Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 14
Evaluation Of HHIP Polymorphisms And Their Relationship With Chronic Obstructive Pulmonary Disease Phenotypes
Authors Bártholo TP , Porto LC , Pozzan R, Nascimento A , Da Costa CH
Received 26 April 2019
Accepted for publication 16 September 2019
Published 3 October 2019 Volume 2019:14 Pages 2267—2272
DOI https://doi.org/10.2147/COPD.S213519
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Thiago Prudente Bártholo,1 Luis Cristovão Porto,2 Roberto Pozzan,3 Adriana Nascimento,2 Claudia Henrique Da Costa4
1Department of Pulmonology and Tisiology, Rio De Janeiro State University (UERJ), Rio De Janeiro, Brazil; 2Laboratory of Histocompatibility and Cryopreservation, UERJ, Rio De Janeiro, Brazil; 3Department of Cardiology, Piquet Carneiro Polyclinic, UERJ, Rio De Janeiro, Brazil; 4Coordinator of the Department of Pulmonology and Tisiology, Faculty of Medical Sciences, UERJ, Rio De Janeiro, Brazil
Correspondence: Thiago Prudente Bártholo
Disciplina de Pneumologia e Tisiologia, Hospital Universitário Pedro Ernesto (UERJ), Boulevard 28 de setembro 77, Vila Isabel, Rio de Janeiro 20551-030, Brazil
Tel +55 21 981088883
Fax +55 21 22688238
Email [email protected]
Purpose: We aimed to correlate three polymorphisms of the Hedgehog Interacting Protein (HHIP) gene with the three main phenotypes of the chronic obstructive pulmonary disease (frequent exacerbator (FE), asthma/COPD overlap (ACO), and emphysema with hyperinflation).
Patients and methods: A cross-sectional study was carried out in the Department of Pulmonology at the Rio de Janeiro State University from February 2015 to July 2018. A total of 81 patients diagnosed with COPD according to the criteria of the Global Initiative for Chronic Obstructive Lung Disease (GOLD) were enrolled. The subjects were divided into three distinct groups according to their phenotypes (FE, ACO and emphysema-hyperinflation). Three polymorphisms of the HHIP gene that are often reported as allegedly involved in the pathogenesis of COPD were analysed: rs1828591, rs13118928, and rs6537296. Real-time PCR - TAQMAN SNP Genotyping Assay was performed. The statistical analysis was carried out with the SPSS program with a multivariate analysis with a 95% confidence interval.
Results: An increase in the frequency of the A allele of the rs13118928 HHIP gene polymorphism was observed in the group of subjects with COPD and emphysema-hyperinflation phenotype when compared with those in the FE phenotype (p=0.019) and subjects with ACO (p=0.04). However, the subjects with emphysema-hyperinflation phenotype presented more often the A allele (p=0.04). The genotypic analysis confirmed the difference between the emphysema-hyperinflation and ACO phenotypes, with a higher prevalence of the AA genotype in the emphysema-hyperinflation group (p=0.04). The ACO and FE phenotype subjects showed no difference in these polymorphisms. No difference was found in the frequency of the polymorphisms rs1828591 (p= 0.552) and rs6537296 (p=0.296) in the three phenotypes evaluated.
Conclusion: The presence of the A allele in the rs13118928 polymorphism of the HHIP gene may be related to the emphysema-hyperinflation phenotype.
Keywords: Chronic obstructive pulmonary disease, ACO, hyperinflation, exacerbator, HHIP polymorphism
Introduction
The COPD presents some distinct phenotypes despite the same risk factors. They are clinically heterogeneous, and the identification of each subgroup seems to be important to define the therapeutic setting. The phenotypes may be related to important prognostic factors such as the presence of symptoms, a higher or lower risk of exacerbation, the response to therapy, the rate of disease progression and mortality.1–3 Several recent studies have attempted to define phenotypes based on various criteria. Miravitlles et al4 described the three main phenotypes: asthma-COPD overlap (ACO), FE and emphysema-hyperinflation.
