Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 12
Evaluation of carbon dioxide rebreathing during exercise assisted by noninvasive ventilation with plateau exhalation valve
Authors Ou YE , Lin ZM, Hua DM, Jiang Y, Huo YT, Luo Q, Chen RC
Received 6 September 2016
Accepted for publication 21 December 2016
Published 16 January 2017 Volume 2017:12 Pages 291—298
DOI https://doi.org/10.2147/COPD.S121637
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Richard Russell
Yong-er Ou,* Zhi-min Lin,* Dong-ming Hua, Ying Jiang, Ya-ting Huo, Qun Luo, Rong-Chang Chen
State Key Laboratory of Respiratory Disease, National Clinical Research Center for Respiratory Disease, Guangzhou Institute of Respiratory Disease, First Affiliated Hospital of Guangzhou Medical University, Guangzhou, People’s Republic of China
*These authors contributed equally to this work
Abstract: Noninvasive ventilation with a plateau exhalation valve (PEV) is often used as an adjunct to exercise to achieve a physiologic training effect in severe chronic obstructive pulmonary disease (COPD) patients. However, during exercise, with the increase of exhalation flow and respiratory rate and limited capability of PEV to exhale gases out of the circuit, it is still unknown whether CO2 rebreathing occurs in COPD patients ventilated during exercise assisted by single-limb circuit with a PEV. A maximal symptom-limited cycle exercise test was performed while ventilated on pressure support (inspiratory:expiratory pressure 14:4 cmH2O) in 18 male patients with stable severe COPD (mean ± standard deviation, forced expiratory volume in 1 s: 29.5%±6.9% predicted). At rest and during exercise, breathing pattern, mean expiratory flow, mean expiratory flow of PEV, and the mean inspiratory fraction of CO2 (tidal fractional concentration of inspired CO2 [FiCO2]) reinsufflated from the circuit was measured for each breath. In comparison with rest, with the significant increase of mean expiratory flow (0.39±0.15 vs 0.82±0.27 L/s), fractional concentration of end-tidal CO2 (2.6%±0.7% vs 5.5%±0.6%), and the significant decrease of mean expiratory flow of PEV (0.41±0.02 vs 0.39±0.03 L/s), tidal FiCO2 significantly increased at peak exercise (0.48%±0.19% vs 1.8%±0.6%) in patients with stable severe COPD. The inflection point of obvious CO2 rebreathing was 0.67±0.09 L/s (95% confidence interval 0.60–0.73 L/s). Ventilated by a single-limb tubing with PEV caused CO2 rebreathing to COPD patients during exercise. Patients with mean expiratory flow >0.60–0.73 L/s may be predisposed to a higher risk of CO2 rebreathing.
Keywords: carbon dioxide rebreathing, noninvasive ventilation, exercise, chronic obstructive pulmonary disease, single-limb circuit, pulmonary rehabilitation
Introduction
Exercise training is a key component of pulmonary rehabilitation. It has demonstrated significant improvements in both exercise tolerance and quality of life in patients with chronic obstructive pulmonary disease (COPD).1 The intensity of exercise training is of great importance to yield a true physiologic effect. However, in patients with severe COPD, exertional dyspnea and leg fatigue make it impossible for the patient to maintain intensity of training for enough time to achieve a physiologic training effect.2 Noninvasive ventilation (NIV) has been reported to be used as support for exercise to improve exercise tolerance and respiratory performances in patients with mild-to-severe COPD with inconsistent results.3–20 In these studies, one of the obvious methodological issues existed with respect to the selection of exhalation valve connected to the single-limb circuit. Evidence from Moga et al19 and Highcock et al20 indicated that NIV with a single-limb circuit with Whisper Swivel II expiratory valve (Respironics Inc., Murrysville, PA, USA) assisting exercise did not improve exercise capacity in COPD patients. Also, as previously reported by Ferguson and Gilmartin,21 the use of Whisper Swivel II expiratory valve during bi-level positive airway pressure (BiPAP) ventilatory assistance causes CO2 rebreathing, which can blunt any effect of BiPAP on partial pressure of CO2 (PaCO2). It could not be dismissed that CO2 rebreathing occurred with the use of the standard exhalation port thus diminishing the efficacy of NIV support. Plateau exhalation valve (PEV) or the Sanders NRV-2 plateau valve (NRV; Respironics Inc.) have been shown to be more effective in eliminating CO2 rebreathing in patients at rest,21 however, the latter one prevented CO2 rebreathing at the expense of increased expiratory resistance and work of breathing22 and sometimes it can potentially malfunction; considering these factors, the former one has been widely used.
