Back to Journals » Journal of Inflammation Research » Volume 16
Evaluation of Biomarkers from Peritoneal Fluid as Predictors of Severity for Abdominal Sepsis Patients Following Emergency Laparotomy
Authors Zhao J , Zhang T, Deng Z, Han X, Ma T, Xie K
Received 22 December 2022
Accepted for publication 17 February 2023
Published 24 February 2023 Volume 2023:16 Pages 809—826
DOI https://doi.org/10.2147/JIR.S401428
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Jie Zhao,1,2 Teng Zhang,2,3 Zhe Deng,1,2 Xia Han,1,2 Tao Ma,2,3,* Keliang Xie1,2,*
1Department of Critical Care Medicine, Tianjin Medical University General Hospital, Tianjin, People’s Republic of China; 2Tianjin Medical University, Tianjin, People’s Republic of China; 3Department of General Surgery, Tianjin Medical University General Hospital, Tianjin, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Tao Ma, Department of General Surgery, Tianjin Medical University General Hospital, 154 Anshan Road, Tianjin, 300052, People’s Republic of China, Tel +86 13702172328, Email [email protected] Keliang Xie, Department of Critical Care Medicine, Tianjin Medical University General Hospital, 154 Anshan Road, Tianjin, 300052, People’s Republic of China, Tel +86 15332112099, Email [email protected]
Purpose: Intra-abdominal infection is considered the second most common cause of sepsis and results in localized or diffused inflammation of the peritoneum. The main treatment for abdominal sepsis is an emergency laparotomy for source control. However, surgical trauma also causes inflammation, and patients become susceptible to postoperative complications. Therefore, it is necessary to identify biomarkers that can be used to distinguish sepsis from abdominal infection. This prospective study investigated whether cytokine levels in the peritoneum could predict complications and indicate severity of sepsis following emergency laparotomy.
Methods: We prospectively observed 97 patients with abdominal infection admitted to the Intensive Care Unit (ICU). After emergency laparotomy,SEPSIS-3 criteria were used for the diagnosis of sepsis or septic shock. Blood and peritoneal fluid samples were drawn at postoperative admission to the ICU and cytokine concentrations were measured by flow cytometry.
Results: Fifty-eight postoperative patients were enrolled. We found significant elevations in the peritoneal concentrations of IL-1β, IL-6, TNF-α, IL-17, and IL-2 in patients with sepsis or septic shock compared to the patients without sepsis after surgery. Positive correlations between levels of these peritoneal cytokines with APACHE II scores were found: IL-6, in particular, had the highest correlation coefficient of 0.833. Meanwhile, IL-10 in blood, MCP-1 and IL-8 in both blood and peritoneum were simultaneously increased in patients with sepsis and septic shock, and also positively correlated with disease severity.
Conclusion: The cytokine storm that occurs in the abdominal cavity after emergency laparotomy may be the main mechanism leading to sepsis. It may be valuable to measure IL-1β, IL-6, TNF-α,IL-17, IL-2, MCP-1, and IL-8 in the peritoneal fluid, combined with serum IL-10, MCP-1 and IL-8, in a panel of cytokines, to assess the severity of sepsis and predict mortality from abdominal infection after emergency laparotomy.
Keywords: abdominal infection, emergency laparotomy, peritoneal cytokine, cytokine storm
Plain Language Summary
Sepsis is an uncontrolled immune response occurring after infection. Abdominal infection is considered the second leading cause of sepsis. Although not familiar to the general public, sepsis has mortality rate as high as 20%. Therefore, early identification and control are particularly important. Currently, there is no single cytokine available to act as a good sepsis biomarker for diagnosing or predicting outcomes. This study was conducted to investigate whether a specific biomarker exists to distinguish sepsis from abdominal infection after surgical intervention. We observed 58 patients with abdominal infection admitted to the Intensive Care Unit (ICU) after an emergency laparotomy. We divided patients into three groups according to severity: from abdominal infection to septic shock. Meanwhile, we collected blood and peritoneal fluid to measure the concentrations of cytokines. Elevations in peritoneal concentrations of IL-1β, IL-6, TNF-α, IL-17, IL-2, MCP-1, and IL-8 were found combined with an increase in serum IL-10, MCP-1, and IL-8 in patients with sepsis or septic shock compared to the patients with no sepsis after surgery. Furthermore, these increases were positively correlated with the severity of abdominal infection.
This study suggests that the cytokine storm that occurred in the abdominal cavity combined with a particular change in serum cytokines may be valuable for assessing the severity of sepsis and predicting mortality from abdominal infection after emergency laparotomy. The cytokine panel both from the abdominal cavity and peripheral blood samples will be useful to identify and control abdominal sepsis after emergency surgery.
