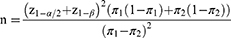Back to Journals » Patient Preference and Adherence » Volume 17
Evaluation of Medicine Information Leaflets for Omeprazole, Safety Knowledge, and Perceptions of Taking the Medication in Thailand
Authors Wongtaweepkij K , Sup-adulchai N, Chanachoat J, Krska J , Jarernsiripornkul N
Received 14 November 2022
Accepted for publication 10 March 2023
Published 27 March 2023 Volume 2023:17 Pages 883—893
DOI https://doi.org/10.2147/PPA.S397557
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Johnny Chen
Kamonphat Wongtaweepkij,1 Nutchwarang Sup-adulchai,2 Jirath Chanachoat,2 Janet Krska,3 Narumol Jarernsiripornkul2
1Division of Clinical Pharmacy, Faculty of Pharmacy, Srinakharinwirot University, Nakhon Nayok, Thailand; 2Division of Clinical Pharmacy, Faculty of Pharmaceutical Sciences, Khon Kaen University, Khon Kaen, Thailand; 3Medway School of Pharmacy, University of Kent, Kent, UK
Correspondence: Narumol Jarernsiripornkul, Division of Clinical Pharmacy, Faculty of Pharmaceutical Sciences, Khon Kaen University, Khon Kaen, 40002, Thailand, Tel +66-4334-8353, Fax +66-4320-2379, Email [email protected]
Purpose: This study aimed to compare package inserts and patient information leaflets for omeprazole in terms of the quality of and satisfaction with the written medicine information, medication safety knowledge, and perceived benefits and risks.
Patients and methods: A cross-sectional, comparative study was conducted at a university hospital in Thailand. Outpatients visiting the pharmacy departments prescribed omeprazole were randomly selected to receive either a package insert or a patient information leaflet. Medication safety knowledge was measured using a set of eight questions. The quality of the written medicine information was measured by the Consumer Information Rating Form. Perceived benefits and risks of the medication were rated using a visual analog scale. Linear regression was used to determine factors associated with perceived benefits and risks.
Results: Of the 645 patients, 293 agreed to answer the questionnaire. 157 and 136 patients were given patient information leaflets and package inserts, respectively. Most respondents were female (65.6%), over half had a degree (56.2%). Patients reading the patient information leaflets had slightly higher overall safety knowledge scores than those reading the package inserts (5.88 ± 2.25 vs 5.25 ± 1.84, p=0.01). Using the Consumer Information Rating Form, the patient information leaflets were given significantly higher scores compared to the package inserts for comprehensibility (19.34± 3.92 vs 17.32± 3.52, p< 0.001) and design quality (29.25 ± 5.00 vs 23.81 ± 5.16, p< 0.001). After reading the leaflets, patients receiving the patient information leaflets had significantly higher satisfaction with the information provided (p=0.003). In contrast, those receiving the package inserts rated the risks of omeprazole higher (p=0.007).
Conclusion: Demonstrable differences were found from the patient perspective between a package insert and a patient information leaflet for the same medicine, mostly in favour of patient information leaflets. Medicine safety knowledge after reading PI and PIL was similar. However, receiving package inserts provided higher perceived risks from taking the medicine.
Keywords: written medicine information, safety knowledge, consumer information rating form, perceptions of benefits and risks
Introduction
Medicine information plays an important role in ensuring that patients are able to use their medications correctly and safely. Some patients forget or misunderstand verbal information provided by healthcare professionals. To improve patients’ understanding of medicines, written medicines information (WMI) has been widely used as an additional source to support patients’ recall.