With the use of the PEV in the circuit, the risk of CO2 rebreathing is presumably low when the expiratory time (Te) is long enough to ensure that evacuation of the circuit is complete. However, our group have previously demonstrated that the mean leak flow of the valve at low pressure (expiratory phase) was 0.43 L/s at rest.23 During exercise, the increase of expiratory flow due to the high levels of ventilatory requirements, and elevated breathing frequency of patients with COPD, may promote the occurrence of CO2 rebreathing. As CO2 rebreathing increases the drive to ventilate and the work of breathing22,24,25 and may have a negative impact on efficacy,26,27 it is important to clarify this risk.
Therefore, the aim of the present study was to evaluate whether CO2 rebreathing occurred in COPD patients ventilated during exercise by the single-limb circuit with a PEV and to estimate a potential threshold of expiratory flow for predicting CO2 rebreathing.
Methods
Study participants
Eighteen COPD patients were recruited from the first affiliated hospital of Guangzhou Medical University from January 2016 to March 2016. The diagnosis of COPD was confirmed by physician’s diagnosis and spirometry. Patients who presented with clinical stability (no exacerbation in the previous 4 weeks and with no change in medications, in the absence of right heart decompensation signs), forced expiratory volume in 1 s (FEV1)<50% predicted, dyspnea as a main symptom that limited daily activities, were included in the study. Excluded criteria: individuals with obvious pulmonary bullae or facial trauma/malformation; cardiovascular disease; a history of uncontrolled hypertension; other respiratory diseases; oxygen saturation (SpO2)<88% at rest; patients with musculoskeletal or neurologic disorders. Ethical approval for this study was obtained from the research ethics committee of the First Affiliated Hospital of Guangzhou Medical University, and all participants gave their written informed consent in accordance with the Helsinki Declaration.
Study design
Patients completed screening tests to determine eligibility for the study. This study comprised of two visits: 1) Visit 1 included a thorough clinical assessment, pulmonary function tests, baseline arterial blood gas sampling, getting used to the application of the ventilator, and completing a maximal symptom-limited-incremental cardiopulmonary exercise test; 2) Visit 2 included a maximal cycle exercise test with the participants assisted by BiPAP (Vision; Respironics Inc.) receiving 10 cmH2O pressure support in addition to oxygen therapy. At rest and during the whole exercise process, breathing pattern, heart rate (HR), flow, pressure, CO2 concentration, and SpO2 were recorded until complete recovery. Before each visit, subjects kept on taking regular medicine and abstained from caffeine, heavy meals, alcohol, and major physical exertion entirely on visit days.
Pulmonary function tests
Pulmonary function tests were performed using a Quark PFT (pulmonary function testing) system (Cosmed, Rome, Italy). FEV1 and forced vital capacity (FVC) were determined from the best of three maneuvers if the variance was within 150 mL or 5% of the FEV1 or FVC, respectively.28 Measurements were expressed as percentages of predicted normal values.29–31
Oxygen delivery and ventilator setting
Oxygen was delivered to the face mask by a tube at a constant rate (5 L/min). Ventilatory assistance was delivered using a BiPAP Vision with a PEV in BiPAP spontaneous/time mode applied via a tightly fitting full face mask (Curative, Beijing, People’s Republic of China). Inspiratory positive airway pressure (IPAP) was set at 14 cmH2O, and the expiratory positive airway pressure (EPAP) was set at 4 cmH2O and a backup respiratory frequency was set at 12 breaths/min.