Introduction
Abdominal sepsis, or secondary peritonitis, is a challenge facing surgeons and Intensive Care Unit (ICU) clinicians worldwide every day. The main treatment involves emergency laparotomy with source control and subsequent return to the ICU ward to correct the altered physiology.1 Despite substantial advances in clinical understanding and disease definition, without timely control, abdominal infection can have a poor prognosis and further deteriorates into sepsis or septic shock, with the potential for extremely high mortality rates for ICU patients.2
Sepsis is an uncontrolled response of the immune system to infection,3 in which high levels of circulating cytokines lead to a generalized inflammatory response with organ failure and, in up to 20% cases, to death.4 In the presence of secondary peritonitis, the abdominal cavity is a rich reservoir of inflammatory cytokines.5 Abdominal visceral damage, peritoneal irritation, and intra abdominal contamination are all potent triggers for the systemic cytokine response, in which immune cells are stimulated to produce a series of serum markers, including cytokines and chemokines.6 Once the inflammatory cytokines from the peritoneum enter the systemic circulation, multi-organ failure may potentially occur. Furthermore, surgical trauma causes inflammation and postoperative immunosuppression. Patients become susceptible to infections, which is a major cause of postoperative morbidity and mortality in this population.7 Therefore, rapid recognition of this inflammatory dysregulation is critical for the management of septic patients to optimize their chance of survival. The value of inflammatory biomarkers for the early identification of sepsis has been investigated. In animals with septic peritonitis, a concentration of cytokines in abdominal fluid has a diagnostic sensitivity and specificity.8 However, these variables have not been evaluated in patients with septic peritonitis who undergo surgery, and the conclusions are controversial.
We conducted studies to evaluate inflammatory biomarkers in both peripheral blood and peritoneal blood as predictors of abdominal septic peritonitis after surgery. Herein, we tested a novel approach of simultaneous measurement of 14 inflammatory-related cytokines/chemokines using the multiplex technique by flow cytometry, to identify useful inflammatory biomarkers capable of distinguishing patients with a high risk of developing sepsis or septic shock and death after abdominal surgery. A further study was conducted that evaluated the association between cytokines and an organ dysfunction assessment score system to determine whether peritoneal or serum cytokines could predict mortality and provide a powerful rapid diagnostic tool for clinical practice in abdominal septic patients.
Materials and Methods
Study Population
This study was conducted at the Department of Critical Care Medicine of Tianjin Medical University General Hospital. From April 2021 to March 2022, a total of 97 patients with abdominal infection were admitted to our department. Fifty-eight of all consecutive patients (59.8%) were included if there was evidence of sepsis or septic shock after emergency laparotomy. The study protocol was approved by the Ethics Committee of Tianjin Medical University General Hospital and written informed consents were obtained from patients, patient-authorized representatives, or legal representatives.
Selection of Cases and Inclusion Criteria
Ninety-seven patients with intra-abdominal infection were tested at our department during the study period. The diagnosis of intra-abdominal infection is primarily clinical. Patients typically present with rapid-onset abdominal pain and signs of local and systemic inflammation (prolonged fever, pain, abdominal tenderness, absence of intestinal sounds, increased white blood cell count, tachycardia, and/or tachypnea). Patients requiring emergency intervention for peritonitis were enrolled in our study.
Surgery was conducted by senior surgeon who had performed more than 500 laparotomies in the last 5 years. During the intra-abdominal operation, the senior surgeon determined the decision based on the degree of abdominal contamination, the clinical status of the patient and the surgeon’s experience. All patients underwent a midline laparotomy with complete exploration of the peritoneal cavity. The gross purulent exudates were aspirated and the debris and particles were removed, followed by abundant peritoneal lavage.
Exclusion criteria were age younger than 18 years or older than 80 years, or a body mass index (BMI) greater than 31. Patients were excluded because they had a history of immunosuppressive therapy within 8 weeks prior to surgery or chemotherapy within 6 months prior to surgery. Transplant recipients were also excluded. Other exclusion criteria for liver cirrhosis, acute non-infectious pancreatitis, and extra-abdominal sepsis were known. Patients were also excluded when disagreement occurred about surgical intervention or lack of informed consent.
Fifty-eight patients were finally included in our study who presented hypotension and signs of hypoperfusion, such as oliguria, acute altered mental state, and lactic acidosis, symptoms which were indicative of sepsis or septic shock. Patients were assigned to three groups according to the severity of infection after surgery. Three groups included abdominal infection group, the abdominal sepsis group, and the abdominal septic shock group separately. Of the 58 patients, 42 were enrolled in the sepsis group because they met two or more criteria of the quick Sequential Organ Failure Assessment (qSOFA) criteria: (1) low mean blood pressure (≤65 mmHg); (2) tachypnea (≥22/min); (3) altered mental status (Glasgow Coma Scale, GCS <15). Furthermore, 17 patients of the 42 patients with sepsis were assigned to the septic shock group. Septic shock was defined as not only meeting more than two criteria for qSOFA, but also having hypotension (mean arterial pressure <65 mmHg) after a minimum of 2 L fluid bolus followed by continuous infusion of vasopressors. The use of vasopressors was defined as therapy with any dose of epinephrine, norepinephrine, dopamine, vasopressin, or other vasopressors. The remaining 16 patients were designated as the abdominal infection control group and did not meet the diagnosis criteria for sepsis or septic shock after surgery.