The asthma-COPD overlap definition is highly variable. Briefly, patients with ACO have a combination of the following factors: history of asthma and/or atopy, reversibility in the bronchodilator test, notable eosinophilia in respiratory and/or peripheral secretions, high IgE, positive prick test to allergens and high concentrations of exhaled NO.4–9 The prevalence of this phenotype is estimated at 20% of COPD patients, and it is more common among the elderly – 50% of the patients are above 50 years.10,11 Furthermore, they commonly present a better response to corticoids.4,12
The exacerbator (FE) phenotype presents occasional episodes of clinical instability of the disease repeatedly within a short period of time. The FE is defined when patients present two or more exacerbations per year and the exacerbations are at least 4 weeks apart after the end of treatment or 6 weeks from the onset of symptoms in case patients have not received any treatment.1,4 Patients who have been hospitalized in the previous year are also considered exacerbators.4,11,13
The emphysema-hyperinflation phenotype has an anatomical-pathological definition at the terminal bronchioles level. These patients clinical characteristics include the presence of dyspnea, exercise intolerance, signs of hyperinflation, and a tendency for low body mass index.1,4 Hyperinflation can be detected by the chest computed tomography scans and/or a respiratory function examination with assessment of the reduced diffusing capacity for carbon monoxide (DLCO). These patients seem to better benefit when treated with bronchodilator therapy involving two long-acting bronchodilators.4,14
The Hedgehog interaction protein is encoded by the 4q31 chromosome and participates in a series of signalling pathways that include pulmonary organogenesis and the response to lung injury in response to cigarette smoking.15 Several studies have been carried out in an attempt to correlate polymorphisms of the Hedgehog Interacting Protein gene (HHIP) with the susceptibility to COPD. The reduced expression of this protein seems to predispose to COPD. Two polymorphisms seem to be related to the reduction of the triggering activity of this protein – rs6537296 and rs1542725.16 Two other polymorphisms that tend to occur together, the rs1828591 and rs13118928, seem to facilitate the expression of an altered and non-functional protein, with a greater risk for the development of the COPD.15,16 The SNP rs11938704 correlates significantly with the forced expiratory volume drop in the first second (FEV1) in the COPD population.17 However, so far, there has been no report of the association of the HHIP polymorphisms with a particular COPD phenotype.17,18
Materials And Methods
A cross-sectional study was carried out with outpatients at the Rio de Janeiro State University (UERJ). Patients enrolled in this study have had the COPD diagnosis in accordance with the Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2018, with the spirometry revealing an obstructive ventilatory disorder (FEV1/FVC after bronchodilator < 0.7) and a smoking history < 20 pack/year. Patients with non-controlled chronic diseases and those with previous lung diseases other than COPD were excluded from the study. The study was approved by the the local ethics committee (Ethic Committee of the Rio de Janeiro State University) – the number of this manuscript is: 39,414,414.8.0000.5259. All individuals signed a free will and information consent form before they became subjects of the study procedures, in compliance with the Helsinki Declaration.
Study Procedures
Patients diagnosed with COPD were invited to participate and answered a questionnaire to enable the collection of epidemiological and clinical data. Patients with smoking history < 20 pack/year, with an alpha1-antitrypsin deficiency history or with acute exacerbation the month before were excluded.
The patients recruited underwent a respiratory function test with a spirometry parameter analysis, a measurement of carbon monoxide diffusion capacity and an assessment of the pulmonary volumes in an HD CPL device (nSpire Health Inc., Longmont, CO, USA), following the standardization and interpretation of the American Thoracic Society, 2005. The Knudson’s equations for flow and volume variables were adopted. Serum samples were obtained for the evaluation of genetic polymorphisms. The polymorphisms studied in the HHIP gene (rs1828591, rs13118928, and rs6537296) were evaluated by the real-time polymerase chain reaction (PCR) through the Taqman SNP Genotyping Assay. These three polymorphisms were selected because of their reported relationship with COPD development.
Subjects were divided into three phenotypes (ACO, FE and emphysema-hyperinflation) based on six parameters: history of atopy, number of exacerbations in the last 12 months, response to bronchodilator test and values of diffusion for CO percentage, total lung volume percentage and residual volume percentage. Subjects who did not fulfil the characteristics of one phenotype or who presented characteristics related to more than one phenotype were excluded. The definition of each phenotype is described in Table 1.
 |
Table 1 Characteristics Considered For Dividing Patients Into The Three Different Phenotypes |
Statistical Analysis
The statistical analysis was performed with the IBM-SPSS statistics 25 program. Age, sex and pulmonary function data (forced vital capacity and forced expiratory volume in the first second) are displayed as mean and standard deviation. Allelic and genotypic frequencies are displayed as absolute numbers and percentages. The differences between groups were assessed using an independent sample T student test, ANOVA and the chi-squared model, where appropriate. Statistical significance was appointed by p values of less than 0.05.
