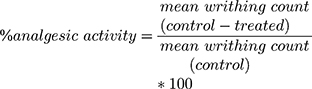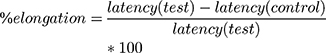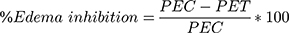Back to Journals » Journal of Inflammation Research » Volume 13
Evaluation of Analgesic and Anti-Inflammatory Activities of 80% Methanol Root Extract of Echinops kebericho M. (Asteraceae)
Authors Yimer T , Birru EM , Adugna M, Geta M , Emiru YK
Received 12 June 2020
Accepted for publication 5 August 2020
Published 30 September 2020 Volume 2020:13 Pages 647—658
DOI https://doi.org/10.2147/JIR.S267154
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Tesfaye Yimer,1 Eshetie Melese Birru,2 Meaza Adugna,2 Mestayet Geta,2 Yohannes Kelifa Emiru3
1Department of Pharmacy, College of Health Science, Debre-Tabor University, Debre Tabor, Ethiopia; 2Department of Pharmacology, School of Pharmacy, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia; 3Department of Pharmacognosy, School of Pharmacy, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia
Correspondence: Tesfaye Yimer Tel +251 921313476
Email [email protected]
Background: Pain and inflammation are the major devastating health problems commonly treated with traditional medicinal plants in Ethiopia. Echinops kebericho M. (Asteraceae) is the one which is frequently used to treat pain and inflammation by traditional healers in Ethiopian folk medicine. However, the plant has not been scientifically evaluated for its traditionally claimed use. The present study aimed at the investigation of analgesic and anti-inflammatory activities of 80% methanol root extract of Echinops kebericho M. in mice model.
Methods: Successive maceration was used as a method of extraction using solvents of increasing polarity: methanol and water. After extraction of the roots with 80% hydro methanol, the crude extract was evaluated for its peripheral and central analgesic activities using acetic acid-induced writhing test and hot plate method, respectively, while its anti-inflammatory activity was evaluated using carrageenan- and formalin-induced paw edema. The extract was evaluated at 100, 200 and 400 mg/kg doses. The positive control groups were treated with ASA 150 mg/kg for writhing test, morphine 10 mg/kg for hot plat method, indomethacin 25 mg/kg and diclofenac 10 mg/kg for paw edema tests and vehicle, distilled water (10 mL/kg) treated mice were assigned as negative controls. All treatment administrations were performed orally.
Results: E. kebericho extract at all test doses showed statistically significant antinociceptive activity in both chemicals-induced peripheral and thermal-induced central pain in a dose dependent manner (p < 0.01 and p < 0.001). The greater analgesic activity was observed by the maximum dose of the extract (400 mg/kg) in both acetic acids-induced writhing test (57.84%) and hot plate method (69.40%). The effect of the extract was also statistically significant (p < 0.01 and p < 0.001) in both carrageenan and formalin-induced paw edema in dose dependent manner. Greater edema inhibition was observed by the highest dose (400 mg/kg) in both observations with the respective percentage values of 70.00% and 79.87%, respectively.
Conclusion: In general, the data obtained from the present study elucidated that the extract possessed a significant analgesic and anti-inflammatory activities and recommended for further studies.
Keywords: analgesic activity, anti-inflammatory activity, Echinops kebericho M., carrageenan, hot plate
Introduction
Pain can be defined as an unpleasant sensory and emotional experience associated with actual or potential tissue damages, or described in terms of such damages.1 It is usually initiated by noxious stimuli and transmitted over specialized neuronal networks to the CNS where, it is interpreted as such. It is a way to protect the body from possible injury.2 Similarly, inflammation, which is employed by both the innate and adaptive immune systems, is a protective response of the body to various obnoxious stimuli such as infections and tissue injury.3,4.
Regardless of the availability of sufficient drugs, pain and inflammation remain the most challenging and devastating health problems which affect 80% of adult population worldwide.5 They are considered as the major clinical, social, and economical problem in most communities around the world.6 Untreated and persistently prolonged pain is the most pervasive disorder that results both physical damage and psychological disorders.7 Non-resolving inflammation also leads to loss of function in terms of missing work, school, or social activities and it results in the progression of serious inflammatory diseases, including asthma, autoimmune disease, chronic inflammation, glomerulonephritis, inflammatory bowel diseases, pelvic inflammatory disease, reperfusion injury, hypersensitivities, hay fever, atherosclerosis, and rheumatoid arthritis. These devastating conditions are the major cause of disabilities and can lead to death unless they are properly managed and controlled.8
The currently available standard drugs for pain and inflammation remain the mainstay for managing and treating these disorders. However, they are associated with many side effects and toxicities, such as gastric irritation, gastric ulcer, alterations in renal function, effects on blood pressure, hepatic injury, and platelet inhibition which may result in increased bleeding. Using NSAIDs results in increased risk of cardiovascular adverse events especially, in patients taking COX-2 inhibitors.8,9 Opioid analgesics are also associated with many unwanted side effects and toxicities, including drowsiness, nausea and vomiting, pruritus, constipation, disturbing hormonal homeostasis, hearing loss, tolerance, physical dependence, addiction, and respiratory problems. In view of this, there is a need for the intensification of research into medicinal plants which are claimed to be effective in the management of pain and inflammation.10
Traditional healers in different parts of Ethiopia use a wide range of traditional medicinal plants to treatand manage pain and inflammation. But, the therapeutic potentials of some of these medicinal plants have not been scientifically evaluated and validated so far.11 Echinops kebericho M. is among the traditional medicinal plants used in Ethiopian folk medicine, and is frequently reported for its antinociceptive and anti-inflammatory potentials.12,13