Maximal cycle exercise test and intervention procedure
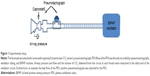
Maximal symptom-limited-incremental cycle exercise tests were conducted on an electronically braked cycle ergometer (Ergoselect 200 K; Cosmed, Rome, Italy). Symptom-limited cycle exercise test consisted of a steady-state resting period of at least 3 min followed by 1 min of unloaded pedaling at 60–65 cycles/min; the load was increased by 10 W each minute, starting at 10 W until a symptom-limited end-point. At rest and during exercise, participants breathed through the facemask attached in series with capnostat5 (Phillips Respironics, Murrysville, PA, USA), pneumotachograph (3830A series; Hans-Rudolph Inc., Shawnee Mission, KS, USA), PEV (flow of the PEV was directly recorded by a pneumotachograph [ADInstruments Inc., Sydney, Australia]), ventilator tubing, and BiPAP ventilator (Figure 1). Mouth pressure was determined using differential pressure transducers (YH; Ying Hui Medical Devices Co. Ltd., Guangzhou, People’s Republic of China) connected to pressure catheters attached to a mask side port. Capnostat5 attached to the Powerlab via NT1D Handheld CO2/SpO2 monitor (Newtech Inc., Shenzhen, People’s Republic of China). The delay representing transit time from the sample point to the analyzer was measured beforehand. Signals of pressure, flow, and CO2 were recorded at a frequency of 200, 200, and 100 Hz, respectively. Signals were recorded continuously using an analogic/numeric Powerlab data acquisition system (ML796; ADInstruments Inc.) running on an iMac computer (Apple Computer Inc., Cupertino, CA, USA). The flow signal was integrated to yield the volume.
Analyses were made breath by breath, after phasing CO2 curves with flow and pressure. For each breath, inspiratory time (Ti), Te, respiratory rate (RR), inspiratory tidal volume (Vti), expiratory tidal volume (Vte), mean expiratory flow (Vte/Te), mean flow vented out from the PEV during expiration (Ex/Te), fractional concentration of inspired CO2 (FiCO2), and fractional concentration of end-tidal CO2 (FetCO2) were measured. The volume of CO2 delivered from the circuit at each breath was calculated by integrating the CO2 flow curve, defined as the product of inspiratory flow and CO2 concentration curves.32 Mean inspiratory fraction of CO2 for each tidal volume (tidal FiCO2), expressed as a percentage, was defined as: inspired volume of CO2/Vti*100.
Using Origin Pro 8 software to draw a scatter plot to determine the relationship between Vte/Te (x values) and the tidal FiCO2 (y values), apply a nonlinear curve fit to the scatter plot, if the nonlinear curve fitness was good (adjusted R2≥0.8, P<0.05), the presence of an inflection point was next determined by assuming that the Vte/Te-tidal FiCO2 curve was made up of two straight lines and that the inflection point represented the point where these lines intersected, to find out the obvious inflection point when CO2 rebreathing appeared. The curve was divided into two parts (A&B) at an arbitrary point, Vk, and a pair of linear regressions were performed using the data above and below this point. This yielded two regression lines, line A and line B, with correlation coefficients, rA and rB, respectively. The process was repeated many times varying the dividing point Vk so that the point where rA*rB was maximal could be determined.
Fingertip SpO2 was measured by pulse oximetry; HR was measured by six-lead electrocardiogram; blood pressure was measured by automatic blood pressure measurement with an arm cuff during the whole process.
Statistical analysis
Data were expressed as mean ± standard deviation after testing for normal distribution (Kolmogorov–Smirnov test) unless otherwise specified. The 95% confidence interval (95% CI) was determined if appropriate. All data were analyzed using paired t-test. Differences were considered significant when P<0.05.
Results
In total, 18 patients with stable severe COPD completed the tests; demographic data of the 18 patients are shown in Table 1. Only one COPD patient had chronic hypercapnic respiratory failure.
As can be seen from Table 2, while ventilated on pressure support (inspiratory:expiratory pressure 14:4 cmH2O) assisted by single-limb circuit with a PEV, in comparison with rest, at peak exercise, with the increase of expiratory tidal volume, elevated breathing frequency, Vte/Te and FetCO2, and the decrease of Ex/Te and Te, tidal FiCO2 significantly increased (all P<0.01).
During expiration, with the increase of Vte/Te, the mean flow vented out from PEV varied from 0.41 to 0.39 L/s throughout exercise in COPD patients (Table 2; Figure 2).
Throughout rest and exercise, tidal FiCO2 varied with Vte/Te in all subjects. In the majority of COPD patients, tidal FiCO2 increased with increasing Vte/Te. After using Origin Pro 8 software to draw a scatter plot to determine the relationship between the Vte/Te (x values) and the tidal FiCO2 (y values), applying a nonlinear curve fit to the scatter plot (Figure 3A), the curve fitness was good (adjusted R2>0.80, P<0.05) in 15 subjects. However, three of the COPD patients with their FEV1 of 0.49, 0.52, and 0.61 L failed the curve fitness (adjusted R2<0.05; Figure 3B). The inflection point of obvious CO2 rebreathing was 0.67±0.09 L/s (95% CI: 0.60–0.73 L/s; Figure 4).
