder at 45°, C is the polar cylinder of astigmatism, and α is the polar axis of astigmatism.
J0 = -(C+0.00001)/2*COS(2*α*PI()/180)
J45 = -(C+0.00001)/2*SIN(2*α*PI()/180)
As Miller explains, these equations include conversion from degrees to radians, as this is the form accepted by Microsoft Excel for transcendental functions, as well as a very small number to negate the need to test for 0 or special handling.
Step 4: Calculation of ORA in Vector Notation
ORA is calculated for each eye in vector notation by subtracting the corresponding J0 and J45 of ACA from RA. In order to evaluate whether accounting for the posterior cornea affects ORA, we also calculated ORA by subtracting TNP from RA.
Step 5: Conversion of ORA from Vector to Clinical Form
ORA is then converted from vector notation to polar notation using the following Microsoft Excel function22 based on the equations by Thibos and Horner.21
C = 2* SQRT(J0^2+J45^2)
α= 0.5*(ATAN2(J0, J45)*180/PI())+90
Step 7: Statistical Analysis
Data analysis was performed using the software SPSS (Statistical Package for the Social Sciences) version 26. Statistical tests were then performed including the Kolmogorov–Smirnov test of normality, the Wilcoxon signed-rank test to compare non-parametric paired sample data, and the Spearman’s rank correlation coefficient for the direction and strength of correlation between non-normally distributed variables. Statistical significance in this study is defined as a p-value <0.05, and was considered highly statistically significant if P ≤ 0.001.
Results
The Kolmogorov–Smirnov test was used to evaluate the normality of the various datasets, which revealed that the spherical equivalent and magnitudes of RA, ACA, PCA, and ORA are all not normally distributed. This falls in agreement with other studies in which the normality of similar data was tested.23–25 As a result, the dataset’s central tendency and spread were expressed in terms of medians and interquartile ranges IQR, and Spearman’s rank correlation coefficient was used to test for correlation between different variables. RA ranged from 0 to −6.5 D with a median of −1 D (IQR: 1.25 D). ACA ranged from 0 to −4.8 D with a median of −1.1 D (IQR: 0.8). PCA ranged from 0 to 1 D with a median of 0.3 D (IQR: 0.3). TNP ranged from −0.1 to −4.3 D with a median of −1.58 (IQR: 0.88) while ORA ranged from 0.1 to 4.1 D with a median of 0.47 D (IQR: 0.56).
On correlating age with different forms of astigmatism, there was no statistically significant correlation between age and any of RA (r=0.017, p = 0.4), ACA (r=−0.16, p = 0.05), PCA (r=−0.16, p = 0.05) or ORA (r = 0.13, p = 0.1) In terms of gender, there is a statistically significant difference between males and females only in terms of RA (p = 0.008) but not ACA (p = 0.17), PCA (p = 0.3), or ORA (p = 0.02). Interestingly, others have also found differences between both genders in terms of astigmatism.26,27
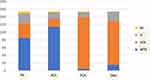
We then looked at the direction of various forms of astigmatism. WTR was the most common form of RA at 55.55% (n = 85), followed by ATR at 24.18% (n = 37) then oblique astigmatism at 16.99% (n = 26), while 3.27% (n = 5) of the eyes showed no refractive astigmatism. As for ACA, WTR was commonest at 74% (n = 114), followed by ATR at 13% (n = 20), oblique at 11.69% (n = 18) and no ACA at 1.3% (n = 2). When it comes to PCA, an opposite pattern is observed where 87.66% of the eyes had an ATR PCA (n = 135), 8.44% oblique astigmatism (n = 13), while only 2.6% of the eyes showed a WTR astigmatism (n = 4) and 1.3% showed no PCA (n = 2). (Figure 1) In terms of the agreement between the directions of RA and ACA, their axes were within 10° of each other in 46.75% of the eyes (n = 72), and within 30° of each other in 75.97% (n = 117), while 20.13% of eyes (n = 31) had axes that were more than 30° away from each other. The mean difference in value between the axis of RA and ACA was 25.64°. As for the axis of ORA, 74.03% of the eyes showed an ATR ORA (n = 114), 9.74% showed WTR ORA (n = 15) and 16.23% had oblique ORA (n = 25). Using the Chi-square test, we detected a statistically significant difference in the frequency distribution of various forms of astigmatism between RA, ACA, PCA, and ORA (p < 0.00001). In terms of magnitude, 71.43% of eyes in the study had an ORA ≥ 0.5D (n = 110), 44.16% had ORA ≥ 0.75D (n = 68) and 26% had ORA ≥ 1D (n = 40).