In a word, among the 58 patients, 25 Patients were enrolled for the sepsis group and 17 patients were enrolled for the septic shock group. Other 16 patients were described as abdominal infection control group without sepsis or septic shock.
Data Collection and Outcome Measurements
Blood samples were taken through an arterial line at admission after surgery. Peritoneal fluid samples were taken from the abdominal drain. All samples were kept on ice and centrifuged within one hour of collection. Blood and peritoneal fluid samples were spun at 1500 g for 20 min. The supernatant was stored at −20 °C in our department, and then transferred to our lab for the −80 degrees °C within 12 hours of sampling.
Routine laboratory and clinical data were collected daily. All treatments were in performed in accordance with standard guidelines under the responsibility of the attending physician, who was usually not the local investigator of the trial. The clinical and severity characteristics at the time of admission to the ICU were evaluated: temperature, white blood cell count (WBC), serum lactate values, surgery location, result of positive pathogens from blood culture, the Acute Physiology, Age, and Chronic Health Evaluation (APACHE-II) score and predicted acute organ dysfunction related to sepsis using Sequential Organ Failure Assessment (SOFA) score.
The primary outcome was 90-day all-cause mortality. Secondary outcomes included the time to discharge from the ICU, the time to wean from mechanical ventilation, the number of days that the patients were free of vasopressors (days without vasopressors) and the time to be free of renal replacement treatment (RRT). Adverse outcomes included gastrointestinal bleeding, incidence of anastomotic fistula or local recurrence, episodes of heart or respiratory failure, and neurologic impairment at the time of ICU and hospital discharge. All adverse events after abdominal surgery were recorded according to the classification of the Medical Dictionary of Regulatory Activities.
Enzyme Linked Immunosorbent Assay
Concentrations of the Interleukin-1β (IL)-1β, IL-2, IL-4, IL-6, IL-10, IL12, IL-17, interferon-γ (IFN-γ), monocyte chemo-attractant protein 1 (MCP-1), tumor necrosis factor-α (TNF-α), C-X-C Motif Chemokine Ligand-10 (CXCL10, IP-10), IL-8, and Free Active Transforming Growth Factor-β (TGF-β) in serum and peritoneal fluid samples were determined using the LEGENDplexTM Human Essential Immune Response Panel (13plex) (BioLegend, San Diego, CA) using flow cytometry. The assays were conducted in strict accordance with the instrument operating instructions, and the data were analyzed using LEGENDplexTM data analysis software. Observed cytokine concentrations were reported in pg/mL or ng/mL. If the observed cytokine concentration of a specific sample was outside the detection range, the maximum or minimum observed cytokine concentration value was reported. IL-3 levels were measured in duplicate using a sandwich enzyme-linked immunosorbent assay (ELISA) using a commercially available kit (NeoBioscience Technology, Beijing, China). All samples were tested by the same investigator, who was blind to the clinical situation. All samples were analyzed in the same assay to avoid variance between assays.
Statistical Analysis
Continuous variables are presented as means and standard deviations, compared using variance and t-test analyses. Categorical variables are presented as the number of patients in each category and the corresponding percentages. The missing data were not replaced. The results obtained for the variables of abdominal fluid and peripheral blood were compared using the Spearman correlation coefficient to identify factors that were significantly associated with the outcomes. All reported P-values were 2-sided, and P-values <0.05 were considered statistically significant. Statistical analyses were performed with SPSS version 25.0 statistical software (IBM Corp).
Results
Baseline Characteristics of Patients
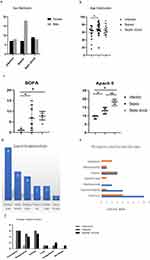
Fifty-eight patients were enrolled in the study and were assigned to the 3 groups: (1) patients with abdominal infection group, 16 patients received surgery in the absence of sepsis or septic shock (age: 58.75 ± 17.52 years); (2) patients with postoperative abdominal sepsis, 25 patients received source control of postoperative sepsis (age: 68.60 ± 14.89 years); and (3) abdominal septic shock group, 17 patients after abdominal surgery were admitted in ICU for septic shock (age: 65.94 ± 12.28 years). Most patients were male, with no differences between the three groups (Figure 1a). Patients with abdominal sepsis were significantly older than the only infection group (p=0.045, Figure 1b). Furthermore, no differences were found in patients with and without cardiovascular, respiratory, hematologic, liver, and kidney dysfunction/failure between the sepsis group and the septic shock group (Table 1, Figure 1f).