Traditionally, E. kebericho M has been used for the relief of different infectious and non-infectious diseases including; fever, headache, cough, diarrhea, malaria, as taenicide, stomach ache, and typhus.14 The infusion and inhalation (after burning) of the roots is used to heal cough and headache respectively, while inhalation of the leaf and steam after burning is used to be relived from inflammation which is conventionally known as “mich” by traditional healers.15 It is reported that echinops species is used by traditional healers for the treatment of a variety of conditions, such as; the dried and/or fresh root is fumigated for the prevention of devil sickness, the dried and/or fresh roots are crushed and mixed with water and then a cup of the mixture is taken orally to treat headache, as well as the fumigation of dried and/or fresh roots are used for treating malaria.16 The dried roots of E. kebericho are crushed and macerated with fresh water and mixed with coffee. The mixture is then taken orally to relievetoothache, headache, and vomiting.12 Traditional healers also use E. kebericho, by inhaling the dried roots to heal inflammation and evil eye.13 Inhalation, infusion, and smoking of the bulbs of E. kebericho is reported to be used for treating cough and headache by traditional healers.17
The species Echinops kebericho is found to possess scientifically approved pharmacological activities. Some of them are as follows; 80% alcoholic root extract of the plant is approved to have anti-schistosomal activity.18 On extracts the essential oils of the roots of E. kebericho is reported that, they exhibited antimicrobial, anthelminthic, molluscicidal and in vivo anti-plasmodial activities,14 and 80% aqueous extract of the roots of this plant is also reported to have in vivo antidiarrheal and ex vivo spasmolytic activities.19
Even though the plant E. kebericho M. is frequently reported for its antinociceptive and anti-inflammatory potentials by traditional healers in different parts of Ethiopia, no any scientific reports concerning the anti-inflammatory and analgesic activity of this plant have been found in literatures yet. So, it is deemed prudent to investigate the anti-inflammatory and analgesic activities of the plant scientifically. The aim of the present study was therefore, to investigate the analgesic and anti-inflammatory activities of 80% methanol root extract of E. kebericho M. in a mice model.
Materials and Methods
Materials and Instruments
Rotary evaporator (yamato, Japan), lyophilizer (OPERON, OPR-FDU-5012, Korea), digital plethysmometer (Ugo Basile-Cat no 7140, Italy) electronic balance (KERN-ALJ 220–4, Germany), tissue Drying Oven (Medite – Medizin technik, Germany), syringes with needles, feeding tube, hot Plate (Orchid Scientific, India.) were used with their respective function.
Drugs and Chemicals
Carrageenan (Sigma Chemicals Co., St Louis, USA), formalin (Taflen Industry, Ethiopia), normal saline (H. R. Leuven, Belgium), distilled water (Ethiopian Pharmaceutical Manufacturing Factory, Ethiopia), absolute methanol (Indenta chemicals, India) and glacial acetic acid (Sigma-Aldrich laborchemikalien, Germany), indomethacin (Cadila pharmaceuticals Ethiopia), aspirin, diclofenac and morphine (Ethiopian Pharmaceutical Manufacturing Factory, Ethiopia) obtained from the respective vendors were used in the experiment.
Collection, Identification and Preparation of Plant Materials
The roots of E. kebericho were collected from around Debre tabor town South Gondar Zone of the Amhara regional state which is located in North West direction and 667km far from the capital city Addis Ababa. The plant was then authenticated by Botanists in the Department of Biology, College of Natural and Computational Sciences, University of Gondar where a specimen with voucher number 002TYT/2019 was deposited for further reference.
Preparation of the Extract
The roots of the plant were washed with tape water to remove dust and any other debris present on it. The roots of E. kebericho were then air dried under a shaded area at room temperature and pulverized using a pestle and mortar to get a coarse powder. A total of 1.50 kg powdered root was macerated using 80% (v/v) methanol. The contents were shaken manually each day and allowed to remain within the solvent for 3 days. After 3 days, the extract was filtered first using gauze and then by Whatman filters paper (No. 1). The marc was re-macerated twice using the same volume of solvent to exhaustively extract the plant material. After extraction was completed, the solvent was evaporated under vacuum using rotary vapor and oven at 40°C. The resulting solution was then placed in a deep freezer operating at −20°C till it forms solid ice and then the remaining solvent (water) was removed using lyophilizer. After all a brownish gummy residue weighing 224gm of crude extract was obtained, giving rise to a percentage yield of 14.93%. Then, the resulting crude extract was kept within deep refrigerator (−20°c) till the commencement of the main procedure.
Experimental Animals
Healthy adult Swiss Albino mice of either sex (20–35g, and 6–8 weeks of age) were purchased from the Ethiopian Health and Nutrition Research Institute (EHNRI) and obtained from School of Pharmacy College of Health Science University of Gondar. All mice were fed with commercial pellets and have had access to water ad libitum. The mice were acclimatized for a week before commencement of the experiment in all procedures to minimize stress. All mice used in this study were handled in accordance with the internationally accepted standard guidelines for use of laboratory animals.20
Preliminary Phytochemical Screening
Standard phytochemical screening test was carried out to detect the presence of secondary metabolites to relate the analgesic and anti-inflammatory of E. kebericho root extract with the presence or absence of these active constituents. Thus, the test for alkaloids, saponins, flavonoids, phenols, steroid, anthraquinone, glycosides and tannins were performed using standard test procedures.14,19.