There are two main types of written medicine leaflets available to patients. Package inserts (PIs) or summaries of product characteristics are aimed at health professionals, but are frequently provided as leaflets in medicine packages, therefore are accessible to patients. Studies show that consumers are aware of and report reading PIs.1,2 A study in Thailand also found that PIs were common sources of information regarding medication, and most patients reported reading them, particularly for newly prescribed medications.3 However, most PIs are considered difficult to understand for patients due to the use of complex medical terms and inappropriate format.4–7 Patient information leaflets (PILs) are another form of WMI produced in simple language and patient-friendly format. Many countries, such as European countries and Australia, promote the development and distribution of PILs as primary information about medicines and support the use of PILs in patient counselling.8,9 Studies in many countries have found that patients often have difficulty in understanding the language and technical terms used in WMI and that level of education is associated with both reading WMI and medication knowledge.10–12 Studies in Thailand in patients using chronic medications showed that they had positive attitudes toward receiving information about medicines safety,13 and did not have anxiety after reading the side effect information in PILs.14
Before distributing to consumers, PILs need to be evaluated using consumer involvement for ease of reading, understanding, and communicative effectiveness.15 Several methods for evaluating these have been studied, covering several dimensions, including understandability, format, readability, satisfaction with information, utility, and actionability.16 The Consumer Information Rating Form (CIRF) was developed originally in English to measure consumers’ perceptions of the comprehensibility, utility, and design quality of written medicine information.17,18 It has been translated and demonstrated feasible for use in other languages.19,20 Previous studies evaluating WMI in Thailand showed that the CIRF could be practically used to assess consumers’ comprehension and the usefulness of written information for chronic medications.20,21
Relatively little research has sought patients’ views on different forms of WMI using standardized methods,21 but given the widespread provision of PIs to patients, is essential to determine whether PILs would offer a better alternative.
In this study, we, therefore, compared patient views on two different forms of WMI, PIs and PILs, for the same medicine. Omeprazole, a proton-pump inhibitor, was chosen as it can be used short or long-term. It is widely prescribed short-term for treating several conditions, including peptic ulcer disease, gastrointestinal reflux disease, and helicobacter pylori infection. It is also prescribed long-term for secondary prevention of gastric ulcers in patients receiving non-steroidal anti-inflammatory drugs.22–25 However, studies have reported gastrointestinal side effects including constipation due to PPI use26 and long-term use of PPIs can potentially cause significant adverse effects, including chronic kidney disease, pneumonia, hypomagnesemia, vitamin B12 deficiency, and Clostridium difficile infection.22,27
Our aim was to compare hospital outpatients’ ratings of the quality of PIs and PILs for omeprazole and to assess their safety knowledge of and perceived benefits and risks of this medicine.
Methods
Study Design and Settings
This was a cross-sectional, comparative study using a self-completed questionnaire performed in the outpatient clinic of Srinagarind Hospital, Khon Kaen University, Thailand, carried out over a three-month period.
Participants
Outpatients aged 18 years old or more and prescribed omeprazole as one of their medications were recruited to the study at the hospital pharmacy department. Participants were excluded from the study if they could not read the written information written provided in the study, were unable to complete the questionnaire themselves or with caregivers’ support and had ever previously assessed the quality of WMI prior to the study. The sample size was calculated to investigate a comparison of patients’ knowledge received from the PI and PIL28 as follows.
Where π1 = patients’ knowledge received from PI (59%) based on Patterson and Teale’s study29
π2 = patients’ knowledge received from PIL (0.75%) based on Jarernsiripornkul et al’s study.30
Since the difference in knowledge received from PI and PIL was 16% (0.16) with alpha error at 5%, and power of the study (beta) at 80%, a total of 300 participants was calculated to obtain enough sample size (each 150 patients in the PI and PIL group).
Study Instruments
Questionnaire
The self-administered questionnaire consisted of three sections. The first section was demographic characteristics of the participants, including gender, age, educational level, income, occupation, number of underlying diseases and prescribed medications, and experience of receiving PI or PIL for omeprazole prior to the study. The second section was the evaluation of written information on omeprazole using the Consumer Information Rating Form (CIRF). The CIRF consists of 4 components that evaluate the quality of WMI: comprehensibility, future use of WMI, utility, and design. The comprehensibility component covers five items on how easy or hard the WMI is to read, understand, remember, locate information, and keep the information for future reference. The future use component contains three items on the likelihood of WMI to read, use, and keep. The utility component has six items of how much information is provided in the WMI and how useful it is. The design component consists of seven items on perceptions about the organization, attractiveness, print size, tone, helpfulness, bias, and spacing between lines.17 The acceptable validity and reliability of a Thai version of the CIRF were also demonstrated.20
The third section was the assessment of safety knowledge of omeprazole use using eight questions with multiple choices covering how to take medicine (1 item), administration (1 item), adverse effects (2 items), overdosage (1 item), contraindication (1 item), precaution (item), and what to do when the dose was missed (1 item). The fourth section contained six items that were summarized into three components: satisfaction of information in the PI or PIL, perceptions of benefits and risks of taking medicine after reading the PI and PIL using a visual analog scale (VAS).
