Back to Journals » Therapeutics and Clinical Risk Management » Volume 18
Evaluating the Safety of Maribavir for the Treatment of Cytomegalovirus
Received 21 December 2021
Accepted for publication 28 February 2022
Published 12 March 2022 Volume 2022:18 Pages 223—232
DOI https://doi.org/10.2147/TCRM.S303052
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Garry Walsh
Ronak G Gandhi,1 Camille N Kotton2
1Department of Pharmacy, Massachusetts General Hospital, Boston, MA, USA; 2Infectious Diseases Division, Massachusetts General Hospital, Boston, MA, USA
Correspondence: Ronak G Gandhi, Senior Attending Pharmacist – Infectious Diseases, Department of Pharmacy, Massachusetts General Hospital, 55 Fruit Street, GRB 005, Boston, MA 02114, USA, Tel +1 617-643-6570, Fax +1 617-726-9232, Email [email protected]
Purpose of Review: Cytomegalovirus (CMV) infections are a common complication in solid organ (SOT) and hematopoietic stem cell transplant (HSCT) recipients, leading to increased morbidity and mortality. Currently available treatment options have reduced the burden of infection, but utilization of these agents can be limited by toxicities such as nephrotoxicity and/or myelosuppression as well as emergence of resistance. The expansion of our current armamentarium towards CMV infection is crucial. Here, we review an emerging therapy, maribavir, and the safety and efficacy of this potential new agent for the prophylaxis and treatment of CMV infections including resistant/refractory disease.
Recent Findings: Maribavir is a novel agent with CMV activity approved by Federal Food and Drug Administration (FDA) in December 2021 for resistant/refractory disease. Compared to currently available treatment for CMV infection, maribavir has a unique mechanism of action, retains activity against most (val)ganciclovir resistant strains, provides a more predictable pharmacokinetic profile, and fewer severe toxicities. Maribavir has been studied in phase 2 and 3 studies with ongoing phase 3 studies. While maribavir failed to meet the primary endpoints in the initial phase 3 study for prophylaxis therapy in allogeneic-HSCT and liver transplant recipients, results from the phase 2 study when used for pre-emptive therapy after HSCT show similar efficacy to valganciclovir, and results from the phase 3 study examining resistant/refractory disease demonstrate superiority to investigator-initiated therapy of (val)ganciclovir, foscarnet, or cidofovir.
Summary: Maribavir provides a new agent for the management of resistant/refractory CMV infection. Results of the recently published phase 3 study provide further insight into the role of this novel therapy.
Keywords: cytomegalovirus, maribavir, safety, efficacy
Introduction
Cytomegalovirus (CMV) infections are among the most common infections after solid organ (SOT) and hematopoietic stem cell transplant (HSCT). Optimal prevention and treatment leads to better overall outcomes.1,2 Clinical symptoms from CMV infection ranges from asymptomatic CMV DNAemia (detectable CMV DNA in plasma, serum, or whole-blood) to CMV syndrome with fevers, malaise, leukopenia, thrombocytopenia, and hepatitis to more severe end organ disease with manifestations such as colitis, retinitis, and pneumonitis.3 The oral prodrug valganciclovir and intravenous ganciclovir are the most common antiviral agents used for prevention and treatment. Although generally well tolerated, side effects such as leukopenia and neutropenia can develop, especially when used in combination with other transplant medicines, which can sometimes be a treatment limiting, although we do not have therapeutic options with a lower toxicity profile. Results from the phase 2 study when maribavir is used for pre-emptive therapy after HSCT show similar efficacy to valganciclovir,4 suggesting that this may be a new treatment option; the phase 3 trial (clinicaltrials.gov NCT02927067) is underway.
