Back to Journals » Clinical Ophthalmology » Volume 15
Evaluation of Anti-SARS-CoV-2 IgA in the Conjunctival Secretions of COVID-19 Patients
Authors Mahmoud H , Hamody A, M Hefny H , Tohamy D , Awny I
Received 26 March 2021
Accepted for publication 14 April 2021
Published 10 May 2021 Volume 2021:15 Pages 1933—1937
DOI https://doi.org/10.2147/OPTH.S312942
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Hany Mahmoud,1 Ahmed Hamody,2 Hesham M Hefny,3 Dalia Tohamy,4 Islam Awny5
1Department of Ophthalmology, Faculty of Medicine, Sohag University, Sohag, Egypt; 2Anesthesia Department, Sohag University, Sohag, Egypt; 3Clinical Pathology Department, Sohag University, Sohag, Egypt; 4Ophthalmology Department, Assiut University, Assiut, Egypt; 5Department of Ophthalmology, Sohag University, Sohag, Egypt
Correspondence: Hany Mahmoud
Department of Ophthalmology, Faculty of Medicine, Sohag University, Egypt
Tel +20 1024368111
Email [email protected]
Purpose: To assess the presence of anti-SARS-CoV-2 IgA in the conjunctival secretions of confirmed COVID-19 patients by nasopharyngeal swabs and correlate its presence with the severity of the disease, patient’s age, sex and ocular symptoms.
Methods: This study included 44 positive COVID-19 patients confirmed with nasopharyngeal swabs during the period 17– 28 February 2021 at Sohag Tropical Medicine Hospital. Tears and conjunctival secretions were examined for the presence of anti-SARS-CoV-2 IgA.
Results: While non-reactive results are strongly correlated to low titre and vice versa, severity showed significant correlation with neither IgA reactivity nor titre. Meanwhile, IgA reactivity did not show significant correlation with either age or sex. The reactivity and IgA titre are correlated with ocular symptoms.
Conclusion: The anti-SARS-CoV-2 IgA could be found in ocular secretions in SARS-CoV-2 patients. There is no correlation with age or sex or severity of the disease; however, they are correlated with ocular symptoms.
Keywords: anti-SARS-CoV-2 IgA, ocular, secretions, swab
Introduction
Since the emergence of SARS-CoV-2 in China in 2019 and the WHO considering it to be a global emergency,1 many studies have evaluated its effects on the eyes, that is, ocular symptoms and signs such as follicular conjunctivitis, subconjunctival hemorrhage, eye congestion or retinal manifestations.2–5
Many studies have evaluated SARS-CoV-2 shedding in conjunctival tears and secretions.6,7 The presence of viral RNA was confirmed by many studies suggesting its transmission through the eyes. This could explain deaths amongst ophthalmologists and support the importance of safety measures when dealing with symptomatic and asymptomatic patients. Many ophthalmologists all over the world have been infected, which supports the suggestion that the virus sheds through tears, although we could not deny that the infection could occur also through breath or other routes.8–10 The American Academy of Ophthalmology (AAO) suspended all ophthalmological interventions except in urgent situations in March 2020, and many ophthalmological societies introduced protective measures such as social distancing in clinics, decreasing the number of patients examined, providing personal protective equipment (PPE) such as masks, gloves, face shields and slit-lamp breath shields, and conducting frequent sterilization.11 The use of 3D display systems could be a solution in both anterior and posterior segment surgeries.
Recently the pathogenesis of the virus was linked to Angiotensin Converting Enzyme 2 (ACE2) receptors, which could be found in many tissues including immune system cells. Many of these receptors were detected in conjunctival tissue, cornea, trabecular meshwork, limbal cells and many ocular tissues, and the spikes in the viral coat attached to these receptors causing the infection suggests their role in viral pathology and transmission. There are two suggested means of transmission: the flow of tears in nasolacrimal ducts to the respiratory system even in case of their obstruction and the tears flowing on the face reaching the nose and the blood flow from the eyes.12–15
IgA is the mucosal response immunoglobulin and is produced in mucosal surfaces such as conjunctiva. Evaluation of its presence in conjunctival secretions could confirm the presence of the SARS-CoV-2 virus and the possibility of its transmission through the eyes.16–18
IgA could be the first immunology response to the coronavirus as it attacks the surfaces of the respiratory, gastrointestinal or ocular systems. It is the rapid immune response, its duration is short and it is the first line of defense locally, so we can suggest that it has a role to play in preventing the worsening of COVID-19 outcomes because it attacks the virus and decreases its load.18
The aim of this study was to evaluate the presence of IgA in conjunctival tears and secretions and correlate its presence with the symptomatology of SARS-CoV-2 in patients.