Animal Grouping and Dosing
Swiss albino mice of either sex weighing 20–35 g were randomly divided into 5 groups of 6 mice per group. Group I was assigned as negative control and received vehicles. Group II was served as positive control and treated with standard drugs; morphine (10 mg/kg) for hot plate test, indomethacin (25 mg/kg) for carrageenan test, ASA (150 mg/kg) for writhing test and diclofenac (10 mg/kg) for formalin test. Groups III–V were used as test groups and given the extract of 100, 200 and 400 mg/kg respectively. Doses were selected based on an acute toxicity study done previously.14,20 All treatment administrations were performed orally and the maximum volume administered “was 0.015 mL/kg.”
Evaluation of Analgesic Activities of the Extract
Acetic Acid-Induced Writhing Test
This method was conducted to detect the peripheral analgesic activity of the extract and was performed by randomly dividing overnight fasted mice with free water access as described in section (2.7). “Acetic acid (0.6%v/v) (10 mL/kg, i.p)” was injected to all groups of mice an hour just after the mice were given the extract, vehicle or standard as per the respective groups. Analgesic activity of the extract was assessed by counting the numbers of writhing which consists of contraction of the abdominal muscle together with stretching of the hind limbs for 30 min after a latency period of 5 min. A reduction in the number of writhes as compared to the control group was considered as evidence for analgesic potential of the extract, and it was expressed as percent inhibition of writhing as follows:21
Hot Plate Method
This method was conducted to evaluate the central analgesic potential of E. kebericho extract and was performed by introducing the mouse into an open-ended cylindrical space with a floor consisting of a metallic plate that was heated by a thermode. A plate was heated to a constant temperature of 55°C ± 1°C producing the behavioral components that were measured in terms of their reaction times, namely paw licking withdrawal of the paw and jumping. All responses were considered to be supraspinally integrated responses. Mice were individually placed on a hot plate with a cut-off time of 15 s to avoid lesions to the animals’ paws. The latency to lick the paw or jump from the hot plate was recorded as the reaction time. The reaction times were noted at 0 and 30, 60, 90 and 120 min after the administration of vehicle (distilled water 10 mL/kg), standard drug (morphine10 mg/kg) and 100 mg/kg, 200 mg/kg and 400 mg/kg of the extract. Percentage increase in reaction time or pain threshold inhibition, was calculated as follows:22
Anti-Inflammatory Activity
Carrageenan-Induced Paw Edema
This method was carried out by inducing of acute inflammation in the paws of overnight fasted mice with free access of water. The mice were injected with carrageenan (1% w/v in normal saline, 0.05mL) into the plantar side of the left hind paw. Just before induction of inflammation, the leg of each mouse was marked on the skin over the lateral malleolus, so that it could be immersed to the same extent in the measurement chamber of the plethysmometer. Carrageenan was injected one hour after administration of the extract, the vehicle and the standard drug with the respective groups of mice. Inflammation was quantitated in terms of mL i.e., displacement of water by edema using a digital plethysmometer at time 0, 1, 2, 3, 4 after carrageenan injection. The percentage inhibition of edema was calculated in comparison to the control mice as follows;23
Where; PEC paw edema in control group
PET paw edema in test group
Formalin-Induced Pedal Edema
In this procedure, 2% v/v formalin was used for the induction of sub-acute inflammation. Freshly prepared 2% v/v formalin with “distilled water (0.02mL)” was injected into the right hind paw of overnight fasted mice by sub-plantar injection at the 1st and 3rd days of observations. The right hind paw of each mouse was marked at the level of lateral malleolus before formalin induction so that it could always be immersed to the same extent in the measurement chamber of the plethysmometer throughout the observation days. The extract, the standard drug and the vehicle were given as per their respective grouping 1hr prior to formalin injection for 7 consecutive days. The mice paw volume was recorded daily using Plethysmometer after 1hr of extract, drug and vehicle administration till the 7th day and the percentage of edema inhibition was calculated using the above formula.24
Statistical Analysis
Analysis of results was done using Statistical Package for Social Sciences (SPSS) software version 21. All results obtained were expressed as mean ± standard error of mean (SEM) of responses. The statistical significance was determined by using One-way Analysis of Variance (ANOVA) followed by a Tukey post hoc test to compare variations among groups and the results were considered significant at p < 0.05. The analyzed data were then presented using tables and graphs where necessary.
Results
Preliminary Phytochemical Screening
Preliminary phytochemical screening for secondary metabolites was carried out to detect the presence or absence of different phytoconstituents from 80% methanol root extract of E. kebericho. The presence of saponins, alkaloids, phenols, tannins, flavonoids, glycoside, and steroids were confirmed through qualitative color changes of test reagents which will give a clue to the possible mechanisms of analgesic and anti-inflammatory effects of the extract.
Analgesic Activity
Acetic Acid-Induced Writhing Test
In this test, E. kebericho extract at all test doses employed (100, 200, and 400m g/kg) showed statistically significant peripheral analgesic activity in a dose dependent manner (p < 0.05, p < 0.01 and p < 0.001 respectively) as compared to the negative control. All 3 test doses of the extract produced increased inhibition of the numbers of writhing with maximum inhibition observed at maximum dose (400 mg/kg) (p< 0.001). The highest dose significantly decreased the number of writhing (p < 0.001) than the lower dose (100 mk/kg) and the middle dose (200 mk/kg) (p < 0.05). The extent of reduction of writhing in the different doses of the extract was different, i.e., significantly lower in 100 mg/kg (p < 0.001) and 200 mg/kg (p < 0.01) and comparable with 400 mg/kg as compared to the standard drug of 150 mg/kg ASA (Table 1).
 |
Table 1 Effect of 80% Hydromethanolic Root Extracts of Echinops kebericho on Acetic Acid-Induced Writhing in Mice |
Inter group comparison among the three doses employed also showed statistically significant difference in 100 mg versus 200 mg/kg (p < 0.05), 200 mg versus 400 mg (p < 0.05) and 100 mg versus 400 mg (p < 0.001). The maximum dose of EK extract (400 mg/kg) showed comparable % inhibition of the numbers of writhing with the standard drug (ASA 150 mg/kg) with respective percentage values of 57.84% and 60.98% respectively (Table 1).