Using the Wilcoxon signed rank test, we identified a statistically significant difference between RA and ACA in terms of magnitude (p = 0.0008) and J0 (p < 0.00001) but not J45 (P = 0.2). ACA is also different from TNP in terms of magnitude (p < 0.00001) and J0 (p < 0.0001) but not J45 (p = 0.9). In addition, ORA calculated as RA-ACA is statistically different from ORA calculated as RA-TNP in terms of magnitude (p < 0.00001) and J0 (p < 0.00001) but not J45 (p = 0.7). Calculating ORA as RA-TNP instead of RA-ACA resulted in an average reduction in ORA magnitude by 0.69D.
There was a statistically significant positive correlation between RA and ACA using Spearman’s rank correlation coefficient in terms of magnitude (r = 0.556, p < 0.001), J0 (r=0.8, <0.001), and J45 (r=0.72, <0.001) (Figure 2). There was also a statistically significant correlation between RA and TNP in terms of magnitude (r = 0.67, p < 0.001), J0 (r = 0.83, p < 0.001), and J45 (r = 0.79, p < 0.001) (Figure 2), which is slightly stronger than the correlation between RA and ACA.
Spearman’s rank correlation coefficient was also used to evaluate the correlation between ORA and various components of astigmatism, including RA, ACA, TNP, and PCA (Table 1). There was a statistically significant positive correlation between ORA and RA for J0 (r = 0.52, p < 0.001) and J45 (r = 0.34, p < 0.001) as well as with magnitude of TNP (r = 0.21, p = 0.01) and J45 of PCA (r = 0.17, p = 0.04). The rest of the correlations are weak and not statistically significant. There is no statistically significant correlation between spherical equivalent and ORA (r = 0.5, p = 0.5).
 |
Table 1 Correlation Between Ocular Residual Astigmatism and Each of Refractive Astigmatism, Anterior Corneal Astigmatism, True Net Power Astigmatism and Posterior Corneal Astigmatism |
To evaluate the influence of ORA on ACA, we adopted the variable termed Compensation Factor CF, as described by Park et al, calculated for both the J0 and J45 components and defined as the ratio of ORA that compensates ACA.28
CF=(ACA-RA)/ACA = (-ORA)/ACA.
According to the value of the resultant CF, various compensation patterns between ORA and ACA can be described. The percentage of eyes belonging to each category was calculated (Table 2).
Same Axis Augmentation SAA: CF < −0.1. ORA increased the amount of RA to values greater than ACA, while maintaining the same axis as ACA.
No Compensation NC: CF −0.1–0.1. ORA has no influence on RA.
Under-Compensation UC: CF 0.1–0.9. ORA decreased the amount of RA to values less than ACA, while maintaining the same axis as ACA.
Full Compensation FC: CF 0.9–1.1. ORA fully compensates for ACA, abolishing RA.
Over-Compensation OC: CF 1.1–2. RA decreased to values less than ACA, and the axis was changed to the opposite angle.
Opposite Axis Augmentation OAA: CF > 2. RA increased to values greater than ACA, and the axis was changed to the opposite angle.
To visualise these data, scatter plots were constructed with ORA on the x axis and ACA on the y axis, both in terms of J0 and J45, on which eyes were colour-coded according to the type of compensation they exhibit (Figure 3).