Furthermore, a small but clinically important number of patients develop resistant and/or refractory CMV infection to valganciclovir, estimated to comprise up to 20% in some series, especially in those who have undergone lung and intestinal transplants.5 Management of resistant and/or refractory CMV has always been clinically challenging, necessitating the use of antivirals with a higher side effect profile such as foscarnet and cidofovir (Table 1).2 Novel anti-CMV drugs in recent clinical development have either a relatively low barrier to developing resistance (letermovir, approved only for prophylaxis, not treatment) or are not in active development (brincidofovir).5 Fortunately, phase 3 data on maribavir shows significant efficacy and tolerability for treatment of resistant and/or refractory CMV infection compared to investigator-initiated therapy.6 In this review, we discuss the use of and evaluate the safety of maribavir for treatment of CMV infection.
 |
Table 1 Agents for CMV Treatment or Prophylaxis |
Mechanism of Action/Pharmacology
Benzimidazoles were the original terminase inhibitors in clinical development for CMV and were considered and attractive option as they did not interfere with host-cellular DNA replication and targeted the core terminase complex of proteins UL51, UL56, and UL89, which are all individually needed for viral replication.7–9 Benzimidazoles such as 2-Bromo-5,6-dichloro-1-β-d-ribofuranosyl-1H-benzimidazole (BDCRB) were unfortunately halted in early clinical development due to unfavorable in vivo metabolism.9 Maribavir is a novel benzimidazole I-riboside compound; although it shares some structural similarities to BDCRB, it acts through a different mechanism. Maribavir competitively inhibits CMV UL97 protein kinase without being an enzymatic substrate of this protein kinase which inhibits DNA synthesis and blocks nuclear egress of viral particles from infected cells.9–12 UL97 is responsible for phosphorylating viral and host cellular proteins but is not absolutely vital for replication into tissue culture as are CMV DNA polymerase UL54 or core terminase complex proteins pUL51, pUL56, and pUL89. This has been noted in mutant viruses that have deleted UL97 gene but remain viable with severe replication defects.9 The complete function of how UL97 and its function on replication and how maribavir impacts inhibition of viral replication remains to be further determined.
Antiviral activity of maribavir inhibits replication of CMV at concentrations of <1µM to ~15µM.9,13 Maribavir is only available as an oral formulation, undergoes rapid absorption with a bioavailability of 30–40% and is highly plasma protein bound (>97%) causing free plasma concentrations to be 100-fold lower than total drug plasma concentrations. Administration with meals high in fat content further decrease maribavir concentrations by 30%. Maribavir primarily exhibits linear pharmacokinetics (PK) achieving a Cmax 1–3 hours after administration with a plasma half-life of 3–5 hours. Maribavir is extensively hepatically metabolized and primarily eliminated via biliary excretion and clearance is not impacted by renal impairment (<3% urinary excretion).14,15
Prophylaxis Trials
Two Phase 1 trials14,15 evaluating the safety, activity, and PK profile of maribavir against CMV were conducted in healthy volunteers and HIV-infected patients. Doses ranged from 50 to 1600 mg with all doses showing no significant adverse events other than headache and dysgeusia occurring in up to 80% of patients, most commonly in those receiving 800 mg or higher. Due to the high degree of protein binding, free drug concentrations were less than 30 ng/mL when doses of ≤200 mg were administered. Based on predictive PK modeling, maribavir doses of 400 mg twice daily would maintain free maribavir plasma concentration above the EC50 (50% effective concentration) of CMV for the entire dosing interval.16
Maribavir was then studied for prophylaxis, in a multicenter, randomized, double-blind, placebo-controlled, dose-ranging phase 2 study evaluating its use for prevention of CMV infection in allogeneic stem cell transplant (HSCT) recipients.17 Maribavir 100 mg twice daily, 400 mg daily, and 400 mg twice daily were evaluated to start at engraftment and could be continued for up to 12 weeks after transplantation. Though higher doses were evaluated in the phase 1 study, 400 mg twice daily was the highest dose allowed due to dysgeusia and concern for nutritional status in these patients.16 Time to onset of CMV infection or diseases was the primary endpoint evaluated. All doses showed similar efficacy in reduction of CMV infection as determined by antigenemia or DNA PCR. CMV disease did not occur in any group receiving maribavir and occurred in three patients in the placebo group only. Patients receiving 400 mg twice daily experienced the highest rates of adverse events with dysgeusia and gastrointestinal side effects being the most common.17 As demonstrated in the phase 1 studies, maribavir displayed linear PK as all dosing schemes had similar trough concentrations, but the 400 mg doses yielded a plasma peak concentration 2.5x higher than the 100 mg dose. Additionally, the 400 mg twice daily doses demonstrated the highest 24-hour drug exposure.9,16,17