Written Information Leaflets
Two different forms of WMI were used in the study. The first was a PI from an omeprazole package produced by the manufacturer. This two-page PI contained information on the indication, the general appearance of the medicine product, the active ingredient, dosage recommendations, pharmacodynamics, pharmacokinetics, contraindications, precautions, drug interactions, adverse effects, overdosage management, storage, use in pregnancy and lactation. The information was written as descriptive text, and categorized into topics. Some medical terms were used.
The second was a one-page PIL developed by the Thai Food and Drug Administration (FDA). The PIL contained six main topics regarding what is the medicine and what is it used for; precautions before using the medicine; how to take the medicine; things to do while using the medicine; possible side effects; and how to store the medicine. The information was written in short sentences, with headings and subheadings to help patients read and find the information easily. Simple language was used, and medical terms were avoided. User testing was used to ensure that readers could read and understand the information.
Data Collection
After patients had received their medicines, the questionnaire together with a cover page explaining the study was directly distributed to the outpatients by researchers trained to ensure consistency in approach. Each participant who was willing to take part in the study received an explanation of the study’s objectives. They were randomly assigned to receive either a copy of the PI or PIL for omeprazole using permuted block technique. Those who agreed were asked to read the leaflet provided carefully and then complete the questionnaire. The questionnaires were returned directly to the researchers after completion.
Data Analysis
Data analyses were performed using IBM SPSS version 25.0. Descriptive characteristics were summarized as frequencies. The CIRF responses were summarized as total scores; perceptions of benefits and risks of taking medicines were presented as VAS scores ranging from 0 (least) to 10 (most); safety knowledge was summed by allocating one point for each correct answer (total score=8). Medicine safety knowledge scores were summed and classified into three categories: poor (percentage of correct answers less than 50% or less than 4 of 8 points), moderate (percentage of correct answers 50–80% or 4–6 points), and good (percentage of correct answers more than 80% or more than 6 of 8 points).
Independent t-tests and one-way ANOVA were used to compare subgroups for the CIRF and perception scores between the PI and PIL groups. Pearson chi-square or Fisher’s exact test was used to compare subgroups for categorical data. Linear regression was used to determine factors associated with the perceptions of benefits and risks of taking the medicines after reading the PI and PIL. After applying the Bonferroni adjustment, results with a p-value less than 0.0006 were considered statistically significant.
Ethical Approval
The study protocol was approved by the Khon Kaen University Ethics Committee for Human Research (HE611500) and conducted in accordance with the Declaration of Helsinki. All participants provided verbal informed consent before joining the study. The Khon Kaen University Ethics Committee approved verbal informed consent from study participants.
Results
Demographic Characteristics of Patients
A total of 645 outpatients were verbally invited by the researchers, of which 347 (53.8%) refused to participate. Five of the returned questionnaires were completed with less than 70% of all items, so were excluded from analysis. Hence, 293 questionnaires were analyzed, giving an overall response rate of 45.4%. Around two-thirds of participants (n = 191, 65.6%) were female, and 59.4% (n = 174) were aged 30–60. More than half had educational levels at a bachelor’s degree or higher (n = 164, 56.2%). The majority of respondents had never received any form of written information about omeprazole (n = 197, 67.5%). Table 1 describes the characteristics of 157 patients who received the PI and 136 who received the PIL. Respondents in the PIL group were likely to be slightly older and had a greater number of underlying diseases and medications than those in the PI group (p<0.001, p=0.001, and p<0.001).
 |
Table 1 Demographic Characteristics of Patients Receiving PI and PIL |
Quality Evaluation of the Written Medicine Information
A comparison of the CIRF scores between respondents reading PI and PIL is presented in Table 2. For overall scores, PILs were rated higher than PI for comprehensibility (mean difference, MD = 2.02), future use (MD = 1.01), and design quality (MD = 5.44) (p<0.0006). No significant differences in any aspects of the utility component, which included contraindications, precautions, adverse effects, and special instructions were found between the PI and PIL groups.