Discussion
Refractive surgeons are often faced with a discrepancy between a subject’s manifest refractive astigmatism and their corneal topographic astigmatism, making accurate operative refractive planning a complex matter. This discrepancy can be represented as the Ocular Residual Astigmatism ORA. This form of astigmatism bears clinical significance in the context of refractive correction. It has been shown that eyes with low ORA have an astigmatic correction success that is twice as high as those with high ORA, with LASEK, LASIK, and SMILE.28–30 It has been argued in those studies that this could be due to a mismatch between the predominant origin of manifest astigmatism and the location of treatment in eyes with high ORA, where most of manifest astigmatism originates from non-corneal sources with a complex interplay with topographic astigmatism both in magnitude and axis. In those eyes, fully correcting refractive astigmatism on a corneal level would potentially result in the creation of astigmatism on the anterior corneal surface to compensate for the high ORA. It was thought that this will impact postoperative visual quality by adversely affecting corneal sphericity and inducing a postoperative ATR astigmatism. This conceptualization suggests that it is not only the magnitude and direction of astigmatism that impact refractive correction, but also its origin, and it has been suggested to adjust manifest refraction values with topographic data to enhance surgical correction.31 ORA must also be taken into consideration when correcting refraction with contact lenses. For instance, spherical rigid contact lenses eliminate the contribution of the anterior corneal surface into manifest astigmatism by creating a new refractive interface between the anterior ocular surface and air, and compensating anterior corneal astigmatism by generating a tear lens between cornea and contact lens. On the other hand, this essentially unmasks the ORA, which means that manifest astigmatism could change in unexpected ways both in magnitude and axis.32,33 The same concept applies when planning toric contact lenses, where the interaction between ORA and the toricity of the contact lens needs to be carefully considered if manifest astigmatism is to be fully corrected. For example, in eyes with low ACA but high ORA, a rigid toric contact lens can be employed with a spherical back optic surface to mask ACA and a toric front optic surface to correct ORA.32 A similar concept applies when planning toric IOL implantation. Historically, toric IOL power calculation formulae were designed so that the toricity of the implanted IOL would neutralize the keratometric anterior corneal astigmatism in terms of magnitude and axis, with the aim of eliminating preoperative manifest astigmatism.33,34 These formulae assume that the anterior corneal surface is the main refractive interface of the eye, and that astigmatism from other sources can be neglected. It has however been shown that this approach can result in inaccurate correction,11 and that including the posterior corneal astigmatism into the power calculation formula results in superior results.35–37 Therefore, newer toric IOL power calculation formulae incorporate posterior corneal astigmatism either estimated or measured, and some include predictions of astigmatism that is surgically induced by corneal incisions.38
The results of our study can be used to highlight the clinical significance of the concept of ORA. We demonstrated a statistically significant difference between RA and ACA in terms of the magnitude and axis. RA and ACA coincided in axis in only less than 50% of the eyes, with just over 20% of the eyes exhibiting a disagreement of more than 30° between the axis of RA and ACA. Such discrepancies pose the challenging question of which magnitude and axis to target when planning astigmatic correction, especially that it has been shown that uncorrected astigmatism as low as 1D in magnitude, as well as misalignments of as little as 10° degrees in the correction of astigmatism can cause a significant reduction in visual acuity.39,40 We also demonstrated that the prevalence of ORA in myopic eyes should not be underestimated. In our sample, almost three quarters of the eyes had ORA magnitudes of ≥0.5D, and just over a quarter had ORA magnitudes of ≥1D, and up to 4D. Similar results have been reported in the literature,41,42 with ORA magnitudes exceeding 1D in up to 46% of eyes.43
In addition, we found a distinct pattern of frequency distribution for the directions of different astigmatic components. Even though WTR was the most common form of both RA and ACA, followed by ATR, there was a statistically significant difference in the frequency distribution between the two components, where 74% of eyes showed WTR ACA, but only 55.55% showing WTR RA, suggesting that around 20% of the eyes with WTR ACA had their ACA influenced by other astigmatic components, ie, ORA in a way that moves the total RA into the oblique or ATR direction, especially considering that ORA shows an opposite pattern of frequency distribution with 74% of the eyes exhibiting an ATR ORA. Others have also noted that ORA is most commonly ATR in direction.41
We studied the interaction between ACA and ORA by implementing the concept of the compensation factor. This highlighted a complex relationship between the two, both in the 0°/180° and the 45° directions. No compensation is a rare occurrence in our sample at 5% and 7% for J0 and J45 respectively. On the other hand, compensations were more common than augmentations in J0, while augmentations were slightly more common than compensations in J45. Under-compensation was the most common pattern observed for J0, while same-axis augmentation was the most common pattern for J45. These results suggest that a complex interplay exists in most eyes between ORA and ACA, both in terms of magnitude and axis, which cannot be neglected in any form of refractive planning. Our results echo those from other studies which demonstrate comparable compensation patterns between ORA and ACA.44,45 The nature of this interplay could also differ according to the refractive state of the eye, as well as the size of the pupil.44