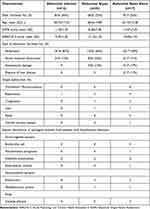 |
Table 1 Baseline Characteristics |
The distribution of causes in abdominal sepsis is shown in Table 1 and Figure 1d. Perforation, especially in the stomach (22/58), was the most frequent cause of abdominal infection. Other causes included acute intestinal obstruction, anastomotic leakage, or purulent collection. Only half of the patients (30/58) had positive blood microbiological data, especially in the sepsis and septic shock groups, respectively. Gram-positive bacteremia (GPB) was the main cause of bloodstream infection after abdominal surgery. Escherichia coli (40%) was the predominant organism in Gram-negative bacteremia (GNB), while Enterococci were the leading GPB type. Other organisms included Enterobacter cloacae, Pseudomonas aeruginosa, Klebsiella pneumoniae, and Staphylococcus aureus. The incidence of bloodstream infection was much higher in postoperative patients with GNB compared to those with GPB infection (20/30 vs 8/30), but there was no significant difference in microorganism cultures between the abdominal sepsis group and the septic shock group (Table 1, Figure 1e).
Outcomes and Serious Adverse Events
The results showed that the APACHE II score was statistically higher in the sepsis and septic shock groups compared to the abdominal infection group (p=0.001). Furthermore, the septic shock group showed a higher score than the patients with sepsis (p=0.001). (Table 1, Figure 1c) Analysis of the SOFA score showed that the sepsis (p=0.008) and septic shock groups (p=0.001) had a significantly higher score than the infection group, but there were no differences between each group. (Table 1, Figure 1c).
On day 90, deaths had occurred in 5 of 17 (29.4%) patients in the septic shock group versus the abdominal infection group (p=0.025). Furthermore, death occurred in 2 of 25 patients (8%) in the abdominal sepsis group versus 0 of 16 patients in the control group (p=0.017). Significant differences were observed after surgery between the only infection patients and those patients who developed sepsis or septic shock postoperatively (Figure 2a). There were no significant differences in length of stay in the ICU, ventilation-free days or days of renal replacement (Table 2). However, there were more episodes of administered for vasopressors (vasopressor free days) in the sepsis (p=0.009) and septic shock groups (p=0.01) compared to the only infection group (Table 2, Figure 2b). Secondary abdominal infections, intestinal obstruction, gastrointestinal bleeding, anastomotic fistula, cerebrovascular disease, or other adverse events were not significantly different across the three groups (Table 2, Figure 2c).
 |
Table 2 Primary and Secondary End Points |
Nine Biomarkers of the Abdominal Cavity Increased in the Sepsis or Septic Shock Groups After Abdominal Plasma
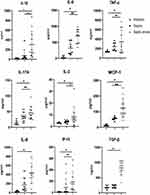
We investigated the role of the cytokine storm in both the abdominal surgical location and the peripheral plasma during the pathophysiology of sepsis and their association with severity. The concentrations of 14 biomarkers in the peritoneal fluid in the abdominal infection, abdominal-sepsis, and abdominal-septic shock groups are shown in Table 3. Nine cytokines, including IL-1β, IL-17, IL-2, TNF-α, IL-8, IL-6, MCP-1, IP-10, IL-8, and TGF-β, revealed a statistically significant increase in sepsis groups compared to the only infection control group. IFN-α, IL-3, IL-4, IL-10, and IL-12 were not detected differences among the three groups (Table 3, Figure 3).
 |
Table 3 Levels of 14 Biomarkers in the Abdominal Cavity of Enrolled Patients |
Furthermore, we found an intermediate relationship, with correlation coefficients ranging from 0.417 to 0.833, between the evaluation of the severity of the disease and levels of peritoneal biomarkers. Analysis of these cytokines, especially for concentrations of IL-1β (Spearman correlation coefficient, R=0.429, p=0.033), IL-2 (R=0.417, p=0.04), TNF-α (R=0.766, p=0.001), IL-8 (R=0.686, p=0.001), IL-6 (R=0.833, p=0.001), MCP-1 (R=0.677, p=0.001), and IL-17 (R=0.622, p=0.001) showed positive correlations with APACHE II scores. Among these, IL-6 had the highest correlation coefficient of 0.833 (Table 4, Figure 4).
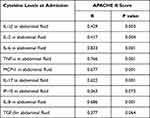 |
Table 4 Correlation Between Peritoneal Cytokines and Severity of Illness Parameters |
Six Biomarkers of Circulation Blood Increased in the Sepsis or Septic Shock Groups After Abdominal Surgery
Based on the same analysis, we also compared the same cytokines in plasma among the three groups (Table 5). Serum levels of IL-10, IL-12, MCP-1, IL-6, IL-8, and TGF-β were increased significantly in septic shock, especially for levels of IL-10, IL-12, and MCP-1 that showed statistical differences among the three groups (Table 5, Figure 5). The analysis showed that five cytokines showed a positive correlation with the APACHE II score (Table 6, Figure 6): IL-10 (R=0.592, p=0.002), IL-12 (R=0.638, p=0.001), MCP-1 (R=0.748, p=0.001), IL-8 (R=0.507, p=0.01), and TGF-β (R=0.543, p=0.001). Meanwhile, MCP-1 in plasma showed the strongest correlation with disease severity. However, both IL-3, IL-4, and IFN-γ both from plasma and peritoneal lavage were not significantly different between the three groups (P>0.05) (Table 3, Table 5).