 |
Table 2 Comparison of Patients Rating the Quality of PI and PIL |
Assessment of Safety Knowledge of Omeprazole Use
The question about how to take medicine was answered correctly by almost all respondents in both the PI and PIL groups (96.2% and 94.9%), while the question about serious adverse effects achieved the least correct response (24.8% for the PI group and 53.7% for the PIL group). While the average score of safety knowledge about omeprazole for the PIL group was slightly higher than the PI group (5.88 ± 2.252 and 5.25 ± 1.835, p=0.01), only one question showed a significantly higher proportion of correct scores in the PIL group compared to the PI group, relating to side effects requiring cessation of treatment (53.7% vs 24.8%, p<0.001) (Table 3). Around half of the PI group had moderate safety knowledge, while half of the PIL group had good safety knowledge. Patients in the PIL group were likely to show higher levels of safety knowledge, but there was no statistically significant difference between the two groups (Table 4).
 |
Table 3 Comparison of Safety Knowledge of Omeprazole Between Reading PI and PIL |
 |
Table 4 Comparison of Safety Knowledge Level Between Reading PI and PIL |
Perceived Satisfaction, Benefits and Risks
Respondents in the PIL group had a significantly higher score for satisfaction with information than in the PI group (7.64 ± 2.057 and 6.92 ± 2.087, p = 0.003). For the risks associated with taking omeprazole, perceptions were higher in those receiving the PI compared to the PIL (14.43 ± 5.304 and 12.67 ± 5.837, p=0.007) (Figure 1). However, no significant difference in benefit perceptions was observed between the two groups. Multiple linear regression showed no statistically significant differences between reading PIs and PILs in perceptions, however type of material was the factor most highly associated with all three aspects (Table 5).
 |
Table 5 Linear Regression for Satisfaction of Information, Perceived Benefits and Risks from Taking the Medicine |
 |
Figure 1 Comparison of satisfaction of information, perceived benefits and risks from taking the medicine between reading PI and PIL. |
Discussion
This study describes patients’ evaluation of two different forms of WMI and compares medication safety knowledge, perceived benefits and risks and satisfaction with information. PILs were rated superior to the PIs for comprehensibility, future use of information, design and satisfaction, but the content was rated similarly for utility and both safety knowledge and perceived benefits were similar in both groups. Risks were perceived as higher in those receiving PIs.
Our respondents rated PIs and PILs similarly in terms of the utility of their content, despite the information in PIs being more lengthy and complex. This finding differs from our previous study in which all aspects of utility were rated higher by those receiving PILs for medicines used long-term compared to PIs.21 The group receiving PIs were younger, had fewer underlying conditions and therefore used fewer regular medicines than those receiving PILs. It is possible that those receiving the PIL had therefore been exposed to more information about medicine safety in general and omeprazole in particular. Both groups had similarly high education levels and reported previous experience with WMI, factors which have been shown to affect reading and understanding of WMI.31,32 Overall, respondents were slightly younger than in our previous study, and age has been shown to affect views on information.32–34
Safety knowledge, assessed by eight questions, was also similar in the two groups, with the exception of symptoms requiring stopping therapy, which was answered more correctly by receiving a PIL. However, the risks associated with the medicine were perceived as higher in those receiving PIs. One possible reason for these differences is the presentation of adverse effect information in the two documents. In the PI, all possible adverse effects were listed together, whereas in the PIL serious and non-serious effects were separated and the language differed. In addition, since PIs are aimed at health professionals, they frequently do not offer advice on what to do if symptoms occur.35 Benefit of reading PIL was demonstrated in a pre-post study in Thailand in which patients had more knowledge about taking atorvastatin after reading the PIL.14 A survey in Thailand found that consumers did not want to read the PIs because of difficult content and small print.36 After being promoted by the Thai FDA, the consumers were more aware of and need to read the medicine information from PILs.3,37 A qualitative study also revealed that PILs were organized and written in short, clear, and easy to understand, that had influenced patients’ interest to read the information.6
Several studies have demonstrated that grouping information and language used in WMI is important in ensuring understanding.38 Simple, short, patient-centered information can result in better recall and understanding of information compared to standard information.7,39 Given the higher ratings for comprehensibility of the PILs, the lack of significant differences in knowledge between the two groups appears surprising. This differs from our previous study in which respondents receiving PILs also rated comprehensibility higher than for PIs, but in addition, had greater safety knowledge.21 One possible explanation could be the pre-existing knowledge of the participants in our study, which was not assessed.