We investigated PCA as a potential source of ORA. We demonstrated a statistically significant correlation between RA and ACA, which parallels well-established results in the literature.46–49 This is to be expected given the widely spread notion that the anterior corneal surface is the main source of total ocular astigmatism due to the difference of refractive index between air and the corneal tissue. Taking the posterior corneal astigmatism into account by representing corneal astigmatism as TNP instead of ACA resulted in a statistically significant difference in the measured corneal astigmatism, as well as a stronger correlation with RA, suggesting a role for PCA in dictating the total ocular astigmatism, by bringing corneal astigmatism more in agreement with refractive astigmatism. In addition, using TNP instead of ACA to calculate ORA resulted in a statistically significant reduction in ORA magnitude by 0.69D on average. This suggests that PCA is a significant contributor to ORA. Our conclusions are similar to those drawn by other researchers, implying that the role of the posterior corneal surface in refractive planning can no longer be neglected.45
There were varying degrees of correlation between ORA and other forms of astigmatism including RA, ACA, TNP, and PCA. This could mean that eyes with more overall astigmatism tend to have more ORA. However, this correlation is not consistent across components such as magnitude, J0 and J45. Similarly, Mohammadpour et al found a weak correlation between the magnitudes of ORA and RA, and an even weaker correlation between the magnitudes of ORA and corneal astigmatism, which was not demonstrated when using analysis of variance in subgroups of astigmatism of different magnitudes. They found a stronger correlation between J0 and J45 components of ORA and corneal astigmatism, but this correlation applied only to patients with WTR astigmatism, but not ATR astigmatism.48 Other works of literature also report variable and non-conclusive degrees of correlation between ORA, RA and ACA.42,45
This work is not without its limitations. The study was carried out on refractive surgery candidates presenting to a single center in Benha, Egypt. It therefore could be argued that the results of the study cannot be generalized, as the study sample might not be adequately representative of the general population. It also excluded eyes with hypermetropic and mixed astigmatism, limiting the scope of its findings to those with myopic astigmatism. Nonetheless, this has the advantage of providing more homogenous data for statistical analysis. Similar study designs can be implemented in the future to investigate ORA in hypermetropic and mixed astigmats.
The study included both the right and left eyes of the same subjects. This poses a risk of bias due to the generally accepted notion that eye pairs are not completely independent. In addition, some degree of parallel or mirror astigmatism axis symmetry has been shown to exist between right and left eye pairs, including for ORA,50,51 a finding that was later challenged.52 A larger sample size could allow using only one eye per subject for statistical analysis.
Refractive astigmatism in this study was derived from autorefractometry measurements from non-dilated eyes. This has the advantage of taking the full extent of astigmatism originating from the lenticular system into account when calculating ORA, including any potential contribution from ciliary body tone, but it also has the potential to induce variability, given the fact that it has been shown in previous studies that astigmatism can dynamically change with accommodation.53,54 Studies show variable degrees of agreement between subjective refraction and objective autorefractometry,55–59 and some argue autorefractoemeters should be used as a complement but not a replacement for subjective refraction.60
Vector decomposition of astigmatism is not without its limitations. J0 and J45 are studied independently, each representing a component of astigmatism in one direction, but not the whole astigmatism. This makes it difficult to extrapolate how the whole eye might be behaving and can sometimes manifest in interesting ways statistically. For instance, we found the difference between RA and ACA, as well as ACA and TNP to be statistically significant in terms of magnitude and J0 but not J45. There was also a statistically significant correlation between ORA and RA for both J0 and J45, but not overall magnitude. Similarly, the frequency distribution for various forms of compensation between ORA and ACA is different for both J0 and J45. An eye could have a compensation factor that suggests under-compensation for the J0 components, but a same-axis augmentation for the J45 component. This makes it difficult to understand how the eyes astigmatism behaves as a whole from a clinical point of view.61–63 In addition, many clinicians, especially those whose scope of practice does not encompass refractive surgery, remain unfamiliar with vector analysis, making literature utilizing such representations of astigmatism hard to digest. We could argue that vector analysis of astigmatism should be included in ophthalmology and optometry postgraduate training and teaching curricula. On the same time, taking posterior corneal astigmatism into account brought total corneal astigmatism closer to refractive astigmatism, confirming that posterior corneal astigmatism contributed to ORA.19
Conclusion
The role of ORA in dictating the accuracy of astigmatic correction means that not taking ORA into account when planning corneal refractive surgery can result in unwanted surprises for both the patient and the surgeon, in a field where subjects expect absolute accuracy and perfect outcomes. This is especially true in eyes with higher levels of ORA. At the least, recognizing the role of ORA should result in adjusting the expectations for subjects with high ORA, or even advising against surgery altogether instead of risking patient dissatisfaction. In addition, pre-operative ORA should be considered, not only when choosing the modality of treatment, be it corneal or lenticular, topography- or wavefront-guided, but also when refractively planning the magnitude and axis of correction.