Discussion
Exercise training is a key component of pulmonary rehabilitation. In patients with severe COPD, exertional dyspnea makes it impossible for the patient to maintain intensity of training for enough time to achieve a physiologic training effect.2 NIV has been reported to be used as support for exercise to improve exercise tolerance and respiratory performances in patients with mild-to-severe COPD, with inconsistent results.3–20 In these studies, one of the obvious methodological issues existed with respect to the selection of exhalation valve connected to the single-limb circuit. PEV or NRV have been shown to be more effective in eliminating CO2 rebreathing in patients at rest,21 however, because NRV has the disadvantages of increased expiratory resistance and work of breathing22 and potential malfunction, PEV has been widely used. In the single-limb tubing for inspiration and expiration, PEV serves to eliminate CO2 from the breathing circuit and is an important part for decreasing dead space ventilation and improving ventilation efficiency. Former studies have found that leak flow rates of PEV remained nearly constant throughout the specified pressure range.23 With the increasing minute ventilation during exercise, NIV with a PEV might cause CO2 rebreathing. As far as we know through the search of Medline, there has been no report on evaluating CO2 rebreathing in COPD patients assisted by NIV in BiPAP mode with a PEV on single-limb circuit during maximal cycle exercise test.
The main findings of this study are as follows: 1) during exercise, with the increasing total expiratory flow of the subject, the flow vented out of PEV decreased (Figure 2); 2) compared to at rest, tidal FiCO2 increased significantly at peak exercise in COPD patients (P<0.01), indicating CO2 rebreathing occurred; 3) ventilated by a single-limb tubing with PEV during exercise, patients with Vte/Te >0.67±0.09 L/s (95% CI: 0.60–0.73 L/s) may be predisposed to a higher risk of CO2 rebreathing.
With regard to patients with severe COPD ventilated by a single-limb tubing during exercise, obvious methodological issues existed and they resulted in inconsistent outcomes for relieving dyspnea or improving exercise tolerance. Different exhalation valves and different levels of pressure support have been reported. In previous studies, investigating the effects of a single-limb circuit with a Silentflow Exhalation valve (Weinmann, Hamburg, Germany) to deliver ventilatory assistance (IPAP: 29.5±4.1 cmH2O, EPAP: 4.38±0.82 cmH2O) during exercise in COPD with hypercapnia,16,17 there were controversial outcomes on the alleviation of dyspnea. Moreover, in studies by Moga et al19 and Highcock et al,20 they found that BiPAP with Whisper Swivel II did not improve exercise capacity. These findings, in combination with the result found by Ferguson and Gilmartin21 that significant CO2 rebreathing could occur at low expiratory pressure levels (≤4 cmH2O) on single-limb circuit with Whisper Swivel Valve or other fixed-resistance exhalation devices, suggested that CO2 may not be adequately cleared with the fixed-resistance exhalation valve both at rest and during exercise and that this blunted the efficacy of NIV. Former study has implied that such CO2 rebreathing and dead space ventilation could be effectively eliminated by the use of NRV or the PEV.21 However, NRV eliminated CO2 at the expense of increasing expiratory resistance and work of breathing,22 and sometimes it can potentially malfunction; considering these factors, PEV has been the most widely used. NIV with single-limb circuit in BiPAP mode is often used,11–17 pressure support (PS) setting of 10 cmH2O has been proven to be effective to reduce dyspnea in COPD patients,4,5,18 and based on the mechanism of action of PEV, leak flow rates were greater at around 4–5 cmH2O, therefore, single-limb tubing with PEV in BiPAP mode with PS setting of 10 cmH2O (IPAP:EPAP 14:4 cmH2O) was used in this study.