The CIRF again proved suitable for evaluating the quality of WMI in a Thai population and was able to detect differences in views among those receiving different forms of WMI. Prior studies in Australia and Belgium have assessed only patient-centered leaflets.17,19 Previous studies in Thailand have shown that the CIRF is easy to read and well-organized20 and demonstrated differences in ratings for PILs and PIs for chronic medicines.21 This study now demonstrates the application of the CIRF in evaluating the quality of a PI and a PIL for a medicine which can be used short term. Given the widespread practice of distributing PIs to patients in many other countries,2 further studies which evaluate the quality of WMI from a patient perspective are needed. Indeed, patients should be involved in evaluating WMI before a product is launched, as occurs in many countries.40,41 The CIRF is a valuable addition to user testing as a mechanism for such evaluation.
This study has shown that PILs offer advantages over PIs, in line with other studies in Thailand3,21 and elsewhere.7,42,43 There is clearly a need for easier, wider access to patient-centred WMI. Manufacturers should be encouraged to produce and test such materials, but health professionals could also assist by advocating and making greater use of the FDA-produced PILs for frequently used medicines.
Strengths and Limitations of the Study
This study used the CIRF, which was previously evaluated for validity and reliability in a Thai population. It compared leaflets which were produced by a manufacturer and the Thai FDA, not leaflets specifically designed for the study. However, it is a cross-sectional study, and we did not determine the extent of previous exposure to omeprazole or specific information received about this medicine. Therefore, the minor differences found in knowledge and perceived benefits and risks between the two forms of WMI could be related to these factors. In addition, the study was conducted in the outpatient setting of only one university hospital and most participants had a high level of education. Also, despite random allocation, participants in the PIL group were older and took more medications than those in the PI group.
Conclusion
Written medicine information for omeprazole is a useful source of the medication use for patients and customers. Using the CIRF showed that PIL provided better comprehensibility, usability, design quality and satisfaction compared to the PI for the same medicine. However, the PI was associated with higher perceived risks, but overall safety knowledge was similar in both groups. The CIRF could be practically used for evaluation of PI and PIL before distributing to the consumers.
Acknowledgments
We would like to express our thanks to all participants in this study and the Pharmacy department staff at Srinagarind Hospital, who assisted in data collection.
Funding
This study received financial support from the faculty of Pharmaceutical Sciences, Khon Kaen University. The funding organization had no role in the design or conducting of the study.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Fuchs J, Hippius M, Schaefer M. A survey of package inserts use by patients; 2005. Available from: https://www.paint-consult.com/fileadmin/editorial/downloads/publikationen/PAINT-Consult_package_inserts_use_patients.pdf.
2. Nualdaisri P, Corlett SA, Krska J. Provision and need for medicine information in Asia and Africa: a scoping review of the literature. Drug Saf. 2021;44(4):421–437. doi:10.1007/s40264-020-01038-8
3. Pongpunna S, Pratipanawatr T, Jarernsiripornkul N. Survey of outpatient’s use and needs of patient medicine information leaflets in Thailand. Int J Clin Pharm. 2019;41(1):141–150. doi:10.1007/s11096-018-0748-z
4. Fuchs J, Hippius M. Inappropriate dosage instructions in package inserts. Patient Educ Couns. 2007;67(1–2):157–168. doi:10.1016/j.pec.2007.03.009
5. Pires C, Vigário M, Cavaco A. Readability of medicinal package leaflets: a systematic review. Rev Saude Publica. 2015;49:4. doi:10.1590/s0034-8910.2015049005559
6. Wongtaweepkij K, Corlett S, Krska J, Pongwecharak J, Jarernsiripornkul N. Patients’ experiences and perspectives of receiving written medicine information about medicines: a qualitative study. Patient Prefer Adherence. 2021;15:569–580. doi:10.2147/PPA.S298563
7. Nualdaisri P, Corlett SA, Krska J. The Effectiveness and value of written medicine information across Asia and Africa: systematic review. Drug Saf. 2021;44(12):1283–1295. doi:10.1007/s40264-021-01114-7
8. The Pharmaceutical Society of Australia. Australian Pharmaceutical Formulary and Handbook.
9. The Medicines and Healthcare Products Regulatory Agency. Best practice guidance on patient information best practice guidance on patient; 2012. Available from: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/328405/Best_practice_guidance_on_patient_information_leaflets.pdf.