Based on the results of this phase 2 study, a subsequent phase 3 randomized, double blind, place-controlled study assessing maribavir prophylaxis for prevention of CMV disease in allogeneic HSCT patient was conducted comparing maribavir 100 mg twice daily to placebo.18 The dose chosen in this study was based on results of the phase 2 study, demonstrating similar efficacy as the 400 mg twice daily with lower incidence of adverse events.9,16 Similar to the phase 2 study, maribavir was initiated following engraftment and continued for up to 12 weeks after HSCT. Unfortunately, maribavir did not reduce CMV infection or disease compared to placebo at day 100 or at 6 months post HSCT.18 Maribavir 100 mg twice daily was also evaluated in a randomized, double-blind, multicenter controlled trial, but this time for prophylaxis against CMV in high-risk (CMV D+/R-) liver transplant recipients against oral ganciclovir.19 Disappointingly, maribavir did not meet the non-inferiority endpoint. After failing to meet the primary endpoint in multiple randomized controlled trials, use of maribavir as prophylaxis against CMV in HSCT and SOT recipients were no longer pursued and was only available under compassionate use.9
The study design of the phase 2 and 3 studies may have contributed to negative results of both studies. In both studies, patients with any evidence of CMV infection during randomization were excluded to ensure subject safety in the placebo group but may have blunted the response to those at higher risk that may have benefited from prophylactic maribavir.16–18 In the phase 2 study, the sample size was likely too small causing a probable overestimation of the overall effects of the 100 mg twice daily dosing scheme compared to the higher doses. This is evident by the lack of dose effect in the overall incidence curve as well as in the rates of acute grade II or higher graft versus host disease (GVHD) being 46% in the placebo group but only 14% in the 100 mg twice daily group and 29 and 23% in the 400 mg daily and twice daily groups, respectively.16,17 The higher rates of GVHD may also have contributed to decreased absorption of oral medications leading to a potential decrease in efficacy. In regards to the phase 3 study, 100 mg twice daily was chosen based on the dose response in the phase 2 study and less adverse events, primarily dysgeusia, even though 400 mg twice daily was statistically (not significant) the best regimen in the phase 2 study and provided free maribavir concentrations above the EC50 based on the phase-1 studies.9,16,18 Additionally, investigators were allowed to use local CMV testing to decide if pre-emptive CMV therapy should be initiated. This included both CMV PCR or CMV antigenemia, though CMV PCR is more sensitive in detecting early disease, it also more prone to false-positive results, which may have impacted study results.16
Pre-Emptive/Primary Treatment Trials
Given that transplant patients often have pre-existing leukopenia and are not always able to tolerate (val)ganciclovir, a phase 2, open-labeled study was conducted comparing maribavir to valganciclovir for pre-emptive treatment of CMV reactivation in HSCT and SOT recipients.4 Patients received 400, 800, and 1200 mg twice daily of maribavir or valganciclovir 900 mg twice daily for weeks 1–3 then 900 mg daily for up to 12 weeks. The primary endpoint was undetectable viral load within 3 and 6 weeks after treatment initiation. Response to treatment was 62% and 79% at 3 and 6 weeks for maribavir, respectively, compared to 56% and 67% for valganciclovir. Recurrence rates during the 12-week trial period were similar, 22% for maribavir and 18% for valganciclovir. Adverse events leading to discontinuation were more common in the maribavir group (23%) compared to valganciclovir (12%). Among the most common side effects were dysgeusia with maribavir (40%, none needing discontinuation) and leukopenia with valganciclovir (8%).4 Overall, this trial demonstrated that maribavir’s efficacy was similar to that of valganciclovir for clearing CMV DNAemia among transplant recipients. The phase 3 trial,
(clinicaltrials.gov NCT02927067) evaluating maribavir 400 mg twice daily compared to valganciclovir for treatment of first-episode asymptomatic CMV infection in HSCT recipients, is still recruiting patients, with an estimated enrollment of 550 patients.
