Back to Journals » Journal of Experimental Pharmacology » Volume 12
Evaluation of Diuretic Activity and Phytochemical Contents of Aqueous Extract of the Shoot Apex of Podocarpus falcactus
Authors Meharie BG , Tunta TA
Received 17 October 2020
Accepted for publication 25 November 2020
Published 16 December 2020 Volume 2020:12 Pages 629—641
DOI https://doi.org/10.2147/JEP.S287277
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Bal Lokeshwar
Birhanu Geta Meharie,1 Tewodros Agedew Tunta2
1Department of Pharmacy, Wollo University, Dessie, Amhara, Ethiopia; 2Department of Pharmacy, Arba Minch University, Arba Minch, SNNPR, Ethiopia
Correspondence: Birhanu Geta Meharie
Department of Pharmacy, Wollo University, Dessie, Amhara, Ethiopia
Email [email protected]
Background: In Ethiopian folk medicine Podocarpus falcactus is used to treat stomachache, cancer, diabetes, and difficulty of urination. However, its diuretic activity has not been proven scientifically.
Objective: To determine the diuretic activity and phytochemical contents of the aqueous extract of the shoot apexes of Podocarpus falcactus.
Methods: The coarse powder of Podocarpus falcactus shoot apex was extracted by cold maceration using distilled water. Male rats were treated with distilled water, the standard drug (furosemide 10 mg/kg), and three different doses (100, 200, and 400 mg/kg) of the aqueous extract. The diuretic activity was determined by measuring parameters such as time to the first urination, volume, electrolyte concentration, and pH of urine. Electrolyte indices were calculated to elucidate the possible mechanism of diuresis. Additionally, qualitative and quantitative determination of phytochemicals in the plant extract was carried out.
Results: The aqueous extract induced diuresis, natriuresis, and kaliuresis in a dose- and time-dependent manner as compared to the negative control. The extract at 200 and 400 mg/kg doses produced significant diuresis (p< 0.001) by the end of the fifth hour compared to the negative control. Excretion of sodium, potassium, and chloride also significantly (p< 0.001) increased following extract administration. In addition, there was a significant change in the pH of urine samples of the extract-treated group compared with the negative control. Qualitative and quantitative determination of phytochemicals revealed the presence of alkaloids, flavonoids, phenolics, and tannins with the value of 128.4 mg atropine equivalents (AE)/g, 142.23 mg quercetin equivalents (QE)/g, 196.84 mg gallic acid equivalents (GAE)/g, and 25.5 mg tannic acid equivalents (TAE)/g, respectively. The aqueous extract exhibited significant diuretic activity due to its phytochemical content, which could be used as a starting point for further studies.
Conclusion: The aqueous extract showed significant diuretic activity and confirmed the folkloric use of Podocarpus falcactus.
Keywords: alkaloids, flavonoids, phenolics, Podocarpus falcactus, rats, saluretic, tannins
Background
Podocarpus falcactus, also known as Afrocarpus falcactus (Podocarpus gracilior or Afrocarpus gracilior),1 “Zigiba” in Amharic,2 is a species of coniferous tree in the family Podocarpaceae, commonly known as the East African yellowwood.1 It is found in mountain forests and is native to eastern Africa; in Ethiopia, Kenya, Tanzania, Uganda, and Mozambique.1 It is also distributed to eastern and southern South Africa, in Swaziland and Lesotho.3 It is planted as an ornamental tree in South Africa, and occasionally outside its natural area of distribution.4
Podocarpus falcactus is a medium-sized tree, growing 20–40 m tall, rarely to 50 m, with a trunk diameter of 50–80 cm. The leaves are spirally arranged, lanceolate, 2–6 cm long and 3–5 mm broad on mature trees, larger, to 10 cm long and 6 mm broad on vigorous young trees.5,6 The family Podocarpaceae consists of important timber trees used by many communities in traditional medicine for the treatment of fever, asthma, cough, cholera, distemper, chest complaints, hepatitis, venereal disease,7 vomiting, febrile illness, and inability to urinate.8
Traditionally, all parts of Podocarpus falcactus have been used for the treatment of several ailments. The bark and stem extracts are used as remedies for stomachache, gonorrhea, deworming, and cancer.9 The gum and shoot parts of the plant are used for the treatment of diabetes, cough, lung problems,2 and vomiting.10 The shoot apex is crushed, mixed with water, and taken orally as a diuretic to treat unable to urinate.8
Podocarpus falcactus also has several pharmacologically proven activities. The leaves showed significant antioxidant, antimicrobial,11,12 and stimulatory activities to nitric oxide release on the macrophage cell line. It also has a cytotoxic effect against breast adenocarcinoma cell lines. Even the known anticancer drug taxol is found in Podocarpus falcactus extract.11
Phytochemical investigation of the leaf extract of the plant revealed the presence of polyphenols and diterpenoids, including podolactones (nor- and bis-norditerpenoid dilactones), totarol, abietane, sempervirol diterpenoids, flavonoids,13,14 and Quercetin-3-O-Glycoside or Quercetin-3-O-β-D-Glucopyranoside (QG) flavonol glycosides.15 The di-terpenoid taxol and polyphenols from the plant Podocarpus falcactus have shown potent antioxidant activity.16
Materials and Methods
Chemicals and Reagents
The chemicals and reagents used were distilled water (EPHARM, Ethiopia), normal saline (EPHARM, Ethiopia), methanol (Sigma-Aldrich, Germany), chloroform (Sigma-Aldrich, Germany), Folin–Ciocalteu reagent (Sigma-Aldrich, Germany), gallic acid (Sigma-Aldrich, Germany), quercetin (Sigma-Aldrich, Germany), atropine (Sigma-Aldrich, Germany), tannic acid (Sigma-Aldrich, Germany), the standard drug furosemide (Denk Pharma GmbH & Co. KG, Germany), and sodium pentobarbitone (Taj Pharmaceuticals Limited, India). Besides, other chemicals and reagents used for phytochemical analysis such as – aluminum chloride, ammonium hydroxide, sodium carbonate, sodium hydroxide, sodium nitrate, dimethyl sulfoxide (DMSO), bromocresol green (BCG), ferric chloride, lead acetate, sulfuric acid, hydrochloric acid, acetic acid, and isopropyl alcohol, potassium hydroxide, mercuric chloride, potassium iodide, iodine, bismuth nitrate were obtained from Fisher Scientific (UK). All chemicals and reagents used were of analytical grade.