 |
Table 5 Levels of 14 Biomarkers in the Serum of Enrolled Patients |
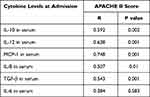 |
Table 6 Correlation Between Serum Cytokines and Severity of Illness Parameters |
Discussion
Mortality rates remain extremely high for abdominal sepsis in ICU patients, and surgery often provides the only opportunity for definitive treatment. Dysregulated inflammation after abdominal surgery may induce a cytokine storm, with signs of excessive inflammation, as well as immune suppression.9 To date, no single cytokine has been identified to act as a good sepsis biomarker to diagnose or predict severity and outcomes. As a result, researchers have focused on a combined cytokine panel having a stronger association with mortality than any individual cytokine during sepsis to predict the severity results of abdominal sepsis.10
Previous studies have paid greater attention to cytokine storms in serum. However, surgeons and researchers believe that peritoneal cytokines respond more extensively compared to systemic cytokines.11 Furthermore, emergency abdominal surgery is characterized by a severe disorder of peritoneal cytokine storm during the postoperative course. Hendriks demonstrated that peritoneal cytokine levels, especially IL-6, TNF-α, and IL-10, were dramatically different in rats model of intraperitoneal sepsis that survived or died.12 Visceral ischemia-reperfusion injury after sepsis up-regulates the expression of both proinflammatory (eg, IL-6 and TNF-α) and anti-inflammatory (eg, IL-10) cytokines in the intestine.13 Since animal models have shown that peritoneal cytokine storms are correlated with clinical outcomes, the peritoneal cytokine response may be more severe than the systemic response after abdominal surgery. However, very few studies have demonstrated the role of peritoneal cytokine levels in predicting the severity of the disease and mortality in patients with sepsis. Therefore, the aim of this study was to compare peritoneal cytokine levels with serum cytokine storms to predict the severity of disease and the risk of death of patients with abdominal sepsis patients following emergency surgery.
Our primary hypothesis was confirmed by significant elevations in peritoneal concentrations of IL-1β, IL-6, TNF-α, IL-17, and IL-2 in sepsis patients compared to the non-sepsis patients. TNF-α, IL-1β and IL-6 are prominent pro-inflammatory cytokines released during infection. Previous studies have suggested that elevated serum levels of IL-1, IL-6, and TNF-α are typical characteristics of cytokine storms.14,15 We interpreted an increase in peritoneal cytokine levels of TNF-α, IL-1β, and IL-6 as indicative of an initial inflammatory response of the peritoneal and further inducing cytokine storms in the abdomen during the postoperative course. IL-17 and IL-2 are important links between innate and adaptive immune processes. IL-17 is recognized as a hallmark molecule of CD4+ T helper 17 (Th17) cells,16 and IL-2 is a marker of strong and long-lasting activation within the lymphocyte immune responses.17 Significant increases in peritoneal levels of IL-17 and IL-2 can interact with other proinflammatory cytokines, especially TNF-α, IL-1β, and IL-6, to drive an amplified immune response, and contribute to the development of sepsis.18 According to our finding, higher levels of peritoneal IL-1β, IL-6, TNF-α, IL-17, and IL-2 observed in this study may support the idea that the abdomen can mount a pro-inflammatory response while avoiding a state of immune paralysis, which may a dominant anti-inflammatory cytokine response pattern after surgery.
Surgeons and researchers believed that the peritoneal cytokine storms in the original infectious cavity were faster and greater and seemed to be more sensitive in determining postoperative inflammatory reaction. Previous studies demonstrated correlations between the severity of abdominal sepsis and systemic cytokine storms.19 There is also a correlation between cytokine disorder and duration of surgery, blood loss, and bacterial count. Furthermore, a correlation has been shown between cytokine storms and postoperative complications.20,21 Bozza et al demonstrated that high IL-1β, IL-4, and IL-6 significantly predicted mortality.22 We observed that not only did the same cytokine storms occur in the abdominal cavity, but also peritoneal IL-1β, IL-6, TNF-α, IL-17, IL-2 had a positive correlation with APACHE II scores. This phenomenon supported the notion that elevated levels of peritoneal cytokine storms were apparently related to the severity of the disease in abdominal sepsis. Furthermore, the regression coefficient of IL-6 in the abdominal cavity was the highest, which supported IL-6 as the center of cytokine storms as in previous studies.23 Our findings convinced patients who underwent abdominal sepsis that they had a more hostile peritoneal cavity and provided a panel of pro-inflammatory cytokines to potentially predict worse outcomes after surgical management.