Funding
The authors did not receive any financial support from any public or private sources. The authors have no financial or proprietary interest in a product, method, or material described herein.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Fotouhi A, Hashemi H, Yekta AA, Mohammad K, Khoob MK. Characteristics of astigmatism in a population of schoolchildren, Dezful, Iran. Optom Vis Sci. 2011;88(9):1054–1059. doi:10.1097/OPX.0b013e318221727d
2. Wolfram C, Höhn R, Kottler U, et al. Prevalence of refractive errors in the European adult population: the Gutenberg Health Study (GHS). Br J Ophthalmol. 2014;98(7):857–861. doi:10.1136/bjophthalmol-2013-304228
3. Xiao X, Liu WM, Ye YJ, et al. Prevalence of high astigmatism in children aged 3 to 6 years in Guangxi, China. Optom Vis Sci. 2014;91(4):390–396. doi:10.1097/OPX.0000000000000221
4. Galvis V, Tello A, Niño CA, Parra MM. Total corneal astigmatism measurement precision. Invest Ophthalmol Vis Sci. 2015;56(10):5912. doi:10.1167/iovs.15-17735
5. Naeser K, Savini G, Bregnhøj JF. Age-related changes in with-the-rule and oblique corneal astigmatism. Acta Ophthalmol. 2018;96(6):600–606. doi:10.1111/aos.13683
6. Jiang Y, Qin Y, Bu S, Zhang H, Zhang X, Tian F. Distribution and internal correlations of corneal astigmatism in cataract patients. Sci Rep. 2021;11(1):11514. doi:10.1038/s41598-021-91028-2
7. Alpins NA. New method of targeting vectors to treat astigmatism. J Cataract Refract Surg. 1997;23(1):65–75. doi:10.1016/S0886-3350(97)80153-8
8. Godefrooij DA, Galvis V, Tello A. Von Helmholtz’s ophthalmometer: historical review and experience with one of the last surviving original devices. Acta Ophthalmol. 2018;96(3):314–320. doi:10.1111/aos.13493
9. Grosvenor T, Quintero S, Perrigin DM. Predicting refractive astigmatism: a suggested simplification of Javal’s rule. Am J Optom Physiol Opt. 1988;65(4):292–297. doi:10.1097/00006324-198804000-00009
10. Koch DD, Ali SF, Weikert MP, Shirayama M, Jenkins R, Wang L. Contribution of posterior corneal astigmatism to total corneal astigmatism. J Cataract Refract Surg. 2012;38(12):2080–2087. doi:10.1016/j.jcrs.2012.08.036
11. Tello A, Galvis V, Lugo IK, et al. Comment on: predicted vs measured posterior corneal astigmatism for toric intraocular lens calculations. J Cataract Refract Surg. 2022;48(10):1227–1228. doi:10.1097/j.jcrs.0000000000001049
12. Ueno Y, Hiraoka T, Miyazaki M, Ito M, Oshika T. Corneal thickness profile and posterior corneal astigmatism in normal corneas. Ophthalmology. 2015;122(6):1072–1078. doi:10.1016/j.ophtha.2015.01.021
13. Sano M, Hiraoka T, Ueno Y, Itagaki H, Ogami T, Oshika T. Influence of posterior corneal astigmatism on postoperative refractive astigmatism in pseudophakic eyes after cataract surgery. BMC Ophthalmol. 2016;16(1):212. doi:10.1186/s12886-016-0391-1