Plant Material
The shoot apexes of Podocarpus falcactus were collected in January 2020 from Hawassa, about 275 km south of Addis Ababa. The plant specimen was identified by a taxonomist and a voucher specimen (BG03) was deposited as a future reference. Shoot apexes were thoroughly washed with tap water and dried under shade at room temperature for 2 weeks. Then, dried shoot apexes were manually pulverized into coarse powder using a mortar and pestle.
Experimental Animals
Both male and female Sprague Dawley rats aged 6–10 weeks and with a weight range of 260–300 g inbred in the animal house of the School of Pharmacy, Addis Ababa University, were used for the experiment. The animals were housed in polypropylene cages (6 rats per cage) under standard environmental conditions (25 ± 2°C, 55 ± 5% humidity, and 12 h/12 h light/dark cycle). The animals were allowed free access to tap water and laboratory pellet and acclimatized to laboratory conditions for one week before the experiment. Each rat was placed in an individual metabolic cage (Techniplast, Italy) 24 h before the commencement of the experiment for adaptation.
Preparation of Plant Extract
The shoot apexes of Podocarpus falcactus were extracted using the cold maceration technique. Two hundred grams of the coarse powder was soaked with distilled water in a conical flask and placed on a shaker at 120 rpm for 3 days at room temperature. The extract was filtered using muslin cloth and Whatman® grade 1 filter paper and the marc was re-extracted for the second and third times by adding another fresh solvent. The fluid extract was dried in a lyophilizer (OPERON, OPR-FDU-5012, Korea) and stored in a desiccator until used for the experiment.
Acute Oral Toxicity Test
Female rats were used for the acute oral toxicity test of the aqueous extract of Podocarpus falcactus as per the Organization for Economic Co-operation and Development guidelines.17 They fasted overnight before and 4 h after administration of the extract. First, a sighting study was performed to determine the starting dose, and a female rat was given 2000 mg/kg of the aqueous extract as a single dose by oral gavage. Since no death was observed within 24 h, an additional four rats were used and dosed as mentioned above. Rats were observed continuously for 4 h at 30 min intervals and then for 14 consecutive days with an interval of 24 h for the general signs and symptoms of toxicity (diarrhea, weight loss, tremor, lethargy, and paralysis), food and water intake, and mortality. Then, three dose levels were chosen for the extract: a middle dose, which is one-tenth of the maximum dose during the acute toxicity study; a low dose, which is half of the middle dose, and a high dose that is twice the middle dose.17
Grouping and Dosing of Animals
Rats were randomly assigned into five groups (negative control, positive control, and three test groups) comprising 6 rats per group. Negative control rats were treated with the vehicle used for reconstitution of the aqueous extract, distilled water (DW), and positive controls were treated with the standard drug, furosemide 10 mg/kg (F10), and three test groups were treated with 100, 200, and 400 mg/kg of the aqueous extract as AQ100, AQ200, and AQ400, respectively. Doses were determined using data from the acute toxicity test. All groups were dosed orally using oral gavage and the volume administered was 1 mL/100 g. At the end of the experiment, all rats were killed by intraperitoneal injection of sodium pentobarbital at a dose of 150 mg/kg.
Diuretic Activity
The method used by Hailu and Engidawork18 was employed to determine the diuretic activity of the plant extract. All animals were subjected to fasting overnight with free access to water. Rats were pretreated with normal saline at an oral dose of 15 mL/kg to impose uniform water and salt load. Rats were divided into groups comprising six animals per group and dosed as described under the grouping and dosing of animals section. Immediately after administration, animals were placed in metabolic cages (a rat in a cage). Then, urine was collected and measured at the time of first urination, 1, 2, 3, 4, and 5 h after dosing and stored at –20°C for electrolyte analysis. At the time of urine collection, no food or water was made available to the rats.
The parameters recorded for each rat were time to the first urination, urine volume, urine concentration of Na+, K+, and Cl−, and urine pH. To compare the effects of three different doses of the aqueous extract with the negative and positive controls, parameters such as percent urinary excretion, diuretic action, and diuretic activity were also calculated using the following formulas. Percent urinary excretion = 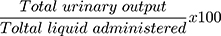 , diuretic action =
, diuretic action = 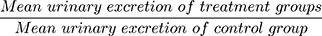 , and diuretic activity =
, and diuretic activity = 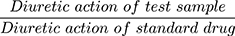 18,19
18,19
Electrolyte Level Analysis
The electrolyte levels of the aqueous extract of Podocarpus falcactus and urine of rats were analyzed. The concentrations of Na+ and K+ were determined by using a high-precision flame photometer (FP8400 Flame Photometer, A.KRUSS OPTRONIC GmbH, Germany), whereas, the concentration of Cl− was determined by using an ion-selective electrode analyzer (AVL 9181 Electrolyte Analyzer, Roche, Germany). The ratios of electrolytes (test/control), Na+/K+, and Cl−/[K++Na+] were calculated to evaluate the saluretic, natriuretic, and carbonic anhydrase inhibitory activity of the aqueous extract of Podocarpus falcactus. Besides, pH was directly determined on fresh urine samples using a pH meter.
Phytochemical Screening
Qualitative phytochemical investigation of the aqueous extract was carried out to determine the presence of secondary metabolites like alkaloids, cardiac glycosides, flavonoids, polyphenols, saponins, steroids, tannins, and terpenoids using standard methods.20–24
Tests for Alkaloids: about 10 mg of the aqueous extract was dissolved in 2 mL of 5% hydrochloric acid and filtered. Filtrates were taken separately in different test tubes. Then, drops of Bouchardat, Dragendorff, Wagner, and Mayer reagents were added to each. The formation of a brown precipitate, red-orange precipitate, yellowish-white precipitate, and red-brown precipitate indicates the presence of alkaloids.
Tests for Anthraquinone Glycosides:
- Borntrager’s test: about 3 mL of chloroform was added to 3 mL of the aqueous extract and the chloroform layer was separated. Then, a 5% potassium hydroxide solution was added to the mixture. The formation of red color indicates the presence of anthraquinone glycosides.