Patients and Methods
Forty-four COVID-19 patients confirmed by nasopharyngeal swab were enrolled in the study. They were recruited during the period 17–28 February 2021 from Sohag Tropical Medicine Hospital. All were subjected to an anti-SARS-CoV-2 IgA test of their tears and conjunctival secretions.
Ocular manifestations including conjunctival, corneal, or retinal issues were examined using a portable slit-lamp and indirect ophthalmoscopy.
Inclusion criteria were all COVID-19 patients confirmed by nasopharyngeal swab attending Sohag Tropical Medicine Hospital during the period 17–28 February 2021.
There were no exclusion criteria.
Ethical Consideration
Ethical guidelines were followed and comprehensive written informed consent was taken from all patients involved in this study. Our study adhered to the tenets of the Declaration of Helsinki and the ethical board committees of our respective institutions (Sohag Faculty of Medicine, Assiut Faculty of medicine, Al-Shahid hospital-Sohag) granted approval.
Method of Tear Collection
On provision of informed consent and under total aseptic conditions and the wearing of PPE, tears were collected in the following manner:
- One drop of Proparacaine 0.5% was installed and the eye was closed for two minutes.
- For 30–60 seconds tears were collected from the lower fornix of the eye using a 2.0 mm x 10.0 mm polyester fiber rod (Transorb Wick, Filtrona and Richmond, VA).
- The sample was put into a 1.5-mL Eppendorf tube.
SARS-CoV-2 IgA Antibody by Chemiluminescent Immunoassay (CLIA) Method
SARS-CoV-2 IgA antibody in tear samples was detected using an iFlash 3000 CLIA analyzer and YHLO‐CLIA‐ 2019-nCoV IgA kits CATALOG #: C86095A supplied by YHLO (Shenzhen Yhlo Biotech Co., Ltd., Shenzhen, China), according to the manufacturer’s instructions. In the first incubation, Anti-2019-nCoV-2 IgA in the sample, sample diluent and 2019-nCoV antigen-coated paramagnetic microparticles reacted to form a complex, followed by washing in which the unbound materials were washed away from the solid phase in a magnetic field. In the second incubation, Acridinium-labeled anti-human IgA conjugate was added to form a reaction complex followed by another wash.
The pre-trigger and trigger solutions were added to the reaction mixture. The resulting chemiluminescent reaction was measured as relative light units (RLUs). A direct relationship exists between the amount of anti-2019-nCoV-2 IgA in the sample and the RLUs detected by the iFlash optical system. Results were determined by comparing the RLU detected by the iFlash optical system with the cut-off calculated from 2019-nCoV IgA calibrators.
The antibody levels were expressed as AU/mL and a non-reactive value was considered to be < 8.0 AU/mL. A value range from ≥ 8 to < 12 AU/mL was considered intermediate, while a value of ≥ 12.0 AU/mL was considered reactive.
We collected the data and recorded them.
Statistical Analysis
Statistical analysis was done using IBM SPSS version 25 (IBM corporation, Chicago, USA, August 2017). Data was expressed as Mean±SD for quantitative data and numbers and percentages for qualitative data. Correlation was done by Spearman test; the strength of correlation was defined as negligible (r < 0.20), weak (0.21 < r <0.40), moderate (0.41 < r < 0.70) or strong (0.71 < r < 1).
Results
Using the Shapiro–Wilk test, the sample was normally distributed (p > 0.05). The mean age of patients was 53.64±14.19 (mean±SD) and there were 26 males (26, 59.1%) and 18 females (40.9%). Patient data is summarized in Tables 1 and 2 and Figure 1.