10. Ahmadi P, Badri S, Zargarzadeh A. An investigation on patient attitudes toward package inserts and their accessibility in Iran. J Res Med Sci. 2018;23(1):100. doi:10.4103/jrms.JRMS_67_18
11. Saleem H, Ghoto MA, Memon N, Dayo A, Suheryani I, Shafique S. Assessment of patient’s awareness towards pharmacotherapy and their sources of drug information in different areas of Hyderabad, Pakistan. Int Res J Biol Sci. 2015;4(8):56–59.
12. Ndwiga JM, Kikuvi G, Omolo JO. Factors influencing knowledge on completion of treatment among tb patients under directly observed treatment strategy, in selected health facilities in embu county, Kenya. Pan Afr Med J. 2016;25:234. doi:10.11604/pamj.2016.25.234.8761
13. Jarernsiripornkul N, Phueanpinit P, Pongwecharak J, Krska J. Experiences of and attitudes towards receiving information about non-steroidal anti-inflammatory drugs: a cross-sectional survey of patients in Thailand. Expert Opin Drug Saf. 2016;15(4):417–426. doi:10.1517/14740338.2016.1139571
14. Pongpunna S, Pratipanawatr T, Wongtaweepkij K, Jarernsiripornkul N. Evaluation of patient’s knowledge of atorvastatin information in patient information leaflets: a pre-post intervention study in Thailand. Patient Prefer Adherence. 2021;15:2377–2387. doi:10.2147/PPA.S334668
15. Garner M, Ning Z, Francis J. A framework for the evaluation of patient information leaflets. Heal Expect. 2012;15(3):283–294. doi:10.1111/j.1369-7625.2011.00665.x
16. Luk A, Aslani P. Tools used to evaluate written medicine and health information: document and user perspectives. Heal Educ Behav. 2011;38(4):389–403. doi:10.1177/1090198110379576
17. Koo MM, Krass I, Aslani P. Evaluation of written medicine information: validation of the consumer information rating form. Ann Pharmacother. 2007;41(6):951–956. doi:10.1345/aph.1K083
18. Krass I, Svarstad BL, Bultman D. Using alternative methodologies for evaluating patient medication leaflets. Patient Educ Couns. 2002;47(1):29–35. doi:10.1016/S0738-3991(01)00171-9
19. Desplenter F, Laekeman G, Demyttenaere K, Simoens S. Medication information for Flemish inpatients with major depression: evaluation and construct validity of the consumer information rating form. J Clin Pharm Ther. 2009;34(6):645–655. doi:10.1111/j.1365-2710.2009.01039.x
20. Wongtaweepkij K, Krska J, Pongwecharak J, Pongpunna S, Jarernsiripornkul N. Development and psychometric validation for evaluating written medicine information in Thailand: the consumer information rating form. BMJ Open. 2021;11(10):e053740. doi:10.1136/bmjopen-2021-053740
21. Wongtaweepkij K, Krska J, Pongpunna S, Pongwecharak J, Jarernsiripornkul N. Thai patients’ drug safety knowledge and perceptions relating to different forms of written medicine information: a comparative study. Patient Prefer Adherence. 2022;16:1141–1152. doi:10.2147/ppa.s361447
22. Kinoshita Y, Ishimura N, Ishihara S. Advantages and disadvantages of long-term proton pump inhibitor use. J Neurogastroenterol Motil. 2018;24(2):182–196. doi:10.5056/jnm18001
23. Khan MA, Howden CW. The role of proton pump inhibitors in the management of upper gastrointestinal disorders. Gastroenterol Hepatol. 2018;14(3):169–175.