Resistant/Refractory Trials
Despite the failed prophylaxis studies, maribavir was still being utilized under compassionate use for resistant/refractory or salvage CMV cases. A case series of six patients including both SOT and HSCT received maribavir 400 mg twice daily as salvage therapy.20 In the case series, four patients had ganciclovir-resistant disease and five patients had tissue invasive disease. All patients had a detectable viral load at the initiation of maribavir. Three patients cleared their CMV DNAemia on monotherapy between days 6–41 and one patient cleared their CMV DNAemia while receiving concomitant foscarnet.
Based on early reports of maribavir’s efficacy for treatment of resistant/refractory disease, a phase 2 study was conducted. This study was a randomized, double-blind, dose-ranging study in HSCT and SOT recipients with resistant/refractory CMV.21 Patients were randomized in a 1:1:1 fashion receiving 400, 800, or 1200 mg twice daily for up to 24 weeks with the primary endpoint of undetectable viral load at 6 weeks. There were 40 patients in each arm and approximately 60% were SOT recipients. The majority of patients (64.2%) had asymptomatic CMV DNAemia and 13.3% of patients had tissue invasive disease. At the 6 and 24-week mark, 67% and 72% of patients had an undetectable viral load, respectively, with the proportion being similar across all doses. Recurrence of CMV occurred in 29% of patients and of those 52% had developed maribavir-resistant virus. Recurrence was more common in SOT than HSCT, even though rates of clearance were similar between both groups. Similar to previous studies, dysgeusia was the most common adverse event occurring in 65% of patients, but only led to one patient discontinuing therapy.21
Subsequently, a phase 3 study was initiated, evaluating maribavir for resistant/refractory CMV infection in both HSCT and SOT recipients.6 The study assessed maribavir 400 mg twice daily to investigator-assigned therapy (IAT) including (val)ganciclovir, foscarnet, or cidofovir. Patients were randomized in a 2:1 fashion. The primary endpoint was confirmed CMV clearance at the end of week 8. Secondary endpoints assessed achievement of CMV clearance and symptoms control at week 8 and if maintained through week 16. The study included 235 patients in the maribavir arm and 117 patients in the IAT group, with ~60% SOT/40% HSCT recipients in both groups. There was a rescue arm for patients in the IAT arm who had an increase in CMV DNA ≥1 log10 from baseline, < 1 log10 decrease from baseline or new/continued symptomatic tissue-invasive infection or did not achieve clearance of CMV DNAemia and demonstrate intolerance to IAT at ≥3 weeks. Overall, 183 (77.9%) of patients in the maribavir arm completed 8 weeks of study assigned treatment compared to only 37 (31.6%) in the IAT group of whom 22 (18.8%) proceeding to maribavir rescue. The primary endpoint was achieved in 131 (55.7%) of patients receiving maribavir compared to 28 (23.9%) in the IAT group (p<0.001). Of those requiring maribavir rescue, 11 of 22 (50%) achieved clearance of CMV DNAemia at week 8. This trend continued through week 16, with 44 (18.7%) in the maribavir arm with CMV clearance and maintenance compared to 12 (10.3%) in the IAT group (p<0.013). Overall, serious treatment-emergent adverse effects were similar between the maribavir and IAT arm, however study drug discontinuation occurred in only 13.2% in the maribavir arm compared to 31.9% in the IAT arm.22,23 Overall, maribavir was superior to IAT for CMV clearance as well as tolerability, symptom control, with post-treatment maintenance of these effects. The results of this phase 3 trial, as well as prior data, were taken into consideration by the FDA when they recently approved Takeda’s new drug application for resistant/refractory disease.