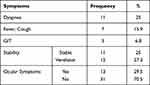 |
Table 1 Participant Data |
 |
Table 2 Results of IgA Detection and the Detection Titre |
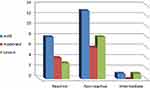 |
Figure 1 Severity and IgA reactivity in the study group. |
The correlation between the severity and presence of ocular symptoms and IgA titre is summarized in Table 3 and the correlations between IgA reactivity and both age and sex are summarized in Table 4. While non-reactive results are strongly correlated to low titre and vice versa, severity did not show a significant correlation with either IgA reactivity or titre. Moreover, the presence of ocular symptoms is strongly correlated with reactive results of IgA and moderately correlated with high IgA titre. Meanwhile, the presence of ocular symptoms did not show a significant correlation with the severity of general symptoms. On the other hand, IgA reactivity did not show a significant correlation with either age or sex.
 |
Table 3 Correlation Between Symptom Severity and IgA Titre |
 |
Table 4 Correlation Between IgA Reactivity and Age and Sex |
Discussion
It is known that SARS-CoV-2 infection causes activation of innate and adaptive immunity, and activated B cells and T cells produce specific SARS-CoV-2 antibodies including IgM, IgG, and IgA. IgA could be detected in thbody secretions, including tears.
Many studies found that IgA could be found in the tears of COVID-19 patients.18 The use of different medications on positive COVID-19 patients could have ocular manifestations rather than anti-glaucoma medication or tear substitutes. These drugs have an effect on virus activity, either increasing or decreasing it, and so ocular medications and their effect on viral expression should be further studied, drug by drug, to identify their potential effects.19 In the current study, IgA could be detected in 15 of the 44 tested patients (34.1%). The presence of IgA in the tears suggests the potential shedding of the virus through tears and infectivity through tears and conjunctival secretions.20
The integrity of the tear film is important for healthy corneas. The tear film is activated during blinking and eye movements. Tears flow through the nasolacrimal ducts and when there is a problem in the lacrimal pathway tears flowing on the face could carry the virus with IgA to the mucous membranes of the respiratory system.21
A non-significant correlation was found between severity of symptoms and IgA titre, which suggests that even mild cases could shed the virus and be a source of infection.22 IgA could be secreted regardless of the presence of ocular symptoms.
This study showed that ocular symptoms are strongly correlated with reactive results of IgA and moderately correlated with high IgA titre. Meanwhile, the presence of ocular symptoms did not show significant correlation with the severity of general symptoms. This differs from other studies that show no correlation.20 This can be explained by the idea that ocular symptoms could occur as an immune response and become more evident when there is a greater immune response in the form of virus/IgA interaction. Confirmation of this explanation requires further investigation. On the other hand, IgA reactivity did not show a significant correlation with either age or sex, a finding demonstrated in many previous studies.
Conjunctival tissue is an optimal site for virus replication and so the conjunctival route of infection should be confirmed as leading to the infection and even death of ophthalmologists.6
Limitations and Recommendations
The limitations of this study were its small sample size and the small number of previous studies regarding the presence of IgA in tears. We recommend conducting further studies with a larger number of participants.
Acknowledgment
This is to certify that the article has not been presented in a meeting.
Funding
This is to certify that the authors did not receive any financial support from any public or private sources.
Disclosure
The authors reported no conflicts of interest for this work and no financial or proprietary interest in the product, method, or material described herein.