Results
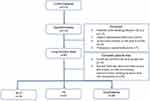
A total of 81 patients with a confirmed COPD diagnosis met the criteria to become subjects included in the study and were recruited. However, 20 patients could not be evaluated because they could not be classified in 1 of the 3 phenotypes studied (n = 6) or because they had been included in more than one phenotype (n = 14). Of the 61 subjects included in the study, 21 were FE phenotypes, 26 emphysema-hyperinflation phenotypes, and 14 ACO phenotypes. Figure 1 presents the flow chart of the recruitment. There was no statistically significant difference between the groups regarding age, sex and forced vital capacity. The forced expiratory volume in the first second was statistically different because the ACO phenotype had higher values demonstrating a mild disease in our sample when compared to other phenotypes (Table 2).
 |
Table 2 Subjects’ Demographic And Functional Characteristics |
When comparing these three phenotypes, there was no statistically significant difference when regarding the genotypic analysis of the rs1828591 (p= 0.552) and rs6537296 (p=0.296) HHIP polymorphisms. However, there was a trend for statistical difference regarding the rs13118928 (p = 0.058) polymorphism. Although not very prevalent in the 3 groups, the G allele was not found in patients in the emphysema-hyperinflation group. In the genotypic analysis, we observed a higher percentage of patients with the emphysema-hyperinflation phenotype with AA genotype (Table 3). Thus, we performed a 2 × 2 analysis to find out a possible implication of the emphysema-hyperinflation phenotype in this statistical trend.
 |
Table 3 Genotypic Frequency In The Phenotypes Studied |
The separate 2 × 2 analysis of the 3 phenotypes revealed a statistically significant difference in the A allele when comparing FEs with emphysema-hyperinflation (p = 0.019), in which the latter group presented this allele more frequently. The analysis between ACO and emphysema-hyperinflation also revealed a statistically significant difference in the comparison between AA × AG × GG genotypes (p = 0.04), with a higher prevalence of the AA genotype in the emphysema-hyperinflation group, and a statistically significant difference in the G allele (p = 0.04) in this same group, and it was less frequently found in patients with the emphysema-hyperinflation phenotype. No statistically significant difference was found in allelic and genotypic analysis between ACO and FEs. Therefore, the presence of the A allele may be related to the trend to develop the emphysema-hyperinflation phenotype and the presence of the G allele is possibly not related to this phenotype (Table 4).
 |
Table 4 Allelic And Genotypic Frequency In 2 × 2 Comparisons With The Rs 13118928 HHIP Gene Polymorphism |
Discussion
Much has been discussed about the different COPD phenotypes, specially the ACO, the emphysema-hyperinflation, and the FE phenotypes. In addition to these 3 phenotypes, Miravitlles et al described four other clinical phenotypes with their clinical nuances and the main therapeutic options for each one.4 Other authors have also reported several phenotypes, in an attempt to identify a clinical spectrum to a better target therapy.6
Nevertheless, there are no studies that discuss a possible distinct genetic origin of each of these phenotypes, especially the three most frequent ones already cited above. The logic of this discussion is based on the fact that individuals exposed to the same risk factor – smoking – developed distinct spectra of COPD. Thus, the correlation of genetic polymorphisms and the different COPD phenotypes becomes imperative.
Several studies report polymorphisms possibly associated with the genesis of COPD, including those in the HHIP gene (rs1828591, rs13118928, and rs6537296).17,18
After a statistical analysis comparing these three polymorphisms with the three major COPD phenotypes, novel results were observed, the possible relationship of the presence of the A allele and absence of the G allele in the rs13118928 polymorphism of the HHIP gene with the emphysema-hyperinflation phenotype.
Our study presented some limitations, mainly regarding the determination of the phenotype within the daily basis clinical context. Some subjects were excluded from the sample for not meeting the criteria of a pure phenotype. Another limitation is related to the fact that the history of atopy and asthma were based on patient-based history. The number of exacerbations that had occurred in the year before the recruitment was confirmed in most cases by medical notes, but some patients had been treated in the emergency room during the episodes of exacerbation, and for this study, all episodes in which the patients had received antibiotics or systemic corticosteroids were considered exacerbations, even if they had not happened in our institution.