14. Ho JD, Tsai CY, Liou SW. Accuracy of corneal astigmatism estimation by neglecting the posterior corneal surface measurement. Am J Ophthalmol. 2009;147(5):788–795. doi:10.1016/j.ajo.2008.12.020
15. Zhang L, Wang Y. The shape of posterior corneal surface in normal, post-LASIK, and post-epi-LASIK eyes. Invest Ophthalmol Vis Sci. 2010;51(7):3468–3475. doi:10.1167/iovs.09-4811
16. Dong J, Zhang Y, Wang X. Calculation of toric intraocular lens power with the Barrett calculator and data from three keratometers. J Trop Med. 2021;2021:7712345. doi:10.1155/2021/7712345
17. Koch DD, Jenkins RB, Weikert MP, Yeu E, Wang L. Correcting astigmatism with toric intraocular lenses: effect of posterior corneal astigmatism. J Cataract Refract Surg. 2013;39(12):1803–1809. doi:10.1016/j.jcrs.2013.06.027
18. Reitblat O, Levy A, Megiddo Barnir E, Assia EI, Kleinmann G. Toric IOL calculation in eyes with high posterior corneal astigmatism. J Refract Surg. 2020;36(12):820–825. doi:10.3928/1081597X-20200930-03
19. Holladay JT, Dudeja DR, Koch DD. Evaluating and reporting astigmatism for individual and aggregate data. J Cataract Refract Surg. 1998;24(1):57–65. doi:10.1016/S0886-3350(98)80075-8
20. Næser K. Assessment and statistics of surgically induced astigmatism. Acta Ophthalmol. 2008;86(thesis1):1–28. doi:10.1111/j.1755-3768.2008.01234.x
21. Thibos LN, Horner D. Power vector analysis of the optical outcome of refractive surgery. J Cataract Refract Surg. 2001;27(1):80–85. doi:10.1016/S0886-3350(00)00797-5
22. Miller JM. Clinical applications of power vectors. Optom Vis Sci. 2009;86(6):599–602. doi:10.1097/OPX.0b013e3181a6a211
23. Lin J. The contribution of ocular residual astigmatism to anterior corneal astigmatism in refractive astigmatism eyes. Sci Rep. 2021;11(1):1018. doi:10.1038/s41598-020-80106-6
24. Lin J, An D, Lu Y, Yan D. Impact of ocular residual astigmatism on anterior corneal astigmatism in children with low and moderate myopia. Res Square. 2021.
25. McKendrick AM, Brennan NA. Distribution of astigmatism in the adult population. J Opt Soc Am a Opt Image Sci Vis. 1996;13(2):206–214. doi:10.1364/JOSAA.13.000206
26. Chen Z, Liu L, Pan C, et al. Ocular residual and corneal astigmatism in a clinical population of high school students. PLoS One. 2018;13(4):e0194513. doi:10.1371/journal.pone.0194513
27. Schuster AKG, Pfeiffer N, Schulz A, et al. Refractive, corneal and ocular residual astigmatism: distribution in a German population and age-dependency - The Gutenberg health study. Graefes Arch Clin Exp Ophthalmol. 2017;255(12):2493–2501. doi:10.1007/s00417-017-3775-x
28. Kugler L, Cohen I, Haddad W, Wang MX. Efficacy of laser in situ keratomileusis in correcting anterior and non-anterior corneal astigmatism: comparative study. J Cataract Refract Surg. 2010;36(10):1745–1752. doi:10.1016/j.jcrs.2010.05.014