As to the measurement of CO2 rebreathing, some research indicated a preference for measurement of CO2 partial pressure in an arterial blood gas sample (PaCO2),21,22 whereas others argued that the value of CO2 inhaled (FiCO2)33–35 offered more accurate and beneficial information. In fact, these values could be regarded as interdependent values. According to our point of view, FiCO2 is the most representative value in rebreathing as it shows the CO2 amount in the patient’s mask at the proto-inspiratory phase. It was shown that during exercise, with the increase of expiratory tidal volume, elevated breathing frequency, and Vte/Te, and the decrease of Ex/Te, tidal FiCO2 significantly increased (Table 2), indicating that CO2 rebreathing occurred during exercise in this setting, which needs to be paid attention to.
Understanding the mechanisms related to the occurrence of CO2 rebreathing is important. CO2 rebreathing consists of rebreathing previously expired gas by the patient if such gas is not appropriately eliminated from the circuit during the ventilatory cycle, as a result of an accumulation of this gas in the circuit. CO2 rebreathing takes place mainly in single-limb circuits. Besides, the type of mask and expiratory port,23,33,34,36–38 and the level of EPAP used,21,34 end-tidal CO2 concentration (EtCO2), RR, tidal volume (Vt), were also the factors influencing CO2 rebreathing.35 However, the increasing level of EPAP was not as important as fixed-resistance exhalation devices since the leak flow rate of PEV was greater at ~4–5 cmH2O. During exercise, with the use of single-limb circuit with a PEV and nonvented mask, with the increasing Vt, RR, and EtCO2, these could predispose patients to CO2 rebreathing.
However, in this study, three of the COPD patients with their FEV1 of 0.49, 0.52, and 0.61 L failed the curve fitness (adjusted R2<0.05; Figure 3B), suggesting that there was no CO2 rebreathing. As their FEV1 (the maximum amount of air a person can expel in 1 s of forceful exhalation) were lower or within the inflection point (0.60–0.73 L/s), this in some extent supports our results. To ensure that no exhaled gas remains in the tubing at the end of expiration, the Vte/Te has to be lower than the intentional leak obtained through PEV (0.39–0.41 L/s) during expiration. Therefore, in some pulmonary rehabilitation programs, with the setting of pressure support similar to our study, with the increasing Vte/Te, it may not be suitable for patients whose FEV1 exceeds 0.73 L to be included; it might be indicated as a reference value for patient inclusion in pulmonary rehabilitation programs with similar study setting.
This study has some limitations which need to be addressed. On the one hand, a limitation of this study is its small number of study patients. However, in this study, we have compared a large number of individual breaths for each respective subject. The comparison of individual breaths, rather than for example patients, is appropriate since the amount of rebreathing may depend on numerous factors, such as Vt, and timing of the respiratory cycle (RR and inspiratory duty cycle). Most of these exhibited large variations (in the same patient) on a breath-by-breath basis during spontaneous breathing with pressure support.
On the other hand, a nonlinear curve fit was applied to the scatter plot, CO2 rebreathing inflection point was determined based on the nonlinear curve fit, and it was an indirect and rough estimation. R2 is a number that indicates how well data fits a curve. In practice, an adjusted R2 was usually close to 1 if the curve perfectly fitted the data and close to 0 when the curve did not fit the data at all, so the choice of 0.8 of R2 was not critical. However, with the increase of ventilatory requirement, expiratory tidal volume, elevated breathing frequency and Vte/Te, tidal FiCO2 increased significantly, indicating that obvious CO2 rebreathing existed in the single-limb circuit.
Since CO2 rebreathing increased respiratory motor output, ventilation and work of breathing, reducing the efficiency of NIV to assist exercise,24,25 the current authors would suggest that the present findings would help design future studies on NIV-assisted exercise in COPD patients. First, our group previously found that CO2 rebreathing could be minimized in COPD patients treated with NIV with the path of exhalation connected to the side hole on the mask at rest. In order to avoid CO2 rebreathing, maybe such a setup modification could be used during exercise.23 Second, an NRV applied on the single-limb during exercise will also be worth testing in future study. Third, use of double-limb ventilatory circuit other than the PEV to prevent CO2 rebreathing should be considered during BiPAP ventilatory assistance.
Conclusion
Our results indicated that ventilatory assistance delivered in BiPAP mode with PS setting of 10 cmH2O (IPAP:EPAP 14:4 cmH2O) with single-limb tubing with PEV during exercise could produce significant CO2 rebreathing. CO2 rebreathing should be paid attention to in the pulmonary rehabilitation exercise programs that deliver bi-level pressure support ventilation with a single-limb circuit with PEV.