Place in Practice: Narrow Spectrum of Coverage
Maribavir primarily provides highly specific antiviral activity against CMV. Clinically, this more narrow spectrum of coverage may be important, as (val)ganciclovir has broader coverage against human herpesviruses, including herpes and varicella (Table 1) in addition to CMV. Clinicians wishing to prevent other human herpesvirus infections in patients on treatment with maribavir would need to include another antiviral agent such as (val)acyclovir or famciclovir. This more narrow coverage of the primary therapy both necessitates an additional pill burden, and when overlooked, may result in a higher rate of human herpesvirus infections such as disseminated zoster. Maribavir has potent efficacy against Epstein Barr virus (EBV) infection in vitro, via inhibition of viral DNA replication and of virus transcription, although there are no clinical data suggesting efficacy.24
Safety and Side Effects
In the phase 2 trial of maribavir for resistant/refractory disease, the most common side effects were gastrointestinal in nature, including dysgeusia (65.0%), nausea (34.2%), vomiting (29.2%), diarrhea (23.3%), fatigue (20.8%), anemia (20.0%), peripheral edema (19.2%), headache (15.8%), and renal impairment (15.8%).21 In general, side effects were less with lower doses of maribavir. Although common, dysgeusia, often described as a “metallic” or “bitter” taste, was tolerable in most patients, with only one patient discontinuing treatment in the phase 2 trial and very few patients across multiple maribavir trials. Gastrointestinal adverse events such as nausea, vomiting, and diarrhea resulted in treatment cessation in three patients. Alterations in vital signs, clinical laboratory data, and electrocardiograms were generally small. Neutropenia was present in 16% of patients at baseline and occurred at least once in 11% of patients during the study; significant myelosuppression due to maribavir was not noted. In the prior study of lower dose maribavir for prophylaxis in bone marrow transplant patients, similar side effects including dysgeusia, nausea, vomiting and rash were seen in a minority of patients.17 In the randomized phase 3 trial of 352 patients comparing maribavir with investigator initiated treatment, maribavir was associated with less acute kidney injury compared with foscarnet (8.5% vs 21.3%) and neutropenia compared with (val)ganciclovir (9.4% vs 33.9%), and fewer patients discontinued maribavir due to treatment-emergent adverse events (13.2% vs 31.9%). Dysgeusia was the most frequently reported treatment-emergent adverse event in the maribavir group (maribavir: 37.2% versus 3.4%) but led to discontinuation in only 0.9% of patients.6 Overall, no severe side effects were found in either trial for maribavir.
Drug Interactions
Clinically significant drug-interactions are of particular interest as maribavir would be an agent primarily used in the SOT and HSCT patient population. Transplant recipients receive numerous medications including immunosuppressants as well as other therapies to manage their co-morbidities of which majority are hepatically metabolized and can be significant substrates, inducers, or inhibitors of the cytochrome P-450 (CYP-450) system.25,26 Maribavir is primarily hepatically metabolized, specifically through CYP-3A4 (70–85%) and CYP-1A2 (15–30%). Maribavir is also a substrate of P-glycoprotein and uridine diphosphate glucuronosyltransferases (UGTs).27 Initial studies conducted in healthy volunteers determined that maribavir did not impact N-acetyltransferase-2 or xanthine oxidase activities.26 Additionally, maribavir had minimal interactions with drugs that are substrates of CYP-450 isozymes. Specifically, maribavir is a weak inhibitor of CYP-2C19, P-glycoprotein, and potentially UGT1A1 and is not considered to be an inducer of CYP-450 isozymes.26–28 Though maribavir should have limited interactions with most medications, Table 2 summarizes some clinically relevant interactions and how to manage them. As a substrate of CYP-3A4 and CYP-1A2, antifungal agent ketoconazole increased maribavir area under the curve (AUC) by 54%, but maribavir was not affected by voriconazole and is unlikely to be impacted by posaconazole, isavuconazole, or itraconazole.25,27–29 Rifampin decreased maribavir exposure by 61%. Antacids had no effect of maribavir metabolism.30 As a weak inhibitor of P-glycoprotein, a randomized, double-blind PK study in renal transplant recipients demonstrated that maribavir increased tacrolimus AUC by 51% leading to significant increase in both peak and trough concentrations.26–30 In the phase 3 trial for resistant/refractory disease, increased blood immunosuppressant drug levels were reported in 21 (9.0%) patients in the maribavir group (tacrolimus: n=19, sirolimus: n=2) and in 1 (0.9%) patient in the IAT (valganciclovir/ganciclovir) group.6 Monitoring tacrolimus as well as other similar agents (cyclosporine, sirolimus, and everolimus) concentrations is prudent while on maribavir and after discontinuation to prevent supra-and-subtherapeutic concentrations.