Hot Plate Method
The hot plate method was performed to determine the central analgesic activity of EK extract in mice. In this model all the three test doses of EK extract and the standard drug morphine produced significant central analgesic activity (p < 0.05, p < 0.01 and p < 0.001) by delaying the reaction time at all-time intervals of observation when compared with the negative control (Table 2). The maximum analgesic activities of all doses of the extract (100, 200 and 400 mg/kg) was observed at 120 mins of observation with the respective values of 63.93%, 66.94%, and 69.40% as compared with the standard drug (morphine 10 mg/kg) that produced 70.81% analgesic activity (Figure 1).
 |
Table 2 Effect of 80% Hydromethanolic Root Extract of Echinops kebericho on Hot Plate Latency Time in Mice |
The latency delayed by the lower and middle doses of the extract was significantly lower (p < 0.001) than that of the standard drug, while the reaction time increased by the higher dose was comparable with the standard drug at all-time intervals of observation (Figure 1).
Anti-Inflammatory Activity
Carrageenan-Induced Paw Edema
This model was conducted to evaluate the anti-inflammatory potential of the extract in acute phase of inflammation. Sub plantar injection of 0.05 mL of 1% carrageenan to the mice hind paw produced a progressive increment of paw thickness that reached its maximum value after 3hrs of induction in negative control (Table 3). All test doses of EK extract (100, 200, and 400 mg/kg) produced statistically significant inhibition of paw thickness starting from 1 hr (p < 0.01 and 0.001) and the effects persisted till the fourth hour of observation (p < 0.01 and p < 0.001) post carrageenan induction as compared to the negative control.
 |
Table 3 Effect of 80% Hydromethanolic Root Extract of Echinops kebericho on Carrageenan-Induced Paw Edema Model in Mice |
The maximum percent of anti-inflammatory activity of the extract at all test doses was observed at the 4th time with the respective percentage values of 54.62%, 61.54% and 70.00% and the effect at this time was found to increase in dose dependent manner. The maximum dose (400 mg/k) of the extract produced higher paw edema inhibition than the lower dose (100 mg/kg) and middle dose (200 mg/kg) of the extract (p < 0.01 and p < 0.05 respectively), whereas its edema inhibition effect was comparable with the positive control (indomethacin 25 mg/kg) at the 4th time of observation (Table 3).
Formalin-Induced Paw Edema Model
Formaldehyde-induced pedal edema method in mice was conducted to detect the anti-inflammatory potential of 80% hydro methanol root extract of EK in sub-acute phase of inflammation. Sub planar injection of 0.02mL of 2% v/v formaldehyde with distilled water to the mice hind paw at the 1st and 3rd day of observation produced progressive increment of the paw volume of the mice which were treated with the negative control (distilled water 10 mL/kg) (Table 4). E. kebericho extract at all test doses (100, 200 and 400 mg/kg) significantly decreased (p<0.05, p < 0.01, p < 0.001 respectively) paw edema volume in mice starting from the 1st day of formalin induction and then continued to the 7th day (p < 0.01, p < 0.001) post-formalin induction as compared with the negative control (Table 4).
 |
Table 4 Effect of 80% Hydromethanolic Root Extract of Echinops kebericho in Formalin-Induced Paw Edema in Mice |
The maximum percent of edema inhibition in all test doses of the extract was observed at the 7th day of observation with the respective values of 69.46%, 74.83%, and 79.87% (Figure 2) and the effect at this time was found to be dose dependent. Inter group comparison among the different doses of the extract showed statistically significant different effect in 100 mg/kg versus 400 mg/kg at the 1st (p < 0.01), 2nd (p<0.05), 3rd (p < 0.05), 5th (p < 0.05), 6th (p < 0.01), and 7th (p < 0.001) days. The higher dose showed comparable edema inhibition with the standard drug at D2, D4, D5, D6 and D7, while its effect was higher at D1 and lower at D3 (Figure 2).
Discussion
Considering the socioeconomic impacts of pain and inflammation and having the knowledge potential herbal medicines from traditionally claimed plants, the need for searching effective antipain and anti-inflammatory drugs with minimal untoward effects from traditional medicinal plants seems reasonable.7,25 Thus, searching for medicinal plants which have been widely used in the community to treat different pain conditions and inflammation with antipyretic activities are essential concern in this regard. Echinops kebericho is among the widely used traditional medicinal plants in Ethiopian folk medicine for treating pain, and different inflammatory conditions.14,15 However, no any scientific reports have been found in the literature about its analgesic and anti-inflammatory activities in experimental animal models so far. It may therefore be worthwhile scientifically investigating the analgesic and anti-inflammatory activities of the root extract of E. kebericho M. in the mice model to substantiate its claimed traditional use.