29. Nöthel J, Katz T, Druchkiv V, Frings A. Effect of postoperative ocular residual astigmatism (ORA) on treatment outcome after myopic laser in situ keratomileusis (LASIK). OPTH. 2022;16:2079–2092. doi:10.2147/OPTH.S352410
30. Qian Y, Huang J, Chu R, et al. Influence of intraocular astigmatism on the correction of myopic astigmatism by femtosecond laser small-incision lenticule extraction. J Cataract Refract Surg. 2015;41(5):1057–1064. doi:10.1016/j.jcrs.2014.09.036
31. Abdelwahab SM, Hamed AM, Bayoumy ASM, Elfayoumi MA. Topography-Guided Femto-LASIK in Virgin Eyes: treating Manifest versus Measured Astigmatism. Clin Ophthalmol. 2020;14:4423–4430. doi:10.2147/OPTH.S281736
32. Morgan PB, Efron SE, Efron N, Hill EA. Inefficacy of aspheric soft contact lenses for the correction of low levels of astigmatism. Optom Vis Sci. 2005;82(9):823–828. doi:10.1097/01.opx.0000177792.62460.58
33. Abulafia A, Barrett GD, Kleinmann G, et al. Prediction of refractive outcomes with toric intraocular lens implantation. J Cataract Refract Surg. 2015;41(5):936–944. doi:10.1016/j.jcrs.2014.08.036
34. Abulafia A, Koch DD, Wang L, et al. New regression formula for toric intraocular lens calculations. J Cataract Refract Surg. 2016;42(5):663–671. doi:10.1016/j.jcrs.2016.02.038
35. Goggin M, Zamora-Alejo K, Esterman A, van Zyl L. Adjustment of anterior corneal astigmatism values to incorporate the likely effect of posterior corneal curvature for toric intraocular lens calculation. J Refract Surg. 2015;31(2):98–102. doi:10.3928/1081597X-20150122-04
36. Hoffmann PC, Wahl J, Hütz WW, Preußner P-R. A ray tracing approach to calculate toric intraocular lenses. J Refractive Surg. 2013;29(6):402–408. doi:10.3928/1081597X-20130515-04
37. Savini G, Hoffer KJ, Ducoli P. A new slant on toric intraocular lens power calculation. J Refract Surg. 2013;29(5):348–354. doi:10.3928/1081597X-20130415-06
38. Savini G, Næser K. An analysis of the factors influencing the residual refractive astigmatism after cataract surgery with toric intraocular lenses. Invest Ophthalmol Vis Sci. 2015;56(2):827–835. doi:10.1167/iovs.14-15903
39. Kane JX, Connell B. A comparison of the accuracy of 6 modern toric intraocular lens formulas. Ophthalmology. 2020;127(11):1472–1486. doi:10.1016/j.ophtha.2020.04.039
40. Sha J, Fedtke C, Tilia D, et al. Effect of cylinder power and axis changes on vision in astigmatic participants. Clin Optom. 2019;11:27–38. doi:10.2147/OPTO.S190120
41. Wolffsohn JS, Bhogal G, Shah S. Effect of uncorrected astigmatism on vision. J Cataract Refract Surg. 2011;37(3):454–460. doi:10.1016/j.jcrs.2010.09.022
42. Piñero DP, Ruiz-Fortes P, Pérez-Cambrodí RJ, Mateo V, Artola A. Ocular residual astigmatism and topographic disparity vector indexes in normal healthy eyes. Cont Lens Anterior Eye. 2014;37(1):49–54. doi:10.1016/j.clae.2013.07.006
43. Srivannaboon S. Internal astigmatism and its correlation to corneal and refractive astigmatism. J Med Assoc Thailand. 2003;86:166–171.