- Ammonium hydroxide test: about 10 mg of the aqueous extract was dissolved in 10 mL of isopropyl alcohol. To this mixture, a drop of concentrated ammonium hydroxide was added. After two minutes, the formation of red color indicates the presence of anthraquinone glycosides.
Test for Cardiac Glycosides (Keller-Kiliani test): about 0.25 g of aqueous extract was dissolved with distilled water. To 5 mL of aqueous extract solution, 2 mL of glacial acetic acid containing one drop of ferric chloride solution was added. This was underplayed with 1 mL of concentrated sulfuric acid. The formation of the brown-ring at the interface indicates the presence of cardiac glycosides.
Tests for Flavonoids:
- Alkaline reagent test: about 1 mL of the aqueous extract was treated with a few drops of 20% sodium hydroxide solution. The formation of intense yellow color, which becomes colorless on the addition of hydrochloric acid, indicates the presence of flavonoids.
- Shinoda test: to 5 mL of the aqueous extract, pieces of magnesium ribbon and concentrated hydrochloric acid were added. The appearance of red to pink color after a few minutes indicates the presence of flavonoids.
Tests for Polyphenols:
- Lead acetate test: few drops of 10% lead acetate solution were added to the aqueous extract solution. The occurrence of a white precipitate indicates the presence of phenolic compounds.
- Ferric chloride test: to 2 mL of the filtered solution of the aqueous extract, four drops of 10% ferric chloride solution were added. The formation of blue-green color indicates the presence of phenolic compounds.
Test for Saponins (Frothing test): to 0.25 g of the aqueous extract, 20 mL of distilled water was added and shaken vigorously for 15 minutes. The formation of a stable persistent froth indicates the presence of saponins.
Test for Steroids (Libermann-Buchard test): about 1 mL of the aqueous extract was treated with 10 mL of chloroform and filtered. The filtrate was treated with a few drops of acetic anhydride and then boiled and cooled. To this mixture, concentrated sulfuric acid was added. The formation of brown-ring at the interface indicates the presence of steroids.
Test for Tannins (Ferric chloride test): about 10 mg of the aqueous extract was dissolved in 2 mL of distilled water and filtered. To the filtrate, a few drops of 10% ferric chloride were added. The formation of a blue or green color indicates the presence of tannins.
Test for Terpenoids (Salkowski test): about 2 mL of chloroform was added to 10 mg of aqueous extract. To this mixture, 3 mL of concentrated sulfuric acid was carefully added. A reddish-brown coloration at the interface indicates the presence of terpenoids.
Quantitative Determination of Phytochemical Contents
Phytochemicals obtained in the qualitative screening test were undergone quantitative estimation using standard methods shown below.
Total Alkaloid Content Determination
The total alkaloid content of the aqueous extract of Podocarpus falcactus was determined using the spectrophotometric method based on the reaction of alkaloid with BCG.25 One mg of the plant extract was dissolved in DMSO. One mL of 2N hydrochloric acid was added and the mixture was filtered. The pH of this solution was adjusted to neutral with 0.1 N sodium hydroxide. Then, the mixture was transferred into a separatory funnel, and 5 mL of BCG solution and 5 mL of phosphate buffer (pH 4.7) were added. The mixture was vigorously shaken with 1, 2, 3, and 4 mL of chloroform. Then, it was diluted to 10 mL with chloroform. Similarly, a set of standard solutions of atropine (12.5, 25, 50, 100, 200, and 400 μg/mL) were prepared by adding 5 mL of BCG solution and 5 mL of 4.7 pH phosphate buffer to accurately measured aliquots of atropine and the mixture was vigorously shaken with 1, 2, 3, and 4 mL of chloroform and collected in a 10 mL volumetric flask and diluted with chloroform up to 10 mL. Then, the absorbance of atropine and test solutions were determined on the reagent blank at 470 nm with a UV-Visible spectrophotometer (SHIMADZU UV-1800, Kyoto, Japan). For accuracy, all procedures were done on the same day and in triplicate. The total alkaloid content was expressed as mg of atropine equivalents per gram of the dry plant extract (AE/g).
Total Flavonoid Content Determination
The aluminum chloride colorimetric method26 was employed to determine the total flavonoid content of the aqueous extract of the plant Podocarpus falcactus. Concentrations of quercetin in methanol (12.5, 50, 100, 200, and 400 µg/mL) were prepared. Two hundred fifty mL of each concentration were mixed with 125 mL of water and 75 mL sodium nitrate solution. The mixture was placed in the dark (at 25°C for 6 min.). Then, 150 mL of aluminum chloride was added and stayed in the dark for 5 h. Finally, 500 mL of sodium hydroxide and 275 mL of water were added to each mixture. The absorbance was measured at 510 nm using a UV-visible spectrophotometer and the concentration versus absorbance standard calibration curve was plotted. Similarly, the test sample was also prepared and investigated. All samples were analyzed in triplicate. The total flavonoid content was expressed as milligrams of quercetin equivalent per gram of dry plant extract (QE/g).
Total Phenolic Content Determination
The Folin-Ciocalteu reagent assay27,28 was employed to determine the total phenolic content of the aqueous extract of Podocarpus falcactus. Fifty µL aliquots of 12.5, 25, 50, 100, 200, and 400 µg/mL methanolic gallic acid solutions were mixed with 100 µL (10%) Folin–Ciocalteu reagent and 100 µL (7%) sodium carbonate. The mixture was incubated at room temperature for 30 min, the absorbance against the prepared reagent blank was determined at 765 nm with a UV-visible spectrophotometer. Then the calibration curve was constructed by plotting the absorbance against concentration. In the same manner, the test sample was prepared and investigated. All samples were analyzed on the same day in triplicate. The total phenolic content was expressed as milligrams of gallic acid equivalent per gram of dry plant extract (GAE/g).
Total Tannin Content Determination
The tannins were determined by following the method used by Kavitha Chandran and Indira.29 To 0.1 mL of the sample extract, 0.5 mL of Folin-Ciocalteu reagent, and 1 mL of sodium carbonate solution (35%) were added. Then, the mixture was diluted to 10 mL with distilled water and shaken well, and kept at room temperature for 30 min. Similarly, a set of reference standard solutions of tannic acid (12.5, 25, 50, 100, 200, and 400 μg/mL) were prepared. Absorbance for test and standard solutions were measured against the reagent blank at 700 nm with a UV-visible spectrophotometer. All procedures were done on the same day in triplicate. The total tannin content was expressed in terms of mg of tannic acid equivalents per gram of dry plant extract (TAE/g).