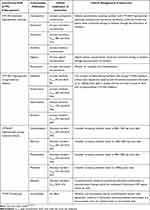 |
Table 2 Clinically Significant Drug Interactions with Maribavir |
Resistance and Antiviral Activity
Maribavir resistance has been noted in vitro as well as in clinical trials caused by UL97 mutations.7,31,32 Mutations specifically at codons 409, 411, and newly characterized codon 480 are major causes of moderate-high grade maribavir resistance in those who had recurrent infection after prolonged maribavir therapy or those who did not clear their infection while on therapy.33,34 Documentation of resistance was not provided in the earlier phase 2 or317,18 prophylaxis studies, however, in the latter phase 2 studies genotypic analysis was conducted on those with recurrent or persistent infections. The analysis overall included 29 patients with recurrent CMV after clearance of infection or those who did not respond to therapy after 14 days; 23 (79%) had UL97 genotyping data available, of which 17 (74%) had mutations at codon 409 or 411 (T409M or H411Y) and 5 (22%) had mutations at codon 480 (C480F).21 Interestingly, development of resistance was uniform across all maribavir doses. Though not as well described as mutations at UL97, resistance to maribavir can occur through another CMV gene, UL27, which confers low-level resistance. However, to date, this has only been described in-vitro and has never been reported in patients receiving maribavir treatment for CMV infection.33,35–37 The cause of this mutation suggests that maribavir inhibition of UL97 kinase activity has led to a compensatory mechanism of resistance.
Uniquely, maribavir typically retain antiviral activity even when resistance is present to conventional anti-CMV agents such as (val)ganciclovir, foscarnet, and cidofovir. (Val)ganciclovir is a substrate for UL97 and relies on initial phosphorylation by the viral UL97 enzyme for its antiviral activity.9,38 Mutations in UL97 at codons 460, 520, and 590–607 are primarily responsible for (val)ganciclovir resistance in CMV and are distinct from the codons that confer resistance to maribavir.34,38,39 Additionally, cross-resistance with foscarnet or cidofovir is not expected as maribavir does not target CMV DNA polymerase, in particular CMV gene UL54, which confers resistance to foscarnet, cidofovir, as well as (val)ganciclovir.34,35
Given the development of maribavir as a therapeutic for resistant/refractory CMV infection, understanding the limitations in that sphere are important. Cross-resistance between ganciclovir and maribavir has been described and thus far at least 10 distinct mutations leading to cross-resistance have been reported.40 Mutation at codon 480 (C480F) that leads to high-level maribavir resistance also confers low-level resistance to (val)ganciclovir.34 Interestingly, one patient in the phase 2 study with resistant/refractory disease who received a prolonged course of ganciclovir prior to receiving maribavir developed a novel UL97 mutation at codon 342 (F342Y). This mutation conferred ganciclovir resistance as well as low-level maribavir cross-resistance, which ultimately promulgated to a mutation at codon H411Y leading to maribavir failure.39 Further research is still needed to better understand this delicate interplay between mainstay of therapy and maribavir.
As the emergence of drug resistant CMV continues to grow, expansion of our armamentarium and understanding antiviral activity of these agents is crucial, especially when used in combination for resistant or refractory disease to improve treatment outcomes. Maribavir has been studied in combination with other anti-CMV agent to understand if synergy or antagonism exists, including both wild type and drug-resistant mutant strains. Overall, maribavir has been shown to be antagonistic and should not be used concurrently with (val)ganciclovir as maribavir inhibits UL97 kinase activity and (val)ganciclovir relies of UL97 kinase mediated phosphorylation for its activity.10 When combined with other anti-CMV agents such as foscarnet, cidofovir, and letermovir, maribavir was shown to have an additive interaction. Interestingly, maribavir in combination with mTOR inhibitor rapamycin (same compound as sirolimus) showed strong synergy, which could ultimately lead to a clinically significant combination.10 Sirolimus is sometimes used in place of calcineurin inhibitors such as tacrolimus in transplant recipients, as it is associated with lower risk of developing CMV infections.10 However, similarly to tacrolimus though not extensively studied, it is expected that sirolimus concentrations will increase when in used in combination with maribavir likely through P-glycoprotein inhibition, so sirolimus monitoring and further studies are warranted.26,27,30
Conclusion and Future Directions
The management and prevention of CMV infection with currently available therapies (val)ganciclovir, foscarnet, cidofovir, and letermovir have drastically improved morbidity and mortality in SOT and HSCT recipients.1,2 Development and approval of novel therapeutics is still critically necessary, however, due to the toxicities and emergence of resistance often limiting their use. Maribavir provides a unique alternative agent to the standard of care for resistant/refractory disease, with a more predictable PK profile and larger margin of safety.4,6,14,15,17,18,21 The approval of maribavir for resistant/refractory disease and phase 3 trial for treatment in pre-emptive therapy could open the door for future real-world studies, including combination therapy for CMV, as well as potentially pave the way for further pipeline agents being approved.