Acetic acid-induced writhing test was selected to detect the peripheral analgesic activity of the extract. Because of its sensitivity and ability to detect antinociceptive effects of natural products and test compounds at dose levels which remains inactive for other methods, acetic acid-induced writhing test is a well recommended model for screening the peripheral analgesic potentials of test compounds.26 Intraperitoneal injection of acetic acid causes irritation and stimulation of the peritoneal cavity that triggers the synthesis and release of various endogenous inflammatory mediators such as histamine, serotonin, bradykinin substance P, and PGs.27 These various endogenous inflammatory mediators elicited chemical-induced visceral pain which is characterized by constriction of abdominal muscles together with the extension of the forelimbs and elongation of the body. That is why the acetic acid-induced writhing test is considered as a model of visceral pain.28 This model has also been associated with increased level of PGE and PGF2a. Increasing PG levels within the peritoneal cavity enhances inflammatory pain by increasing capillary permeability and activating primary afferent nociceptors.29
Echinops kebericho extract at all doses employed (100, 200 and 400 mg/kg) significantly (p < 0.01, and p < 0.001) showed peripheral analgesic activities by reducing the number of writhing with the respective values of 31.01%, 48.43%, 57.84% as compared with the negative control. These findings confirmed that the peripheral analgesic activity of the extract increased from the lower dose (100 mg/kg) to the higher dose (400 mg/kg) in dose dependent manner. The increase in analgesic activity with increasing doses of the extract might be attributed with an increase in concentration of phytoconstituents that possess analgesic activity with the maximum dose.
The possible mechanism by which the extract produced peripheral analgesia in this model might be associated with inhibiting the synthesis and release of various endogenous inflammatory mediators and suppression of sensitivity of peripheral nociceptors in the peritoneal free nerve endings for chemical-induced pain. These proposed mechanisms are in line with the principles that stated, any agent that decreases the number of writhing will demonstrate analgesia by inhibiting the synthesis and release of PGs, and by inhibiting the peripheral pain transmission.28,30
The second model used was the hot plate test, in which the supra-spinal nociception and the involvement of central antinociceptive mechanism were detected.28 Since the paws of mice are very sensitive to heat at temperatures which are not damaging the skin, the central antinociceptive mechanisms of the extract were detected by introducing the mice to the constantly heated plate and by observing the reaction times, namely jumping, withdrawal, and licking of the paws. The time until these responses occured was prolonged after administration of centrally-acting analgesics.31
This model was selected to evaluate the central analgesic potential of the extract because of its sensitivity to strong analgesics, limited tissue damage with a cutoff time of 15 sec, which is usually applied to limit the amount of time the mouse spends on the hot plate. The model also requires less time and measurements are usually accurate.28
The extract at all test doses (100 mg/kg, 200 mg/kg and 400 mg/kg) significantly (p < 0.05, p < 0.01, p < 0.001 respectively) elevated the pain threshold by increasing the reaction time starting from 30 min of observation onwards as compared to the negative control. The maximum analgesic effects of the extract were observed at 120 min of observation time with their respective percentage values of 63.93%, 66.94%, 69.40% as compared with morphine (10 mg/kg) which showed a percentage analgesic value of 70.81% at this time. At all times of observation, the doses of the extract showed analgesic activities in dose dependent manner. The doses of the extract took longer time (peak time) to attain for maximal effect, which for all doses was 120 min. This delay may be due to a probable time lag for the drug to enter in to the central compartment and distribute into the target site or formation of active metabolites that are endowed with analgesic activity with better half-life. A relatively better action of 400 mg/kg at all observation time may be attributed by the presence of high concentration of active metabolites.
The possibly proposed mechanism of central analgesic effects of the extract may be by activating the periaqueductal gray matter (PAG) to release endogenous peptides (i.e., endorphin or enkephalin). These endogenous peptides descend the spinal cord and function as inhibitors of the pain impulse transmission at the synapse in the dorsal horn or via peripheral mechanisms involved in the inhibition of PG, leukotriene, and other endogenous substances that are key players in central pain transmission.32
Previous phytochemical screening on EK has revealed the presence of alkaloids, polyphenols, saponins, phytosterols, carotenoids, lignans, sesquiterpene alcohols, acetylenic and thiophene compounds, terpenoids, and essential oil.33 It is proved that the root extract of EK contained secondary metabolites including saponin, tannin, alkaloids, phenols, flavonoids, glycosides and steroids by preliminary phytochemical screening in the present study. So, it can be said that the analgesic effects shown by the extract may be due to the presence of these aforementioned and currently identified phytoconstituents.34 This suggestion is in line with the reports that stated, phytoconstituents like, alkaloids, flavonoids, steroids, and tannin isolated from medicinal plants have been reported to possess a significant analgesic activity.9,28
In the study of the anti-inflammatory activity, the root extract of EK was detected against carrageenan-induced acute and formalin-induced sub-acute phases of inflammation. Since the inflammatory response is a polyphasic tissue reaction, which involves both short lived increase in vascular permeability and prolonged cellular infiltration and proliferation, it is important to evaluate the potential of the extract for anti-inflammatory effect via a sequential test valid for various phases of inflammation.9
Carrageenan-induced hind paw edema is a prototype model which is employed to evaluate the anti-inflammatory potentials of various natural and synthetic products as well as to determine the possible mechanisms involved in inflammation.4,14 Carrageenan is a phlogistic, non-antigenic agent and is devoid of apparent systemic effect, it is also believed that the experimental model exhibited a high degree of reproducibility in acute phase inflammation. Thus, carrageenan-induced paw edema, is a frequently used method for the screening of acute inflammatory potentials of various natural products.14
In the present study, acute inflammation was induced by sub planar injection of carrageenan (1%v/v in normal saline) in the left hind paws of the mice. Following the induction of carrageenan, an acute localized inflammation was induced through sequential release of various endogenous inflammatory mediators. The release of these endogenous mediators was biphasic. The early phase (0 and 2.5 h) after carrageenan induction is mainly mediated by the release of histamine, serotonin, and bradykinin. These mediators are attributed for inflammation by increasing vascular permeability in the damaged tissue surroundings. The late phase which is sustained by the overproduction of COX-2 and its pro-inflammatory PGs product, with infiltration of polymorphonuclear leucocytes (neutrophils), took place 2.5–6hrs post carrageenan induction.9 There are also other chemicals mediators released during the late phase of inflammation such as, oxygen-derived free radicals like superoxide anion (O2-) and hydroxyl radicals (OH−), nitric oxide (NO) which play an important role in the development and progression of acute inflammation.12,35
The extract at all test doses employed (100, 200 and 400 mg/kg) significantly (p < 0.05, p < 0.01 and p < 0.001 respectively) decreased the formation of edema starting from 1 hr post carrageenan induction and the effects persisted (p < 0.001) till the 4th hr of observation. The effect of the extract started from the 1st phase (1 hr) and continued till the 4th hr (second phase) of inflammation. This observation suggested that bioactive constituents in the extract may suppress both phases of acute inflammation by interfering with the release and/or activity of the chemical mediators.