References
1. Sohrabi C, Alsafi Z, O’Neill N, et al. World Health Organization declares global emergency: a review of the 2019 novel coronavirus (COVID-19). Int J Surg. 2020;76:71–76. doi:10.1016/j.ijsu.2020.02.034
2. Rousseau A, Fenolland JR, Labetoulle M. SARS-CoV-2, COVID-19 et œil: le point sur les données publiées [SARS-CoV-2, COVID-19 and the eye: an update on published data]. J Fr Ophtalmol. 2020;43(7):642–652. French. doi:10.1016/j.jfo.2020.05.003
3. Wu P, Duan F, Luo C, et al. Characteristics of ocular findings of patients with coronavirus disease 2019 (COVID-19) in Hubei Province, China. JAMA Ophthalmol. 2020;138(5):575–578. doi:10.1001/jamaophthalmol.2020.1291
4. Barton LM, Duval EJ, Stroberg E, et al. COVID-19 autopsies, Oklahoma, USA. Am J Clin Pathol. 2020;153(6):725–733. doi:10.1093/ajcp/aqaa062
5. Bertoli F, Veritti D, Danese C, et al. Ocular findings in COVID-19 patients: a review of direct manifestations and indirect effects on the eye. J Ophthalmol. 2020;2020:4827304. doi:10.1155/2020/4827304
6. Xia J, Tong J, Liu M, et al. Evaluation of coronavirus in tears and conjunctival secretions of patients with SARS-CoV-2 infection. J Med Virol. 2020;92(6):589–594. doi:10.1002/jmv.25725
7. Mahmoud H, Ammar H, El Rashidy A, et al. Assessment of coronavirus in the conjunctival tears and secretions in patients with SARS-CoV-2 infection in Sohag Province, Egypt. Clin Ophthalmol. 2020;14:2701–2708. doi:10.2147/OPTH.S270006
8. Qing H, Li Z, Yang Z, et al. The possibility of COVID-19 transmission from eye to nose. Acta Ophthalmol. 2020;98(3):e388. doi:10.1111/aos.14412
9. Hoenig LJ. The eye and COVID-19 pandemic. Clin Dermatol. 2020;38(4):506. doi:10.1016/j.clindermatol.2020.03.013
10. Dockery DM, Rowe SG, Murphy MA, et al. The ocular manifestations and transmission of COVID-19: recommendations for prevention. J Emerg Med. 2020;59(1):137–140. doi:10.1016/j.jemermed.2020.04.060
11. Napoli PE, Nioi M, d’Aloja E, Fossarello M. Safety recommendations and medical liability in ocular surgery during the COVID-19 pandemic: an unsolved dilemma. J Clin Med. 2020;9(5):1403. doi:10.3390/jcm9051403
12. Peng M, Dai J, Sugali CK, Rayana NP, Mao W. The role of the ocular tissue in SARS-CoV-2 transmission. Clin Ophthalmol. 2020;14:3017–3024. doi:10.2147/OPTH.S269868
13. Hikmet F, Méar L, Edvinsson Å, et al. The protein expression profile of ACE2 in human tissues. Mol Syst Biol. 2020;16(7):e9610. doi:10.15252/msb.20209610
14. Pham TL, He J, Kakazu AH, et al. Elovanoid-N32 or RvD6-isomer decrease ACE2 and binding of S protein RBD after injury or INFγ in the eye. Res Sq [Preprint]. 2020:
15. Napoli PE, Nioi M, d’Aloja E, Fossarello M. The ocular surface and the coronavirus disease 2019: does a dual ‘ocular route’ exist? J Clin Med. 2020;9(5):1269. doi:10.3390/jcm9051269
16. Knop N, Knop E. Conjunctiva-associated lymphoid tissue in the human eye. Invest Ophthalmol Vis Sci. 2000;41(6):1270–1279.
17. Kugadas A, Wright Q, Geddes-McAlister J, et al. Role of microbiota in strengthening ocular mucosal barrier function through secretory IgA. Invest Ophthalmol Vis Sci. 2017;58(11):4593–4600. doi:10.1167/iovs.17-22119
18. van Ginkel FW, Gulley SL, Lammers A, et al. Conjunctiva-associated lymphoid tissue in avian mucosal immunity. Dev Comp Immunol. 2012;36(2):289–297. doi:10.1016/j.dci.2011.04.012
19. Napoli PE, Mangoni L, Gentile P, Braghiroli M, Fossarello M. A panel of broad-spectrum antivirals in topical ophthalmic medications from the drug repurposing approach during and after the coronavirus disease 2019 era. J Clin Med. 2020;9(8):2441. doi:10.3390/jcm9082441
20. Caselli E, Soffritti I, Lamberti G, et al. Anti-SARS-Cov-2 IgA response in tears of COVID-19 patients. Biology. 2020;9(11):374. doi:10.3390/biology9110374
21. Napoli PE, Nioi M, Mangoni L, et al. Fourier-domain OCT imaging of the ocular surface and tear film dynamics: a review of the state of the art and an integrative model of the tear behavior during the inter-blink period and visual fixation. J Clin Med. 2020;9(3):668. doi:10.3390/jcm9030668
22. Cervia C, Nilsson J, Zurbuchen Y, et al. Systemic and mucosal antibody responses specific to SARS-CoV-2 during mild versus severe COVID-19. J Allergy Clin Immunol. 2021;147(2):545–557.e9. doi:10.1016/j.jaci.2020.10.040
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