Statistical Analysis
The results of the study are expressed as the mean ± standard error of the mean (S.E.M). Statistical analysis of the data was performed with one-way analysis of variance (ANOVA) followed by Tukey post hoc multiple comparison test. Dose-dependent effects were evaluated using linear regression. Significant differences were set at p values lower than 0.05.
Ethical Consideration
Rats were handled as per the international animal care and welfare,30 and the national institute of health guidelines for the care and use of laboratory animals.31
Results
Percent Yield of the Aqueous Extract
The shoot apexes of Podocarpus falcactus were extracted with distilled water using the cold maceration technique and it was investigated for its diuretic activity and total contents of important secondary metabolites. The percent yield of the aqueous extract of the shoot apexes of the plant was found to be 21.5% (43 g).
Acute Oral Toxicity Test
The acute oral toxicity test of the aqueous extract of the shoot apex of Podocarpus falcactus showed no gross behavioral changes and mortality within 24 h as well as in the next 14 days, referring that the median lethal oral dose of the aqueous extract was greater than 2000 mg/kg in rats.
Diuretic Activity of the Aqueous Extract
Effect on the First Urinary Latency
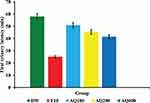
The effect of aqueous extract of Podocarpus falcactus shoot apex on the first urinary latency is presented in Figure 1. Rats treated with AQ100 did not show fast onset in their urination as compared to the negative control. AQ200 and AQ400 showed a minimal delay in the onset of urination, 45.33 ± 1.99, and 41.5 ± 1.57 min., respectively, (p<0.001) as compared to the standard (25.17 ± 1.01 min.).
Effect on Urine Volume
The effect of aqueous extract of Podocarpus falcactus shoot apex on urinary output is shown in Table 1. The aqueous extract produced diuresis which appeared to be a function of dose and time (r2 = 0.855; p<0.001). AQ100 did not produce any detectable difference in urine volume within the first 3 hours after dosing, but it produced a significant increase in urine volume after 4 hours as compared to the negative control (p<0.001). AQ200 and AQ400, however, produced a significant increase in urine volume with maximum diuresis of 120% (p<0.001) and 136% (p<0.001), respectively. AQ200 and AQ400 had a comparable diuretic effect with F10. Among the three doses of the aqueous extract, AQ400 and AQ200 produced significant diuresis compared to AQ100 at all time-points. AQ200 and AQ400 exhibited a diuretic action of 2.20 and 2.31, respectively (Table 1). The percent urinary excretion of AQ200 (96%) and AQ400 (100%) was higher as compared to the negative control (44%).
 |
Table 1 Effect of Aqueous Extract of Podocarpus Falcactus Shoot Apex on Urine Volume in Rats |
Effect on Cumulative Urine Output
The cumulative urine output of rats treated with aqueous extract of Podocarpus falcactus shoot apex is presented in Figure 2. AQ100 had produced nearly equal urine output as a negative control. Whereas, AQ200 and AQ400 had produced significantly comparable urine output as the standard drug. As it is shown in Figure 2, the cumulative urine output produced after administration of AQ400 is larger than the standard within the first 3 hours and after the 5th h.
Effect on Urinary Electrolyte Excretion
The urine samples collected over the five hours were analyzed for the electrolyte content (Na+, K+, and Cl−) and presented in Table 2. A significantly increased sodium loss was observed in rats treated with the aqueous extract at all doses. The increased sodium excretion produced by AQ100, AQ200, and AQ400 was 45%, 94%, and 118%, respectively, (p<0.001), compared to the negative control. F10 produced the maximum sodium excretion (124%, p<0.001), which was significantly greater than the effect produced by AQ100 (p<0.001) and AQ200 (p<0.05). Enhanced potassium loss was observed at the doses of 200 and 400 mg/kg, (55% and 96%, p<0.001), respectively, compared to the negative control. These values were, however, significantly lower than produced by F10 (122%, p<0.001). In the case of chloride excretion, AQ200 (35%, p<0.05) and AQ400 (48%, p<0.001) produced a significant loss than the negative control. In this regard, no apparent differences were observed between the standard and the aforementioned two doses. Table 2 also shows the saluretic indices of the aqueous extract at three different dose levels. AQ400 for Na+ (2.18), K+ (1.96), and Cl− (1.48) were nearly similar to the indices of F10: 2.24, 2.22, and 1.42 for the three ions, respectively. The Na+/K+ ratios of AQ100 (1.64), AQ200 (1.84), and AQ400 (1.64) were higher than the ratio for the standard drug (1.48). The carbonic anhydrase inhibitory activity of AQ200 and AQ400 was 0.62 and 0.58, respectively, (Table 2).
 |
Table 2 Effect of Aqueous Extract of Podocarpus Falcactus Shoot Apex on Urinary Electrolyte Excretion in Rats |
Electrolyte Content of the Extract
The levels of three electrolytes (Na+, K+, and Cl−) were determined to exclude the possibility of interference since water-soluble salts could be present and interfere with the urinary excretion of electrolytes. There were no detectable levels of the three electrolytes in the extracts at all doses tested.
Effect on Urine pH
The urinary pH was measured and different treatment groups had resulted in different urine pH Figure 3. AQ200 and AQ400 produced a relatively alkaline urine (pH = 8.67 ± 0.25, and 8.84 ± 0.35, p<0.05), respectively. The standard drug had also produced slightly basic urine (pH = 8.32 ± 0.39).
Qualitative Phytochemical Screening
The aqueous extract of Podocarpus falcactus was tested for the composition of medicinally active compounds and it was found to be positive for alkaloids, flavonoids, polyphenols, and tannins (Table 3).
 |
Table 3 Phytochemical Screening of Aqueous Extract of Podocarpus Falcactus Shoot Apex |
Quantitative Determination of Phytochemical Contents
Medicinally useful secondary active metabolites were found in the aqueous extract. Therefore the total quantities of these phytochemicals in the aqueous extract were determined using respective standards and UV-visible spectrophotometer.