24. Abdelazim AH, Ramzy S. Application of different quantitative analytical techniques for estimation of aspirin and omeprazole in pharmaceutical preparation. BMC Chem. 2022;16(1):60. doi:10.1186/s13065-022-00854-6
25. El-Olemy A, Abdelazim AH, Ramzy S, et al. Application of different spectrofluorimetric approaches for quantitative determination of acetylsalicylic acid and omeprazole in recently approved pharmaceutical preparation and human plasma. Spectrochim Acta A Mol Biomol Spectrosc. 2021;262:120116. doi:10.1016/j.saa.2021.120116
26. Hanif IF, Syafhan NF, Hersunaryati Y, Andrajati R. Gastrointestinal side effects of Proton Pump Inhibitors on inpatients at Gatot Soebroto Hospital. J Young Pharm. 2017;9(1):s13–s15. doi:10.5530/jyp.2017.1s.4
27. Jaynes M, Kumar AB. The risks of long-term use of proton pump inhibitors: a critical review. Ther Adv Drug Saf. 2018;10:2042098618809927. doi:10.1177/2042098618809927
28. Lemeshow S, Ogston SA, Hosmer DW, Klar J, Lwanga SK. Adequacy of Sample Size in Health Studies. New York: Wiley for the World Health Organization; 1991.
29. Patterson C, Teale C. Influence of written information on patients’ knowledge of their diagnosis. Age Ageing. 1997;26(1):41–42. doi:10.1093/ageing/26.1.41
30. Jarernsiripornkul N, Phueanpinit P, Pongwecharak J, Krska J. Development and evaluation of user-tested Thai patient information leaflets for non-steroidal anti-inflammatory drugs: effect on patient’s knowledge. PLoS One. 2019;14(1):e0210395. doi:10.1371/journal.pone.0210395
31. Bawazir SA, Abou-Auda HS, Gubara OA, Al-Khamis KI, Al-Yamani MJ. Public attitude toward drug technical package inserts in Saudi Arabia. J Pharm Technol. 2003;19(3):209–218. doi:10.1177/875512250301900302
32. Koo MM, Krass I, Aslani P, Guzmàn WM, Le Duff M. Factors influencing consumer use of written drug information. Ann Pharmacother. 2003;37(2):259–267. doi:10.1177/106002800303700218
33. Dodds LJ, King RW. Factors affecting attitudes to the provision of information with prescribed drugs. Pharm J. 1989;242(suppl):R7–R12.
34. Manafo E, Wong S. Exploring older adults’ health information seeking behaviors. J Nutr Educ Behav. 2012;44(1):85–89. doi:10.1016/j.jneb.2011.05.018
35. Al-Aqeel SA. Evaluation of medication package inserts in Saudi Arabia. Drug Healthc Patient Saf. 2012;4(1):33–38. doi:10.2147/DHPS.S29402
36. Burapadaja S, Tantipathananandh P, Sirithunyalug B. Consumer’s opinions on reading a medicine leaflet. Chiang Mai Univ J. 2004;3(2):155–167.
37. Wongtaweepkij K, Krska J, Pongwecharak J, Jarernsiripornkul N. Experiences and views of medicine information among the general public in Thailand. Patient Prefer Adherence. 2020;14:1073–1082. doi:10.2147/PPA.S257454
38. Maat HP, Lentz L, Raynor DK. How to test mandatory text templates: the European patient information leaflet. PLoS One. 2015;10(10):e0139250. doi:10.1371/journal.pone.0139250
39. Morrow DG, Weiner M, Young J, Steinley D, Deer M, Murray MD. Improving medication knowledge among older adults with heart failure: a patient-centered approach to instruction design. Gerontologist. 2005;45(4):545–552. doi:10.1093/geront/45.4.545
40. Raynor DK. User testing in developing patient medication information in Europe. Res Social Adm Pharm. 2013;9(5):640–645. doi:10.1016/j.sapharm.2013.02.007
41. Yamamoto M, Doi H, Yamamoto K, et al. Adaptation of the European Commission-recommended user testing method to patient medication information leaflets in Japan. Drug Healthc Patient Saf. 2017;9:39–63. doi:10.2147/DHPS.S114985
42. Mansoor L, Dowse R. Written medicines information for South African HIV/AIDS patients: does it enhance understanding of co-trimoxazole therapy? Health Educ Res. 2007;22(1):37–48. doi:10.1093/her/cyl039
43. Dowse R, Barford K, Browne SH. Simple, illustrated medicines information improves ARV knowledge and patient self-efficacy in limited literacy South African HIV patients. AIDS Care. 2014;26(11):1400–1406. doi:10.1038/jid.2014.371
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.