Acknowledgments
The authors thank colleagues from the pulmonary function test room (Division of Respiratory Diseases Department, Guangzhou Institute of Respiratory Disease, First Affiliated Hospital of Guangzhou Medical University, People’s Republic of China) for their collaboration, and Doctors Yi Gao, Wei-jie Guan, and Li-ping Zhong for technical support.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure
The authors report no conflicts of interest in this work.
References
Spruit MA, Singh SJ, Garvey C, et al. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188(8):e13–e64. | ||
Maltais F, LeBlanc P, Jobin J, et al. Intensity of training and physiologic adaptation in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1997;155(2):555–561. | ||
van’t Hul A, Kwakkel G, Gosselink R. The acute effects of noninvasive ventilatory support during exercise on exercise endurance and dyspnea in patients with chronic obstructive pulmonary disease: a systematic review. J Cardiopulm Rehabil. 2002;22(4):290–297. | ||
van’t Hul A, Gosselink R, Hollander P, Postmus P, Kwakkel G. Acute effects of inspiratory pressure support during exercise in patients with COPD. Eur Respir J. 2004;23(1):34–40. | ||
van’t Hul A, Gosselink R, Hollander P, Postmus P, Kwakkel G. Training with inspiratory pressure support in patients with severe COPD. Eur Respir J. 2006;27(1):65–72. | ||
Corner E, Garrod R. Does the addition of non-invasive ventilation during pulmonary rehabilitation in patients with chronic obstructive pulmonary disease augment patient outcome in exercise tolerance? a literature review. Physiother Res Int. 2010;15(1):5–15. | ||
Nickol AH, Hart N, Hopkinson NS, et al. Mechanisms of improvement of respiratory failure in patients with COPD treated with NIV. Int J Chron Obstruct Pulmon Dis. 2008;3(3):453–462. | ||
Chen H, Liang BM, Xu ZB, et al. Long-term non-invasive positive pressure ventilation in severe stable chronic obstructive pulmonary disease: a meta-analysis. Chin Med J (Engl). 2011;124(23):4063–4070. | ||
Bianchi L, Foglio K, Porta R, Baiardi R, Vitacca M, Ambrosino N. Lack of additional effect of adjunct of assisted ventilation to pulmonary rehabilitation in mild COPD patients. Respir Med. 2002;96(5):359–367. | ||
Hawkins P, Johnson LC, Nikoletou D, et al. Proportional assist ventilation as an aid to exercise training in severe chronic obstructive pulmonary disease. Thorax. 2002;57(10):853–859. | ||
Johnson JE, Gavin DJ, Adams-Dramiga S. Effects of training with heliox and noninvasive positive pressure ventilation on exercise ability in patients with severe COPD. Chest. 2002;122(2):464–472. | ||
Reuveny R, Ben-Dov I, Gaides M, Reichert N. Ventilatory support during training improves training benefit in severe chronic airway obstruction. Isr Med Assoc J. 2005;7(3):151–155. | ||
Borghi-Silva A, Di Thommazo L, Pantoni CB, Mendes RG, Salvini Tde F, Costa D. Non-invasive ventilation improves peripheral oxygen saturation and reduces fatigability of quadriceps in patients with COPD. Respirology. 2009;14(4):537–544. | ||
Borghi-Silva A, Mendes RG, Toledo AC, et al. Adjuncts to physical training of patients with severe COPD: oxygen or noninvasive ventilation? Respir Care. 2010;55(7):885–894. | ||
Toledo A, Borghi-Silva A, Sampaio LM, Ribeiro KP, Baldissera V, Costa D. The impact of noninvasive ventilation during the physical training in patients with moderate-to-severe chronic obstructive pulmonary disease (COPD). Clinics (Sao Paulo). 2007;62(2):113–120. | ||
Dreher M, Storre JH, Windisch W. Noninvasive ventilation during walking in patients with severe COPD: a randomised cross-over trial. Eur Respir J. 2007;29(5):930–936. | ||
Dreher M, Doncheva E, Schwoerer A, et al. Preserving oxygenation during walking in severe chronic obstructive pulmonary disease: noninvasive ventilation versus oxygen therapy. Respiration. 2009;78(2):154–160. | ||