We found that serum IL-10 overproduction was associated with the severity and fatal outcome of sepsis after emergency surgery. A similar phenomenon was identified in a previous study for patients with postoperative multiorgan failure (cardiovascular), which also showed that the serum level of IL-10 was higher in the sepsis group and in non-survivors at all time points.24 As we mentioned above, peritoneal pro-inflammatory cytokines increased to induce inflammatory responses after abdominal surgery. Our results supported that these pro-inflammatory cytokine storms in the cavity were inhibited primarily by IL-10. High IL-10 in peripheral blood were not only associated with sepsis-induced immunosuppression,25 but also supported the previous understanding that IL-10 has the ability to block the production of inflammatory cytokines even in the abdominal cavity.26,27 Therefore, our results suggest that increased levels of systemic IL-10 combined with storms of pro-inflammatory cytokines in the peritoneum after emergency surgery correlate with a poor outcome of abdominal sepsis. This observation also reflected an inflammatory imbalance between pro- and anti-inflammatory cytokine relationships both in the peritoneum and in the circulation during the postoperative period.
Chemokines are another important category of cytokines in the pathogenesis of sepsis. Our study found that chemokines, especially MCP and IL-8, both in the blood and in the cavity, were extremely high in peritoneal sepsis and septic shock and correlated strongly with the severity of the disease. Several previous studies have shown that serum levels of IL-8 and MCP-1 were significantly higher in non-survivors compared to sepsis survivors.28,29 Previous studies have also observed that IL-8 and MCP-1 have a positive correlation with the SOFA score and were higher in non-survivors within the first 24 hours after admission to the ICU.30 Our results strongly suggested that MCP-1 and IL-8 acted as key chemokines to induce cytokine storms in the abdomen and serum during the progression of sepsis. Unlike other cytokines, which have broad effects on many cells, chemokines are usually cell-specific. MCP-1 is an important molecule for monocyte recruitment by regulating the production of pro-inflammatory cytokines.31 IL-8 plays a critical role in sepsis through neutrophil recruitment to vital organs.32 We found that pro-inflammatory cytokines only increased in the peritoneum, while peripheral blood and peritoneal chemokines increased during the primary phase after surgery. This pattern might represent a peritoneal environment that recruited immune cells to continue the pro-inflammatory cascade within the peritoneal cavity. Furthermore, elevated peritoneal cytokine concentrations, surgical stimulation during damage control surgery, and potential intraoperative physiological changes may all contribute to the trend for increased serum chemokines in the early phase of postoperative care. Therefore, the MCP-1 and IL-8 chemokines in the blood and abdominal cavity could be potential markers for predicting the severity of disease and the risk of death from abdominal infections after emergency surgery.
In contrast to previous research, we found that serum IL-12 concentration increased significantly in patients with sepsis or in non-survivors. However, a previous study suggested that increased IL-12 production was associated with a favorable outcome following sepsis.33 IL-12 is believed to exert a protective effect during the sepsis stage through regulation of cellular immunity and phagocytic functions.34 Conflicting data for IL-12 in the index study could be due to an imbalanced and excessive inflammatory response due to sepsis. Another potential reason why serum IL-12 concentrations were different from previous studies could be attributed to the small sample size in our study. Specifically, the elevated serum concentration of IL-12 observed in patients with sepsis within our cohort warrants further exploration in future.
In this study, we developed a unique strategy targeting a combination of circulation and peritoneal biomarkers to rapidly detect sepsis after surgery. The current study identified several novel and interesting relationships between serum cytokine levels in the peritoneal and circulation released to attenuate abdominal infection injury after surgery. Moreover, the relative ease of measuring cytokine levels allow for a rapid diagnosis at the bedside, which allows doctors to choose or modify the diagnostic algorithm and therapeutic decisions in real time. We believe that the potential utility of the peritoneal cytokine panel will help improve the severity and outcome of abdominal sepsis after emergency surgery.
The present study still has some limitations. First, the sample size of the study was too small to clearly define the diagnostic role of cytokine dosage in serum and abdominal effusion and its real clinical use, but this was a pilot study that provides a good rationale for verification in a large clinical trial; Second, in theory, the stronger the cytokine storm in sepsis, the more severe the damage to multiple organs. However, hundreds of cytokines, which have the potential for substantial adverse effects on the body, were not evaluated. In future research, we anticipate that each cytokine will receive a more reasonable and accurate weight coefficient according to its damage during sepsis. Third, we studied the cytokine profile at a single time point (post-operation at admission). Measurement of cytokines at different time points could have given information about trends of these cytokines in relation to disease progression and better differentiation between survivors and non-survivors. Future studies should expand the sample size, and serial measurement of cytokines at different time points during treatment may achieve a more comprehensive clinical picture.
Conclusions
A cytokine storm was found to develop both in the peritoneum and in the circulation shortly after abdominal surgery in patients with secondary peritonitis. We found increased trends for peritoneal pro-inflammatory cytokines levels of IL-1β, IL-6, TNF-α, IL-17, IL-2, and circulation anti-inflammatory biomarkers of IL-10 in a surgical abdominal sepsis cohort, and MCP-1 and IL-8 increased in both serum and peritoneum. All of the cytokines and chemokines mentioned above increased to aggravate cytokine storms after abdominal infection and could be the main mechanism that leads to sepsis. As a result, we suggested to measure IL-1β, IL-6, TNF-α, IL-17, IL-2, MCP-1, and IL-8 in the peritoneal fluid, combined with serum IL-10, MCP-1, and IL-8, in a panel of cytokines, which may be valuable to assess the severity of sepsis and predict mortality from abdominal infection after emergency surgery.