44. Frings A, Katz T, Steinberg J, Druchkiv V, Richard G, Linke SJ. Ocular residual astigmatism: effects of demographic and ocular parameters in myopic laser in situ keratomileusis. J Cataract Refract Surg. 2014;40(2):232–238. doi:10.1016/j.jcrs.2013.11.015
45. Muftuoglu O, Erdem U. Evaluation of internal refraction with the optical path difference scan. Ophthalmology. 2008;115(1):57–66. doi:10.1016/j.ophtha.2007.02.022
46. Park CY, Oh JH, Chuck RS. Predicting Ocular Residual Astigmatism Using Corneal and Refractive Parameters: a Myopic Eye Study. Curr Eye Res. 2013;38(8):851–861. doi:10.3109/02713683.2013.790976
47. Grosvenor T, Ratnakaram R. Is the relation between keratometric astigmatism and refractive astigmatism linear? Optom Vis Sci. 1990;67(8):606–609. doi:10.1097/00006324-199008000-00009
48. Keller PR, Collins MJ, Carney LG, Davis BA, van Saarloos PP. The relation between corneal and total astigmatism. Optom Vis Sci. 1996;73(2):86–91. doi:10.1097/00006324-199602000-00003
49. Mohammadpour M, Heidari Z, Khabazkhoob M, Amouzegar A, Hashemi H. Correlation of major components of ocular astigmatism in myopic patients. Contact Lens Anterior Eye. 2016;39(1):20–25. doi:10.1016/j.clae.2015.06.005
50. Remón L, Benlloch J, Furlan WD. Corneal and refractive astigmatism in adults: a power vectors analysis. Optom Vis Sci. 2009;86(10):1182–1186. doi:10.1097/OPX.0b013e3181baac2c
51. Dunne MC, Elawad ME, Barnes DA. A study of the axis of orientation of residual astigmatism. Acta Ophthalmol. 1994;72(4):483–489. doi:10.1111/j.1755-3768.1994.tb02802.x
52. Solsona F. Astigmatism as a congenital, bilateral and symmetrical entity (observations based on the study of 51,000 patients). Br J Physiol Opt. 1975;30(2–4):119–127.
53. McKendrick AM, Brennan NA. The axis of astigmatism in right and left eye pairs. Optom Vis Sci. 1997;74(8):668–675. doi:10.1097/00006324-199708000-00029
54. He JC, Burns SA, Marcos S. Monochromatic aberrations in the accommodated human eye. Vision Res. 2000;40(1):41–48. doi:10.1016/S0042-6989(99)00156-X
55. He JC, Wang J. Measurement of wavefront aberrations and lens deformation in the accommodated eye with optical coherence tomography-equipped wavefront system. Opt Express. 2014;22(8):9764–9773. doi:10.1364/OE.22.009764
56. Elliott M, Simpson T, Richter D, Fonn D. Repeatability and accuracy of automated refraction: a comparison of the Nikon NRK-8000, the Nidek AR-1000, and subjective refraction. Optom Vis Sci. 1997;74(6):434–438. doi:10.1097/00006324-199706000-00028
57. Gwiazda J, Weber C. Comparison of spherical equivalent refraction and astigmatism measured with three different models of autorefractors. Optometry Vision Sci. 2004;81(1):56–61. doi:10.1097/00006324-200401000-00011
58. McCaghrey GE, Matthews FE. Clinical evaluation of a range of autorefractors. Ophthalmic Physiol Opt. 1993;13(2):129–137. doi:10.1111/j.1475-1313.1993.tb00441.x
59. Mukash SN, Kayembe DL, Mwanza JC. Agreement between retinoscopy, autorefractometry and subjective refraction for determining refractive errors in Congolese children. Clin Optom. 2021;13:129–136. doi:10.2147/OPTO.S303286
60. Bamdad S, Momeni-Moghaddam H, Abdolahian M, Piñero DP. Agreement of wavefront-based refraction, dry and cycloplegic autorefraction with subjective refraction. J Optom. 2022;15(1):100–106. doi:10.1016/j.optom.2020.08.008
61. Kinge B, Midelfart A, Jacobsen G. Clinical evaluation of the Allergan Humphrey 500 autorefractor and the Nidek AR-1000 autorefractor. Br J Ophthalmol. 1996;80(1):35–39. doi:10.1136/bjo.80.1.35
62. Manny RE, Deng L, Gwiazda J, et al. Internal astigmatism in myopes and non-myopes: compensation or constant? Optom Vis Sci. 2016;93(9):1079–1092. doi:10.1097/OPX.0000000000000946
63. Abdelwahab S, Hamed A, Elshahat A, Rashad S, Elfauyomi M. Outcomes of small incision lenticule extraction with dual-incisions in myopic patients. Clin Ophthalmol. 2020;14:3067–3074. doi:10.2147/OPTH.S270032
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.