Keilty SE, Ponte J, Fleming TA, Moxham J. Effect of inspiratory pressure support on exercise tolerance and breathlessness in patients with severe stable chronic obstructive pulmonary disease. Thorax. 1994;49(10):990–994. | ||
Moga AM, de Marchie M, Saey D, Spahija J. Bi-level Positive Airway Pressure (BiPAP) with standard exhalation valve does not improve maximum exercise capacity in patients with COPD. COPD. 2015;12(1):46–54. | ||
Highcock MP, Shneerson JM, Smith IE. Increased ventilation with NiIPPV does not necessarily improve exercise capacity in COPD. Eur Respir J. 2003;22(1):100–105. | ||
Ferguson GT, Gilmartin M. CO2 rebreathing during BiPAP ventilatory assistance. Am J Respir Crit Care Med. 1995;151(4):1126–1135. | ||
Lofaso F, Brochard L, Touchard D, Hang T, Harf A, Isabey D. Evaluation of carbon dioxide rebreathing during pressure support ventilation with airway management system (BiPAP) devices. Chest. 1995;108(3):772–778. | ||
Chen R, Zhang X, He G. [Modification of facial mask on the dead space effect in non-invasive mask ventilation]. Zhonghua Jie He He Hu Xi Za Zhi. 2000;23(12):734–736. Chinese. | ||
Scheid P, Lofaso F, Isabey D, Harf A. Respiratory response to inhaled CO2 during positive inspiratory pressure in humans. J Appl Physiol. 1994;77(2):876–882. | ||
Georgopoulos D, Mitrouska I, Bshouty Z, Webster K, Patakas D, Younes M. Respiratory response to CO2 during pressure-support ventilation in conscious normal humans. Am J Respir Crit Care Med. 1997;156(1):146–154. | ||
Hill NS. Noninvasive ventilation. Does it work, for whom, and how? Am Rev Respir Dis. 1993;147(4):1050–1055. | ||
Brochard L. Non-invasive ventilation: practical issues. Intensive Care Med. 1993;19(8):431–432. | ||
Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. The Eur Respir J. 2005;26(2):319–338. | ||
Crapo RO, Morris AH, Clayton PD, Nixon CR. Lung volumes in healthy nonsmoking adults. Bull Eur Physiopathol Respir. 1982;18(3):419–425. | ||
Morris JF, Koski A, Temple WP, Claremont A, Thomas DR. Fifteen-year interval spirometric evaluation of the Oregon predictive equations. Chest. 1988;93(1):123–127. | ||
Briscoe WA, Dubois AB. The relationship between airway resistance, airway conductance and lung volume in subjects of different age and body size. J Clin Invest. 1958;37(9):1279–1285. | ||
Lucangelo U, Blanch L. Dead space. Intensive Care Med. 2004;30(4):576–579. | ||
Taccone P, Hess D, Caironi P, Bigatello LM. Continuous positive airway pressure delivered with a “helmet”: effects on carbon dioxide rebreathing. Crit Care Med. 2004;32(10):2090–2096. | ||
Samolski D, Calaf N, Guell R, Casan P, Anton A. Carbon dioxide rebreathing in non-invasive ventilation. Analysis of masks, expiratory ports and ventilatory modes. Monaldi Arch Chest Dis. 2008;69(3):114–118. | ||
Szkulmowski Z, Belkhouja K, Le QH, Robert D, Argaud L. Bilevel positive airway pressure ventilation: factors influencing carbon dioxide rebreathing. Intensive Care Med. 2010;36(4):688–691. | ||
Schettino GP, Chatmongkolchart S, Hess DR, Kacmarek RM. Position of exhalation port and mask design affect CO2 rebreathing during noninvasive positive pressure ventilation. Crit Care Med. 2003;31(8):2178–2182. | ||
Racca F, Appendini L, Gregoretti C, et al. Effectiveness of mask and helmet interfaces to deliver noninvasive ventilation in a human model of resistive breathing. J Appl Physiol (1985). 2005;99(4):1262–1271. | ||
Racca F, Appendini L, Gregoretti C, et al. Helmet ventilation and carbon dioxide rebreathing: effects of adding a leak at the helmet ports. Intensive Care Med. 2008;34(8):1461–1468. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.






 ) represents the curve that fits the scatter plot.
) represents the curve that fits the scatter plot.