Abbreviations
APACHE II, Acute Physiology, Age and Chronic Health Evaluation; SOFA, Sequential Organ Failure Assessment; RRT, Renal Replace Treatment; GNB, Gram Negative Bacteremia; GPB, Gram Positive Bacteremia; IL-1β, Interleukin-1β; IL-2, Interleukin-2; IL-4, Interleukin-4; IL-6, Interleukin-6; IL-8, Interleukin-8; IL-10, Interleukin-10; IL-12,Interleukin-12; IL-17, Interleukin-17; IFN-γ, Interferon-γ; MCP-1, Monocyte Chemo-attractant Protein-1; TNF-α, Tumor Necrosis Factor-α; CXCL10 (IP-10), C-X-C Motif Chemokine Ligand-10; TGF-β1, Transforming Growth Factor-β.
Data Sharing Statement
All data collected or analyzed during this study are included in this article.
Ethics Approval and Consent to Participate
The study protocol was approved by the ethics committee of Tianjin Medical University General Hospital.
Consent for Publication
Written informed consent was obtained from the patients, their authorized representatives, or legal representatives before the samples were obtained from the patients.
Acknowledgments
The authors thank colleagues from the Department of General Surgery of Tianjin Medical General Hospital for patient data assistance.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by grants from the Tianjin Municipal Education Commission of the China Scientific Research Program (No. 2021KJ210).
Disclosure
None of the authors has a commercial relationship or other that might pose a conflict of interest. No part of this article or the information has been presented elsewhere.
References
1. Massimo S, Alain C-M, Francesco ML, et al. The management of intra-abdominal infections from a global perspective: 2017 WSES guidelines for management of intra-abdominal infections. World J Emerg Surg. 2017;12:29. doi:10.1186/s13017-017-0141-6
2. Quirine J, Boldingh J, Fleur E, et al. Abdominal sepsis. Curr Opin Crit Care. 2017;2(3):159–166.
3. Laura E, Andrew R, Waleed A, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021;10(47):1181–1247.
4. Delano MJ, Ward PA. The immune system’s role in sepsis progression, resolution, and long-term outcome. Immunol Rev. 2016;274(1):330–353. doi:10.1111/imr.12499
5. James TR, Michael AM, Hobart WH. Secondary peritonitis: principles of diagnosis and intervention. BMJ. 2018;361:k1407. doi:10.1136/bmj.k1407
6. Bleszynski MS, Chan T, Buczkowski AK. Comparison of inflammatory cytokines in peritoneal fluid at source control surgery for abdominal sepsis. Am J Surg. 2017;213(5):849. doi:10.1016/j.amjsurg.2017.03.037
7. Chao J, Cui S, Liu C, et al. Detection of early cytokine storm in patients with septic shock after abdominal surgery. J Translat Intern Med. 2020;8(2):91–98. doi:10.2478/jtim-2020-0014
8. Guieu LVS, Bersenas AME, Holowaychuk MK, Brisson BA, Weese JS. Serial evaluation of abdominal fluid and serum amino-terminal pro-C-type natriuretic peptide in dogs with septic peritonitis. J Vet Intern Med. 2015;29(5):1300–1306. doi:10.1111/jvim.13575
9. de Pablo R, Monserrat J, Reyes E, et al. Mortality in patients with septic shock correlates with anti-inflammatory but not proinflammatory immunomodulatory molecules. J Intensive Care Med. 2011;26(2):125–132. doi:10.1177/0885066610384465
10. Peter B, Chris F, Keith RW, John B, James AR. Plasma cytokine levels predict response to corticosteroids in septic shock. Intensive Care Med. 2016;42(12):1970–1979. doi:10.1007/s00134-016-4338-z
11. Hecker A, Reichert M, Reuß CJ, et al. Intra-abdominal sepsis: new definitions and current clinical standards. Langenbeck’s Archiv Surg. 2019;404(3):257–271. doi:10.1007/s00423-019-01752-7
12. Hendriks T, Bleichrodt RP, Lomme R, Ben MD, Harry VG, Otmar RB. Peritoneal cytokines predict mortality after surgical treatment of secondary peritonitis in the rat. J Am Coll Surg. 2010;211(2):263–270. doi:10.1016/j.jamcollsurg.2010.03.038
13. Zager RA, Johnson ACM. Renal ischemia-reperfusion injury upregulates histone-modifying enzyme systems and alters histone expression at proinflammatory/profibrotic genes. Am J Physiol. 2009;296(5):F1032–41. doi:10.1152/ajprenal.00061.2009
14. Xi DT, Tian-T J, Jia-R D. Pathogenesis and treatment of cytokine storm induced by infectious diseases. Int J Mol Sci. 2021;22(23):13009. doi:10.3390/ijms222313009
15. Jekarl DW, Kim JY, Ha JH, et al. Diagnosis and prognosis of sepsis based on use of cytokines, chemokines, and growth factors. Dis Markers. 2019;2019:1–11. doi:10.1155/2019/1089107
16. Carvalho N, Barros A, Coelho HO, et al. A Th2 cytokine profile in appendicular lavage fluid suggests allergy as a possible etiology for acute appendicitis. Mediators Inflamm. 2019;4:1–5. doi:10.1155/2019/8146257
17. Zhai GH, Zhang W, Xiang Z, et al. Diagnostic value of sIL-2R, TNF-α and PCT for sepsis infection in patients with closed abdominal injury complicated with severe multiple abdominal injuries. Front Media SA. 2021;12:741268.