Total Alkaloid Content Determination
The total alkaloid content of the aqueous extract was determined by the BCG reaction method using atropine as the standard alkaloid in plotting the calibration curve. The total alkaloid content, calculated from the atropine calibration curve (y = 0.0028x + 0.1874, R2 = 0.9908) and expressed in atropine equivalents was found to be 128.4 mg AE/g.
Total Flavonoid Content Determination
The total flavonoid content of the aqueous extract was determined by aluminum trichloride assay using quercetin as a standard in plotting the calibration curve. The calibration curve of quercetin that its linearity range was from 12.5 to 400 µg/mL and the regression equation of the calibration curve is y = 0.0044x + 0.3862, R2 = 0.9805. The total flavonoid content was 142.23 mg QE/g.
Total Phenolic Content Determination
The total phenolic content in the aqueous extract was determined by the Folin–Ciocalteu method using gallic acid as a standard in plotting the calibration curve. The calibration curve of gallic acid that its linearity range was from 12.5 to 400 µg/mL and the regression equation of the calibration curve is y = 0.005x – 0.0352, R2 = 0.9955. The total phenolic content was 196.84 mg GAE/g.
Total Tannin Content Determination
The total tannin content in the aqueous extract was determined by the Folin–Ciocalteu method using tannic acid as a standard in plotting the calibration curve. The total tannin content, calculated from the tannic acid calibration curve (y = 0.0024x + 0.0078, R2 = 0.992) and expressed in tannic acid equivalents per gram of the dry plant extract was 25.5 mg TAE/g.
Discussion
According to a previous ethnopharmacological survey carried out in Southern Ethiopia, the shoot apexes of Podocarpus falcactus are used to manage the difficulty of urination (as a diuretic),8 but its diuretic activity has not been proven scientifically. This study reports the diuretic and natriuretic effects of the aqueous extract of the shoot apexes of Podocarpus falcactus. The folkloric use of the plant indicates that the shoot apex of Podocarpus falcactus is crushed, mixed with water, and taken orally as a diuretic to treat unable to urinate.8 Therefore, extraction was done using distilled water in a cold maceration technique to simulate the traditional application.
In view of urine output, the aqueous extract of the plant showed an increase in diuresis that appeared to be a function of dose and time (r2 = 0.855; p<0.001). AQ100 did not produce a visible effect throughout the experiment, but AQ200 and AQ400 were able to produce a significant diuresis throughout the observation time (Table 1). This could probably suggest that AQ100 might be below the minimum effective dose, which cannot elicit diuresis, and AQ200 and AQ400 might be large enough to cause significant diuresis. Besides, the diuretic actions of AQ200 and AQ400 are 2.20 and 2.31, respectively, which are nearly similar to the diuretic action of F10 (2.34). The diuretic activity of the aqueous extract in the two effective doses was a mild type since their values were 0.94 and 0.99 for AQ200 and AQ400, respectively (Table 1). Diuretic activity is good if it is more than 1.50, moderate if it is between 1.00–1.50, mild if it is between 0.72–0.99, and nil if it less than 0.72.18
The aqueous extract had a significant delay in the first urinary latency in its lowest dose (AQ100 = 51.0 ± 2.07 min. p<0.001), whereas, the medium (AQ200) and the highest (AQ400) doses had a minimal delay in the onset of urination (45.33 ± 1.99 and 41.5 ± 1.57 min., respectively, p<0.001) as compared to F10 (25.17 ± 1.01 min.) (Figure 1). This is also augmented by the evidence from cumulative urine output (Figure 2). As it is shown in Figure 2, AQ200 and AQ400 caused a significant increase in cumulative urine output at the end of the 3rd hour as compared to the negative control. The delay in the first urinary latency and a significant increase in cumulative urine output after 3 h with the aqueous extract may indicate that its diuretic activity is probably mediated via secondary organic metabolites. Remarkably, the diuretic activity of the plant extract was dose and time-dependent indicating that this effect is intrinsic, genuine, and possibly receptor-mediated.32 Renal excretion of electrolytes is as salient as the excretion of water for treatment of hypertension, peripheral edema, ascites, and congestive heart failure.33 The increase in diuresis caused by the aqueous extract reflected correspondingly in the excretion of electrolytes. It significantly increased the excretion of urinary electrolytes (Na+, K+, and Cl−) in a dose-dependent manner. Although the aqueous extract increased the excretion of K+ as compared to the negative control, it was significantly lower than that induced by the standard drug. The natriuretic activity (aldosterone secretory index) of the plant extract can be determined by taking the ratio of Na+/K+ and values greater than 2.0 indicate a favorable natriuretic effect, whereas ratios greater than 10.0 indicate a potassium-sparing effect.33 Since the aqueous extract did not increase the Na+/K+ ratio, it is not acting as a potassium-sparing diuretic. Because, potassium-sparing diuretics are usually very weak, have a slow onset of action,34 and increase the urinary Na+/K+ ratio.33
Besides, the aqueous extract increased the saluretic index and had a dose- and time-dependent diuresis. This evidence suggests that the aqueous extract might act via the mechanism of loop diuretics. Loop diuretics increase the urinary flow rate and urinary excretion of Na+, K+, and Cl− by inhibiting Na+-K+-2Cl− symporter in the thick ascending limb of the loop of Henle, stimulating the production of renal prostaglandins, and inhibiting carbonic anhydrase enzyme in the proximal convoluted tubule (PCT).34–36
The aqueous extract of the shoot apexes of Podocarpus falcactus used in the present study produced a similar Na+ and Cl− excretion profile to that of the standard. However, there is a difference when K+ excretion is considered. This could suggest that the aqueous extract might act via several mechanisms.