The maximum percentage of edema inhibition by all doses of the extract was observed at the 4th time of observation with the respective values of 54.65%, 61.54% and 70.00%. These effects verified that the extract’s anti-inflammatory effect was in dose dependent manner. Edema inhibition potential showed by the higher dose of the extract (400 mg/kg) was comparable with that of the standard drug (Indomethacin 25mg/kg) with the respective values of 70.00% and 71.54% at the 4th time of observation.
The significant anti-inflammatory effects shown by both the extract and the standard drug (Indomethacin 25 mg/kg) evidenced that their effects started in the early phase of inflammation by inhibiting endogenous inflammatory mediators such as serotonin and histamine as they involve in the early phase of inflammation, while the effects of edema inhibition reached maximum at the 4th time suggests that both the extract and the standard drug have profound anti-inflammatory effects against various endogenous inflammatory mediators that involve in the late phase of inflammation such as, COX, different PG analogues, BK and/or leukotriene or they could have, free radical scavenging activities.8,36
Formaldehyde-induced paw edema model is the well-known model for the evaluation of anti-arthritic and anti-inflammatory potentials of natural products in sub-acute phase of inflammation. Injection of 2% formalin v/v with distilled water subcutaneously in the palm of the mice in the 1st and 3rd days of observation produced progressive paw edema which was characterized by increased migration of leucocytes and phagocytes, infiltrations of neutrophils, macrophages and proliferation of fibroblasts into the surrounding of injured area.35 Hence, the inhibition of formaldehyde-induced edema is one of the most prototype methods to evaluate anti-inflammatory activity in sub-acute phase of inflammation, anti-proliferative activity, and screening anti-arthritic agents from natural products35,36
In this model the three doses of the extract (100, 200 and 400 mg/kg) showed significantly mice paw swelling inhibition (p < 0.05, p < 0.01, p < 0.001 respectively) starting from the 1st day of observation and the effects progressed (p < 0.01 and p < 0.001) till the 7th day. The effect shown by the extract was in dose dependent manner.
The highest percentage of edema inhibition for all doses (100, 200 and 400 mg) was observed at the 7th day of observation with the respective values of 69.46%, 74.83% and 79.87% respectively. The highest dose of the extract showed significantly higher effect (p < 0.05 and p < 0.01) via the lower (100 mg/kg) and the middle dose (200 mg/kg) while its effect was higher at the 1st and 2nd days and remain comparable at the rest days of observation with the standard drug (diclofenac 10 mg/kg). Increasing edema inhibition of the extract as the dose increased can be explained by the possible existence of an adequate concentration of the active metabolite(s) in the maximum dose level (400 mg/kg) when compared to the lower and middle (100 and 200 mg/kg) dose levels of the extract and by the quick metabolism and elimination of the effective phytoconstituents present in inadequate concentrations in the lower and middle dose levels. The observed effects of the extract may be through anti-proliferative activities against fibroblasts, inhibition of infiltrations of neutrophils and macrophages, and antagonizing the migration of leucocytes and phagocytes in to the area of inflammation.35
The anti-inflammatory action of E. kebericho extract in the present study can be supported by previous reports from scientific journals that stated plants which contain mainly alkaloids, flavonoids, saponin, and tannins phenolic compound, glycosides, coumarins and triterpenoid chemical constituents showed strong anti-inflammatory effects. So, it can be deduced that the anti-inflammatory effect of E. kebericho extract in the present study may be due to the presence of alkaloids, flavonoids, saponin, tannin, and triterpenoids. Alkaloids exert its anti-inflammatory activity through interfering with indubitable COX expression and production of PGE2, inhibition of pro-inflammatory cytokines production like IL-1β, IL-6, TNF-α, terpenoids exert its anti-inflammatory effect through inhibition of PLA2 activity, inhibition of TNF-α production, inhibition of iNOS expression, inhibition of COX-2 expression, and inhibition of NF-κB activation, while saponins are believed to interfere with iNOS expression, inhibition of COX-2 expression and subsequent production of PGE2, and exert their sequential anti-inflammatory effects.4,26 Furthermore, polyphenols exert their anti-inflammatory properties through inhibition of the production of inflammatory cytokines and chemokine and suppressing the activity of (COX and iNOS and thereby decreasing the production of reactive oxygen and nitrogen species).8
The results obtained from the present study were in line with the findings of others8,9,28 that demonstrated the analgesic and anti-inflammatory activities of medicinal plants in a dose dependent manner.