18. Leandro AL, Be CF, Ana CO. The interplay between microbiota and inflammation: lessons from peritonitis and sepsis. Clin Transl Immunol. 2016;15(7):e90.
19. Kjell J, Britt R, Lennart T, et al. Intraperitoneal cytokine response after major surgery: higher postoperative intraperitoneal versus systemic cytokine levels suggest the gastrointestinal tract as the major source of the postoperative inflammatory reaction. Am J Surg. 2004;187(3):372–377. doi:10.1016/j.amjsurg.2003.12.019
20. Zhengw X, Crystal W, Helen LR, et al. Inflammatory mediators in intra-abdominal sepsis or injury - a scoping review. Crit Care. 2015;19:373. doi:10.1186/s13054-015-1093-4
21. Liu M, Silva‐Sanchez A, Randall TD, Selene MP. Specialized immune responses in the peritoneal cavity and omentum. J Leukoc Biol. 2015;109(4):717–729. doi:10.1002/JLB.5MIR0720-271RR
22. Bozza FA, Salluh JI, Japiassu AM, et al. Cytokine profiles as markers of disease severity in sepsis: a multiplex analysis. Critical Care. 2007;11(2):R49. doi:10.1186/cc5783
23. Sujin K, Tadamitsu K. Interplay between interleukin-6 signaling and the vascular endothelium in cytokine storms. Exp Mol Med. 2021;53(7):1116–1123. doi:10.1038/s12276-021-00649-0
24. Stanilova SA, Karakolev ZT, Dimov GS, et al. High interleukin 12 and low interleukin 10 production after in vitro stimulation detected in sepsis survivors. Intensive Care Med. 2005;31(3):401–407. doi:10.1007/s00134-005-2575-7
25. Swirski FK, Chousterman BG, Weber GF. Cytokine storm and sepsis disease pathogenesis. Semin Immunopathol. 2017;39(5):517–528. doi:10.1007/s00281-017-0639-8
26. Xu XJ, Luo ZB, Xia T, et al. Comparison of interleukin-6, interleukin-10, procalcitonin and C-reactive protein in identifying high-risk febrile illness in pediatric cancer patients: a prospective observational study - ScienceDirect. Cytokine. 2019;116:1–6. doi:10.1016/j.cyto.2019.01.004
27. Fatime H, Ildikó L, Nándor Ö, Domonkos T, Zoltán O, Zsolt M. Extracorporeal cytokine adsorption in septic shock: a proof of concept randomized, controlled pilot study - ScienceDirect. J Crit Care. 2019;49:172–178. doi:10.1016/j.jcrc.2018.11.003
28. He J, Chen Y, Lin Y, et al. Association study of MCP-1 promoter polymorphisms with the susceptibility and progression of sepsis. PLoS One. 2017;12(5):e0176781. doi:10.1371/journal.pone.0176781
29. Marty C, Misset B, Tamion F, Fitting C, Carlet J, Cavaillon J-M. Circulating interleukin-8 concentrations in patients with multiple organ failure of septic and nonseptic origin. Crit Care Med. 1994;22(4):673–679. doi:10.1097/00003246-199404000-00025
30. Fujishima S, Sasaki J, Shinozawa Y, et al. Serum MIP-1α and IL-8 in septic patients. Intensive Care Med. 1996;22(11):1169–1175. doi:10.1007/BF01709331
31. Tsai H, Chin-Hao C, Wen-J K. Biomarkers of early sepsis may be correlated with outcome. J Transl Med. 2014;12:146. doi:10.1186/1479-5876-12-146
32. Augustina F, Ewurama DAO, Jones AA. Cytokines as potential biomarkers for differential diagnosis of sepsis and other non-septic disease conditions. Front Cell Infect Microbiol. 2022;12:901433. doi:10.3389/fcimb.2022.901433
33. Martin JL, Mario B, Mark G, et al. Sepsis biomarkers in unselected patients on admission to intensive or high-dependency care. Critical Care. 2013;17(2):R60. doi:10.1186/cc12588
34. Carbone F, Bonaventura A, Vecchiè A, et al. Early osteopontin levels predict mortality in patients with septic shock. Eur J Intern Med. 2020;78:113–120. doi:10.1016/j.ejim.2020.04.035
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.