The aqueous extract exhibited a dose- and time-dependent diuresis, and a comparable increase in excretion of Na+ and Cl− as well. Since the aqueous extract did not increase the Na+/Cl− ratio (thiazide-secretory index), it might not act via a thiazide-like mechanism. Thiazide and thiazide-like diuretics the so-called low ceiling diuretics have a rapidly flattening dose-response curve and increase the urinary flow rate and excretion of electrolytes particularly, Na+, K+, and Cl− by interfering with Na+-Cl− co-transporter in the distal convoluted tubule and also to some extent by inhibiting carbonic anhydrase enzyme in the PCT.37
The aqueous extract has a K+ saving effect in comparison to the excretion of Na+ and Cl−. So, this is one advantage of the aqueous extract over the conventional agents. Since one of the main adverse effects of loop and thiazide diuretics is hypokalemia which may require oral administration of potassium supplements or potassium-sparing diuretics that reduce urinary K+ excretion.36,38,39
The aqueous extract induced both water and electrolyte excretion via the non-osmotic mechanism. Osmotic activity is excluded since there was no detectable level of electrolyte in the aqueous extract. So, the aqueous extract possibly exerted a diuretic effect by inhibiting tubular reabsorption of water and electrolytes as such action has been suggested for some other plants.40
The ratio of Cl−/[Na+ + K+] is used to estimate the carbonic anhydrase inhibitory activity of the extract. The values between 1.0 and 0.8 can exclude carbonic anhydrase inhibition. With a decreasing ratio, enzyme inhibitory activity can be assumed.33 The aqueous extract had carbonic anhydrase inhibitory indices of 0.62 and 0.58, at the doses of AQ200 and AQ400, respectively (Table 2). Thus, this study indicates that the aqueous extract might have an inhibitory action on carbonic anhydrase enzyme in the renal tubules. This can be evidenced by the notion that a significantly increased urinary pH was also observed in the aqueous extract treated rats (Figure 3).
The active principle(s) responsible for the diuretic and natriuretic activities of the aqueous extract of Podocarpus falcactus is/are, so far, not known. Qualitative phytochemical screening of the aqueous extract revealed the presence of alkaloids, flavonoids, polyphenols, and tannins (Table 3). It is reasonable to suggest that these secondary metabolites may act individually or synergistically to produce the observed diuretic and natriuretic activities of Podocarpus falcactus.
Phytochemical screening of the aqueous extract of Podocarpus falcactus showed the presence of active phytochemical groups such as polyphenols, flavonoids, alkaloids, and tannins. Previous studies revealed that these phytochemicals had diuretic and natriuretic activities via several mechanisms. For example, alkaloids inhibit carbonic anhydrase,41 stimulate renal blood flow through vasodilation of renal afferent arteries, and possibly inhibit tubular reabsorption of water and electrolytes.42 On the other hand flavonoids and phenolics also inhibit carbonic anhydrase enzyme in the renal tubule.43–45 They increase diuresis because they inhibit angiotensin-converting enzyme (ACE),46,47 increase the bioavailability of bradykinin, prostacyclin, and nitric oxide, or exert an inhibitory effect on Na+/K+-ATPase.47,48 Adenosine A1 receptor antagonists can induce diuresis and Na+ excretion by direct inhibition of Na+ re-absorption in proximal tubules, or indirectly by promoting afferent arteriole dilation.49,50 Flavonoids are one of the natural antagonist ligands for A1 adenosine receptors, while antagonistic activity to the receptor is known to associate with diuretic activity.51 The adenosine A1 receptors are responsible for the reabsorption of 60–70% of filtered sodium and water in the PCT.50 So, adenosine A1 receptor antagonists maintain glomerular filtration rate via vasodilation of renal afferent arteries, stimulate renal blood flow, and cause natriuresis and diuresis.52 Moreover, alkaloids, flavonoids, and phenols have carbonic anhydrase inhibitory activity.41,43–45 The enzyme carbonic anhydrase has a role in the regulation of pH and reabsorption of sodium in the PCT. As it is presented in Figure 3, the pH of urine of rats treated with the aqueous extract is alkaline. This is possibly due to the carbonic anhydrase inhibitory activity of alkaloids, flavonoids, and phenols found in the aqueous extract and it is a circumstantial evidence for the presence of carbonic anhydrase inhibitory activity with that of carbonic anhydrase inhibitory index (Table 2).
Interestingly, flavonoids have both diuretic and potassium-sparing activities.53 The plant extract has less effect on the excretion of potassium (Table 2). So, this might be another evidence for the potassium-sparing activity of the plant extract as it was found to contain flavonoids expressed as 142.23 mg QE/g of dry plant extract. Additionally, tannins are implicated in decreasing blood pressure by promoting the excretion of water and electrolytes.54,55 Collectively, shreds of evidence suggested that the plant Podocarpus falcactus has diuretic activity via several mechanisms due to the phytochemicals it contained.
To sum up, this study provides further evidence that the aqueous extract of the plant Podocarpus falcactus possessed a comparable diuretic activity with furosemide.
Results obtained from qualitative and quantitative phytochemical tests realized that alkaloids, flavonoids, phenolics, and tannins are responsible for the observed diuretic and natriuretic effects. Secondary metabolites have several mechanisms of diuresis and even they spare the wastage of potassium, which is the main side effect of conventional diuretics, especially loop and thiazide diuretics. Hence, considering the claimed mechanisms of natural diuretics, and the anticipated carbonic anhydrase inhibitory activity, the aqueous extract of Podocarpus falcactus has multiple mechanisms of action.
Conclusion
This study provides evidence for the use of Podocarpus falcactus as a diuretic agent through the enhancement of sodium and water excretion. Phenolics, flavonoids, alkaloids, and tannins act individually or in synergy via multiple mechanisms to produce the observed effect, though the specific compound is not yet determined. The maximum dose of the aqueous extract of Podocarpus falcactus produced a remarkable diuresis, which was comparable to furosemide. From the data of electrolyte analysis, urinary pH, and total phytochemical content, it is plausible to assume that the plant could have multiple modes of action.
Data Sharing Statement
All the necessary data and materials can be obtained from the corresponding author with a reasonable request.
Ethics Approval
The study was approved by the Ethical Review Board of the College of Medicine and Health Sciences of Wollo University.
Author Contributions
All authors made a significant contribution to the conception and design of the study, execution, acquisition, analysis, and interpretation of data. Besides, they took part in drafting, revising, or critically reviewing the article, gave final approval of the version to be published, have agreed on the journal to which the article has been submitted, and agree to be accountable for all aspects of the work. The authors have consented to the publication of this manuscript.
Funding
No funding was received for this work.
Disclosure
The authors report no conflicts of interest for this work.
References
1. Abdillahi H, Stafford G, Finnie J, Van Staden J. Ethnobotany, phytochemistry and pharmacology of Podocarpus sensu latissimo (sl). S Afr J Bot. 2010;76(1):1–24. doi:10.1016/j.sajb.2009.09.002
2. Meresa A, Gemechu W, Basha H, et al. Herbal medicines for the management of diabetic mellitus in Ethiopia and Eretria including their phytochemical constituents. AJADD. 2017;5(01):040–58.