In general, it can be concluded that the anti-inflammatory activity of E. kebericho extract may be due to cumulative effects of the presence of different active phytoconstituents in reducing the synthesis, release and action of different endogenous inflammatory mediators that are mentioned above which play key roles for the development and progress of both acute and sub-acute inflammation.
Conclusions
In conclusion, the plant extract possessed peripheral analgesic activity and central pain inhibition potential. It also exhibited an anti-inflammatory activity, in both acute and sub-acute phases of inflammation. These results might imply that the plant extract was involved in inhibition of various endogenous inflammatory mediators, pain transmission and mediators, due to the presence of secondary metabolites including alkaloids, flavonoids, saponins, terpenoids, tannins, and essential oils which are repeatedly reported to possess analgesic and anti-inflammatory activities. The current findings put scientific evidence about the traditional claimed uses of Echinops kebericho M. for painful conditions and inflammation purpose in Ethiopian folk medicines.
Further investigations should be conducted on the fractionation to determine the most active fraction, constituent isolation, binding studies and electrophysiological procedures may also be useful to fully elucidate anti pain and anti-inflammatory and specific mechanisms of E. Kebericho related to these mechanisms.
Abbreviations
ASA, acetyl salicylic acid; EK, Echinops kebericho M; CNS, central nervous system; COX, cyclooxygenase; IL, interleukin; iNOS, inducible nitric oxide synthase; NSAID, nonsteroidal anti-inflammatory drug; NF-κ, nuclear factor kappa; PGs, prostaglandins; TNF, tumor necrosis factor.
Data Sharing Statement
The datasets used and or analyzed during the current work are available from the corresponding author up on a reasonable request.
Ethics Approval and Consent
Ethical clearance was obtained from Department of Pharmacology, School of Pharmacy Collage of Medicine and Health Science University of Gondar.
Acknowledgment
We would like to acknowledge Debre-tabor University for providing financial support. We are also grateful to Mr Zewudu Birhanu (B. pharm, MSc, associate professor) for providing carrageenan.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work. TY conceived the idea, drapted the proposal, collected the plant matterials and conducted the actual laboratory work. TY and YKE prepared and critically reviewed the final manuscript for publication. EMB, YKE, MG and MA were involved in the design and implementation stage of the study, and revising the the manuscript critically for important intellectual content. All authors read and approved the final version of the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Kumar KH, Elavarasi P. Definition of pain and classification of pain disorders. J Advan Clin Res Insights. 2016;3(3):87–90. doi:10.15713/ins.jcri.112
2. Stanton-Hicks M, Jänig W, Hassenbusch S, Haddox J, Boas R, Wilson P. Reflex sympathetic dystrophy: changing concepts and taxonomy. Pain. 1995;63(1):127–133.
3. Tadele A, Asres K, Melaku D, Mekonnen W. In vivo anti-inflammatory and antinociceptive activities of the leaf extracts of Clematis simensis Fresen. Ethiop Pharm J. 2009;27:33–41.
4. Salzano S. Redox Regulation of Inflammation and Immunity. University of Brighton; 2013.
5. WHO. WHO Guidelines on the Pharmacological Treatment of Persisting Pain in Children with Medical Illnesses. Thieme New York: World Health Organization; 2012.
6. Calati RBC, Artero S, Ilgen M, Courtet P. The impact of physical pain on suicidal thoughts and behaviors meta-analyses. J Psychiatr Res. 2015;71:16–32. doi:10.1016/j.jpsychires.2015.09.004
7. Henschke N, Kamper SJ, Maher CG, editors. The epidemiology and economic consequences of pain.
8. Geremew H, Shibeshi W, Tamiru W, Engdawork E. Experimental evaluation of analgesic and anti-inflammatory activity of 80% methanolic leaf extract of Moringa stenopetala Bak. F. in mice. Ethiop Pharm J. 2015;31(1):15–26. doi:10.4314/epj.v31i1.2
9. Tamrat Y, Nedi T, Assefa S, Teklehaymanot T, Shibeshi W. Anti-inflammatory and analgesic activities of solvent fractions of the leaves of Moringa stenopetala Bak. (Moringaceae) in mice models. BMC Complement Altern Med. 2017;17(1):473. doi:10.1186/s12906-017-1982-y
10. Schug SA, Garrett WR, Gillespie G. Opioid and non-opioid analgesics. Best Pract Res Clin Anaesthesiol. 2003;17(1):91–110. doi:10.1053/bean.2003.0267
11. Yonathan M, Asres K, Assefa A, Bucar F. In vivo anti-inflammatory and anti-nociceptive activities of Cheilanthes farinosa. J Ethnopharmacol. 2006;108(3):462–470. doi:10.1016/j.jep.2006.06.006
12. Abera B. Medicinal plants used in traditional medicine by Oromo people, Ghimbi District, Southwest Ethiopia. J Ethnobiol Ethnomed. 2014;10(1):40. doi:10.1186/1746-4269-10-40
13. Getnet Z, Chandrodyam S, Masresha G. Studies on traditional medicinal plants in Ambagiorgis area of Wogera District, Amhara Regional State, Ethiopia. Int J Pure Appl Biosci. 2016;4:38–45. doi:10.18782/2320-7051.2240
14. Ameya G, Gure A, Dessalegn E. Antimicrobial activity of Echinops Kebericho against human pathogenic bacteria and fungi. Afr J Trad Compl Altern Med. 2016;13(6):199–203. doi:10.21010/ajtcam.v13i6.29
15. Teklehaymanot T, Giday M, Medhin G, Mekonnen Y. Knowledge and use of medicinal plants by people around Debre Libanos monastery in Ethiopia. J Ethnopharmacol. 2007;111(2):271–283. doi:10.1016/j.jep.2006.11.019
16. Mengesha GG. Ethnobotanical survey of medicinal plants used in treating human and livestock health problems in Mandura Woreda of Benishangul Gumuz, Ethiopia. Adv Med Plant Res. 2016;4(1):11–26.