Conclusion
In conclusion, this study demonstrates a possible relationship between the presence of the A allele and the absence of the G allele at the rs13118928 HHIP polymorphism with the emphysema-hyperinflation phenotype. This data needs to be corroborated by subsequent studies, but it opens the discussion of the relationship between the COPD phenotype and genotype.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Chair JV, Agusti A, Anzuetto A, et al. The global initiative for Chronic Obstructive Pulmonary Disease-Global strategy for the diagnosis, management and prevention for chronic obstructive pulmonary disease-update. 2018. Available from: www.goldcopd.org.
2. Han MK, Agusti A, Calverley PM, et al. Chronic obstructive pulmonary disease phenotypes: the future of COPD. Am J Respir Crit Care Med. 2010;182:598–604. doi:10.1164/rccm.200912-1843CC
3. Agusti A, Sobradillo P, Celli B. Addressing the complexity of chronic obstructive pulmonary disease – from phenotypes and biomarkers to scale free networks, system biology and P4 medicine. Am J Respir Crit Care Med. 2011;183:1129–1137. doi:10.1164/rccm.201009-1414PP
4. Miravitlles M, Calle M, Soler-Cataluna JJ. Clinical phenotypes of COPD: identification, definition and implications for guidelines. Arch Bronconeumol. 2012;48:86–98. doi:10.1016/j.arbres.2011.10.007
5. Rhee CK. Phenotype of asthma-chronic obstructive pulmonary disease overlap syndrome. Korean J Intern Med. 2015;30:443–449. doi:10.3904/kjim.2015.30.4.443
6. Alshabanat A, Zafari Z, Albanyan O, Dairi M, Fitzgerald JM. Asthma and COPD overlap syndrome (ACOS). A systematic review and meta analysis. PLoS One. 2015;10:e0136065. doi:10.1371/journal.pone.0136065
7. Corhay JL, Schleich F, Louis R. Phenotypes in chronic obstructive pulmonary disease. Rev Med Liège. 2014;69:415–421.
8. Miravitlles M, Soler-Cataluña JJ, Calle M, et al. Spanish COPD guidelines (GesEPOC): pharmacological treatment of stable COPD. Spanish Society of Pulmonology and Thoracic Surgery. Arch Bronconeumol. 2012;48:247–257. doi:10.1016/j.arbres.2012.04.001
9. Piras B, Miravitlles M. The overlap phenotype: the (missing) link between asthma and COPD. Multidiscip Respir Med. 2012;7:8. doi:10.1186/2049-6958-7-8
10. Postma DJ, Rabe KF. The asthma–COPD overlap syndrome. N Eng J Med. 2015;373:1241–1249. doi:10.1056/NEJMra1411863
11. Gibson PG, McDonald VM. Asthma-COPD overlap 2015: now we are six. Thorax. 2015;70:683–691. doi:10.1136/thoraxjnl-2014-206740
12. Chen X, Xu X, Xiao F. Heterogeneity of chronic obstructive pulmonary disease: from phenotype to genotype. Front Med. 2013;7:425–432. doi:10.1007/s11684-013-0295-x
13. Suzuki T, Tada Y, Matsuura Y, et al. Clinical, physiological and radiological features of asthma-chronic obstructive pulmonary disease overlap syndrome. Int J Chron Obstruct Pulmon Dis. 2015;10:947–954.
14. Burge S, Wedzicha JÁ. COPD exacerbations: definitions and classifications. Eur Respir J. 2003;21:46s–53s. doi:10.1183/09031936.03.00102002
15. Vestbo J, Agusti A, Wouters EFM, et al. Should we view chronic obstructive pulmonary disease differently after ECLIPSE? A clinical perspective from the study team. Am J Respir Crit Care Med. 2014;189:1022–1030. doi:10.1164/rccm.201306-1150OC
16. Celli BR. Chronic obstructive pulmonary disease phenotypes and their clinical relevance. Proc Am Thorac Soc. 2006;3:461–466. doi:10.1513/pats.200603-029MS
17. Zhou X, Baron RM, Hardin M, et al. Identification of chronic obstructive pulmonary disease genetic determinant that regulates HHIP. Hum Mol Gen. 2012;21:1325–1335. doi:10.1093/hmg/ddr527
18. Durme YMTAV, Eijgelsheim N, Joos GF, et al. Hedgehog interaction protein is a COPD susceptibility gen: the Rotterdam study. Eur Respir J. 2010;36:89–95. doi:10.1183/09031936.00129509
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.