3. Assefa A, Abate D, Stenlid J. Corynelia uberata as a threat to regeneration of Podocarpus falcatus in Ethiopian forests: spatial pattern and temporal progress of the disease and germination studies. Plant Pathol. 2015;64(3):617–626.
4. Martin A. South African palynological studies. I: statistical and morphological variation in the pollen of the South African species of podocarpus. Grana. 1959;2(1):40–68.
5. Geldenhuys C. Reproductive biology and population structures of Podocarpus falcatus and P. latifolius in southern Cape forests. Bot J Linn Soc. 1993;112(1):59–74. doi:10.1006/bojl.1993.1041
6. Gure A, Wahlström K, Stenlid J. Pathogenicity of seed‐associated fungi to Podocarpus falcatus in vitro. Forest Pathol. 2005;35(1):23–35. doi:10.1111/j.1439-0329.2004.00387.x
7. d’Avigdor E, Wohlmuth H, Asfaw Z, Awas T. The current status of knowledge of herbal medicine and medicinal plants in Fiche, Ethiopia. J Ethnobiol Ethnomed. 2014;10(1):38. doi:10.1186/1746-4269-10-38
8. Regassa R. Diversity and conservation status of some economically valued indigenous medicinal plants in Hawassa college of teacher education campus, Southern Ethiopia. Int J Adv Res. 2013;1(3):308–328.
9. Abdillahi HS, Verschaeve L, Finnie JF, Van Staden J. Mutagenicity, antimutagenicity and cytotoxicity evaluation of South African podocarpus species. J Ethnopharmacol. 2012;139(3):728–738. doi:10.1016/j.jep.2011.11.044
10. Teklehaymanot T, Giday M. Ethnobotanical study of medicinal plants used by people in Zegie Peninsula, Northwestern Ethiopia. J Ethnobiol Ethnomed. 2007;3(1):12. doi:10.1186/1746-4269-3-12
11. El-Sayed AS, Safan S, Mohamed NZ, Shaban L, Ali GS, Sitohy MZ. Induction of taxol biosynthesis by aspergillus terreus, endophyte of Podocarpus gracilior pilger, upon intimate interaction with the plant endogenous microbes. Process Biochem. 2018;71:31–40. doi:10.1016/j.procbio.2018.04.020
12. Abdillahi H, Stafford G, Finnie J, Van Staden J. Antimicrobial activity of South African podocarpus species. J Ethnopharmacol. 2008;119(1):191–194. doi:10.1016/j.jep.2008.06.023
13. Addo EM, Chai H-B, Hymete A, et al. Antiproliferative constituents of the roots of Ethiopian Podocarpus falcatus and structure revision of 2α-hydroxynagilactone F and nagilactone I. J Nat Prod. 2015;78(4):827–835. doi:10.1021/np501062f
14. Liu J, Yang C, Zhang J, Wu J, Chen Y. A new 5 (6→ 7) abeo-sterol from the twigs of. Nat Prod Res. 2017;31(2):175–180. doi:10.1080/14786419.2016.1224870
15. Desai S, Tatke P. Isolation and analytical method development of flavonol glycoside, quercetin-3-o-β-D-glucoside: a review. J Nat Remedies. 2016;15(2):77–85. doi:10.18311/jnr/2015/482
16. Isah T. Natural sources of taxol. Int J Pharm Sci Res. 2015;214–227.
17. Guideline OO. 425: Acute oral toxicity—up-and-down procedure. OECD Guidelines Test Chem. 2001;2:12–16.
18. Hailu W, Engidawork E. Evaluation of the diuretic activity of the aqueous and 80% methanol extracts of Ajuga remota Benth (Lamiaceae) leaves in mice. BMC Complement Altern Med. 2014;14(1):135. doi:10.1186/1472-6882-14-135
19. Geleta B, Eyasu M, Fekadu N, Debella A, Challa F. Evaluation of diuretic activity of hydro-ethanolic extract of Moringa stenopetala leaves in Swiss albino mice. Clin Exp Pharmacol. 2015;5(190):2161.
20. María R, Shirley M, Xavier C, et al. Preliminary phytochemical screening, total phenolic content and antibacterial activity of thirteen native species from Guayas province Ecuador. J King Saud Univ Sci. 2018;30(4):500–505. doi:10.1016/j.jksus.2017.03.009
21. Tiwari P, Kumar B, Kaur M, Kaur G, Kaur H. Phytochemical screening and extraction: a review. Int Pharm Sci. 2011;1(1):98–106.
22. Rohini MV, Padmini E. Preliminary phytochemical screening of selected medicinal plants of polyherbal formulation. J Pharmacognosy Phytother. 2016;5(5):277.
23. Hossain MA, AL-Raqmi KAS, AL-Mijizy ZH, Weli AM, Al-Riyami Q. Study of total phenol, flavonoids contents and phytochemical screening of various leaves crude extracts of locally grown Thymus vulgaris. Asian Pac J Trop Biomed. 2013;3(9):705–710. doi:10.1016/S2221-1691(13)60142-2
24. Gul R, Jan SU, Faridullah S, Sherani S, Jahan N. Preliminary phytochemical screening, quantitative analysis of alkaloids, and antioxidant activity of crude plant extracts from Ephedra intermedia indigenous to Balochistan. ScientificWorldJournal. 2017;2017.
25. Tabasum S, Khare S, Jain K. Spectrophotometric quantification of total phenolic, flavonoid, and alkaloid contents of Abrus precatorius L. seeds. Asian J Pharm Clin Res. 2016;9(2):371–374.
26. Al-Matani SK, Al-Wahaibi RNS, Hossain MA. Total flavonoids content and antimicrobial activity of crude extract from leaves of Ficus sycomorus native to Sultanate of Oman. Karbala Int J Modern Sci. 2015;1(3):166–171. doi:10.1016/j.kijoms.2015.11.007
27. Ainsworth EA, Gillespie KM. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. Nat Protoc. 2007;2(4):875–877. doi:10.1038/nprot.2007.102
28. Adebiyi OE, Olayemi FO, Ning-Hua T, Guang-Zhi Z. In vitro antioxidant activity, total phenolic and flavonoid contents of ethanol extract of stem and leaf of Grewia carpinifolia. Beni Suef Univ J Basic Appl Sci. 2017;6(1):10–14. doi:10.1016/j.bjbas.2016.12.003
29. Kavitha Chandran CI, Indira G. Quantitative estimation of total phenolic, flavonoids, tannin and chlorophyll content of leaves of Strobilanthes Kunthiana (Neelakurinji). J Med Plant. 2016;4:282–286.