17. Mesfin F, Seta T, Assefa A. An Ethnobotanical Study of Medicinal Plants in Amaro Woreda. Ethiopia; 2014.
18. Alemu Y, Mekonnen Z, Zeynudin A, Yohannes M, Biruksew A, Suleman S. Anti-schistosomal activities of Echinops kebericho Mesfin root and Hagenia abyssinica (Bruce) JF Gmel flower part crude extracts in Swiss albino mice. Asian Pac J Trop Med. 2018;11(10):570. doi:10.4103/1995-7645.244517
19. Shiferie F, Shibeshi W. In vivo antidiarrheal and ex-vivo spasmolytic activities of the aqueous extract of the roots of Echinops kebericho Mesfin (Asteraceae) in rodents and isolated guinea-pig ileum. Int J Pharm Pharmacol. 2013;2(7):110–116.
20. Worlein JM, Baker K, Bloomsmith M, Coleman K, Koban TL, editors. The Eighth Edition of the Guide for the Care and Use of Laboratory Animals. Implications for Behavioral Management. Malden MA: wiley-blackwell; 2011.
21. Kumar RS, Rajkapoor B, Perumal P, Dhanasekaran T, Jose MA, Jothimanivannan C. Anti-inflammatory and analgesic activities of ethanol extract of Indigofera trita Linn. Pharmacologyonline. 2009;1:278–289.
22. Neto A, Costa J, Belati C, et al. Analgesic and anti-inflammatory activity of a crude root extract of Pfaffia glomerata (Spreng) Pedersen. J Ethnopharmacol. 2005;96(1–2):87–91. doi:10.1016/j.jep.2004.08.035
23. Rahman M, Chakma J, Islam S, Rana M, Ahmed N. Analgesic and anti-inflammatory effect of Clausena suffruticosa root extract in animal model. J Sci Res. 2011;3(3):631–639. doi:10.3329/jsr.v3i3.7594
24. Liao C-R, Kao C-P, Peng W-H, Chang Y-S, Lai S-C, Ho Y-L. Analgesic and anti-inflammatory activities of methanol extract of Ficus pumila L. in mice. Evid Based Complement Altern Med. 2012;2012.
25. Goldberg DS. Intervening at the right point in the causal pathways: law, policy, and the devastating impact of pain across the globe. Annals Health L. 2013;22:198.
26. Subedi NK, Rahman S, Akbar MA. Analgesic and antipyretic activities of methanol extract and its fraction from the root of schoenoplectus grossus. Evid Based Complement Altern Med. 2016;2016:1–8. doi:10.1155/2016/3820704
27. Konaté K, Bassolé IHN, Hilou A, et al. Toxicity assessment and analgesic activity investigation of aqueous acetone extracts of Sida acuta Burn f. and Sida cordifolia L. (Malvaceae), medicinal plants of Burkina Faso. BMC Complement Altern Med. 2012;12(1):120.
28. Tadiwos Y, Nedi T, Engidawork E. Analgesic and anti-inflammatory activities of 80% methanol root extract of Jasminum abyssinicum Hochst. ex. Dc. (Oleaceae) in mice. J Ethnopharmacol. 2017;202:281–289. doi:10.1016/j.jep.2017.02.036
29. Demsie DG, Yimer EM, Berhe AH, Altaye BM, Berhe DF. Anti-nociceptive and anti-inflammatory activities of crude root extract and solvent fractions of Cucumis ficifolius in mice model. J Pain Res. 2019;12:1399. doi:10.2147/JPR.S193029
30. Debebe E, Makonnen E, Debella A. Anti-nociceptive effect of the methanolic extract of roots of andrachneaspera in three models of Nociception. Pharmacol Online. 2007;1:41–48.
31. Sun K, Song X, Jia R, et al. Evaluation of analgesic and anti-inflammatory activities of water extract of galla chinensis in vivo models. Evid Based Complement Altern Med. 2018;2018. doi:10.1155/2018/6784032
32. Badole SL, Zanwar AA, Ghule AE, Ghosh P, Bodhankar SL. Analgesic and anti-inflammatory activity of alcoholic extract of stem bark of Pongamia pinnata (L.) Pierre. Biomed Aging Pathol. 2012;2(1):19–23. doi:10.1016/j.biomag.2011.11.001
33. Hymete A, Rohloff J, Iversen TH, Kjøsen H. Volatile constituents of the roots of Echinops kebericho Mesfin. Flav Frag J. 2007;22(1):35–38. doi:10.1002/ffj.1746
34. Wolde‑Mariam M, Yarlagadda R, Asres K. In vivo anti‑inflammatory and antinociceptive activities of the aerial part extract of Dicliptera laxata. Int J Green Pharm. 2013;7:3.
35. Çadirci E, Süleyman H, Gürbüz P, Güvenalp UZA, DEMİREZER LÖ. Anti-inflammatory effects of different extracts from three Salvia species. Turk J Biol. 2012;36(1):59–64.
36. Alwashli A, Al-sobarry M, Alnamer R, Cherrah Y, Alaoui K. Analgesic and anti-inflammatory activities of boswellia elongata balf methanolic extracts, as endemic plants in Yemen. J Biol Active Prod Nat. 2012;2(2):90–98. doi:10.1080/22311866.2012.10719114
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.