30. Vogel HG, Vogel WH. Drug Discovery and Evaluation: Pharmacological Assays. Springer Science & Business Media; 2013:1324–1326.
31. National Research Council. Guide for the care and use of laboratory animals. National Academies Press; 2010. Available from: https://grants.nih.gov/grants/.../guide-for-the-care-and-use-oflaboratory-animals.pdf.
32. Al Disi SS, Anwar MA, Eid AH. Anti-hypertensive herbs and their mechanisms of action: part I. Front Pharmacol. 2016;6:323.
33. Hock FJ. Drug Discovery and Evaluation: Pharmacological Assays. Springer; 2016:837–841.
34. Sam R, Ives HE, Pearce D. Diuretic Agents: In Basic and Clinical Pharmacology. Mc Graw Hill; 2018:254–274.
35. Cadwallader AB, De La Torre X, Tieri A, Botrè F. The abuse of diuretics as performance‐enhancing drugs and masking agents in sport doping: pharmacology, toxicology and analysis. Br J Pharmacol. 2010;161(1):1–16. doi:10.1111/j.1476-5381.2010.00789.x
36. Jackson EK. Drugs Affecting Renal Excretory Function: In Goodman & Gilman’s the Pharmacological Basis of Therapeutics. New York: McGraw Hill; 2018:445–470.
37. Hullatti K, Manjunatha J, Kuppasth I. Comparative study on diuretic effect of Buchanania angustifolia Roxb., and Buchanania lanzan Spreng. fruit extracts and fractions. J Appl Pharm Sci. 2014;4(8):59.
38. Mishra S, Sharma SK, Yadav J, Kasana B. A review on †œhow exactly diuretic drugs are working in our bodyâ€. J Drug Deliv Ther. 2013;3(5):115–120. doi:10.22270/jddt.v3i5.594
39. Smith H. Diuretics: a review for the pharmacist. SA Pharm J. 2014;81(7):18–21.
40. Prabhu MN, Pai PG, Kumar SV, et al. Diuretic activity of aqueous ethanolic extract of leaves of Costus speciosusin normal Wistar albino rats. Res J Pharm Biol Chem Sci. 2014;5(3):127–132.
41. Karioti A, Carta F, Supuran T. An update on natural products with carbonic anhydrase inhibitory activity. Curr Pharm Des. 2016;22(12):1570–1591. doi:10.2174/1381612822666151211094235
42. Melendez-Camargo ME, Contreras-León I, Silva-Torres R. Diuretic effect of alkaloids fraction extracted from Selaginella lepidophylla (Hook. et Grev.) spring. Bol Latinoam Caribe Plantas Med Aromát. 2014;13(1):92–99.
43. Aditama R, Mujahidin D, Syah YM, Hertadi R. Docking and molecular dynamics simulation of carbonic anhydrase II inhibitors from phenolic and flavonoid group. Procedia Chem. 2015;16:357–364. doi:10.1016/j.proche.2015.12.064
44. Ekinci D, Karagoz L, Ekinci D, Senturk M, Supuran CT. Carbonic anhydrase inhibitors: in vitro inhibition of α isoforms (hCA I, hCA II, bCA III, hCA IV) by flavonoids. J Enzyme Inhib Med Chem. 2013;28(2):283–288. doi:10.3109/14756366.2011.643303
45. Huyut Z, Beydemir Ş, Gülçin İ. Inhibitory effects of some phenolic compounds on the activities of carbonic anhydrase: from in vivo to ex vivo. J Enzyme Inhib Med Chem. 2016;31(6):1234–1240. doi:10.3109/14756366.2015.1117459
46. Ghosh D, Scheepens A. Vascular action of polyphenols. Mol Nutr Food Res. 2009;53(3):322–331.
47. Perez-Vizcaino F, Duarte J, Jimenez R, Santos-Buelga C, Osuna A. Antihypertensive effects of the flavonoid quercetin. Pharmacol Rep. 2009;61(1):67–75. doi:10.1016/S1734-1140(09)70008-8
48. Junior AG, Prando TBL, Leme T, et al. Mechanisms underlying the diuretic effects of Tropaeolum majus L. extracts and its main component isoquercitrin. J Ethnopharmacol. 2012;141(1):501–509. doi:10.1016/j.jep.2012.03.018
49. Chen J-F, Eltzschig HK, Fredholm BB. Adenosine receptors as drug targets—what are the challenges? Nat Rev Drug Discov. 2013;12(4):265–286.
50. Welch WJ. Adenosine, type 1 receptors: role in proximal tubule Na+ reabsorption. Acta Physiol. 2015;213(1):242–248. doi:10.1111/apha.12413
51. Jacobson KA, Moro S, Manthey JA, West PL. Interactions of flavones and other phytochemicals with adenosine receptors. Adv Exp Med Biol. 2002;505:163–171.
52. Modlinger PS, Welch WJ. Adenosine A1 receptor antagonists and the kidney. Curr Opin Nephrol Hypertens. 2003;12(5):497–502. doi:10.1097/00041552-200309000-00003
53. Junior AG, Gasparotto FM, Boffo MA, et al. Diuretic and potassium-sparing effect of isoquercitrin—an active flavonoid of tropaeolum majus L. J Ethnopharmacol. 2011;134(2):210–215. doi:10.1016/j.jep.2010.12.009
54. Coşan DT, Saydam F, Özbayer C, et al. Impact of tannic acid on blood pressure, oxidative stress and urinary parameters in L-NNA-induced hypertensive rats. Cytotechnology. 2015;67(1):97–105. doi:10.1007/s10616-013-9661-4
55. Yokozawa T, Oura H, Sakanaka S, Ishigaki S, Kim M. Depressor effect of tannin in green tea on rats with renal hypertension. Biosci Biotechnol Biochem. 1994;58(5):855–858. doi:10.1271/bbb.58.855
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.