The FDA approval of maribavir is only for resistant/refractory disease as the treatment trials are not yet complete, and maribavir failed to meet the primary endpoint in the phase 3 prophylaxis study for prevention of CMV disease in allogenic HSCT recipients. Concerns regarding CMV testing and optimal maribavir dosing in the prophylaxis study have been raised.16 The approval of maribavir could lead to real-world experience studies, utilizing 400 mg twice daily rather than 100 mg twice daily as universal prophylaxis in both SOT and HSCT recipients. Studies also evaluating its use as prophylaxis after acute treatment of CMV (especially with resistant/refractory infection) as an alternative to pre-emptive monitoring should also be considered. The safety profile and oral formulation of maribavir compared to DNA polymerase inhibitor agents may encourage further investigator-initiated studies and possible off-label use.
As agents with novel mechanisms of action such as maribavir become available, combination therapy against CMV truly becomes a possibility, for the first time, especially for (val)ganciclovir resistant disease. Though letermovir is currently available for clinical use and has a unique mechanism of action compared to DNA polymerase inhibitors, it is primarily studied as prophylaxis for HSCT recipients rather than the treatment of CMV1,9 and had a very low barrier to development of antiviral resistance. In vitro studies have shown that maribavir is at least additive when combined with foscarnet and cidofovir and potentially synergistic when used with sirolimus.10 Future studies are now needed to determine if combination therapy with directly or indirectly acting CMV agents is beneficial for the treatment of CMV infection or disease compared to monotherapy, as demonstrated in hepatitis C virus or human immunodeficiency virus infections.41,42
The approval of maribavir expands the armamentarium for the management of complex CMV infections. The safety profile of maribavir should provide alternatives to marrow suppressive and nephrotoxic agents such as foscarnet or cidofovir for (val)ganciclovir resistant disease. Maribavir will potentially be the only CMV treatment agent available that is not primarily renally excreted, which will hopefully eliminate sub or supratherapeutic concentrations seen with DNA polymerase inhibitors in those patients with renal impairment leading to further toxicities or development of resistance.1,2 The results of the ongoing phase 3 treatment study (clinicaltrials.gov NCT 02927067) will provide further insight on the role of maribavir for CMV infection. Additionally, monitoring and further understanding of disease recurrence and development of maribavir resistance while on therapy will be crucial, based on the phase 2 study and results from the recent phase 3 study.6,21 Overall, the approval of maribavir will provide clinicians with another treatment option as well development of newer pharmacologic and non-pharmacologic interventions for the management of CMV.
Funding
There is no funding to report.
Disclosure
Ronak G. Gandhi has been a paid consultant to Takeda and Shionogi. Camille N. Kotton has been a paid consultant to Biotest, GSK, Merck, Takeda, Roche Diagnostics, and Hookipa. The authors report no other conflicts of interest in this work.
References
1. Kotton CN. CMV: prevention, Diagnosis and Therapy. Am J Transplant. 2013;13(Suppl 3):
2. Kotton CN, Kumar D, Caliendo AM, et al. The Third International Consensus Guidelines on the Management of Cytomegalovirus in Solid-organ Transplantation. Transplantation. 2018;102(6):900–931. doi:10.1097/TP.0000000000002191
3. Ljungman P, Boeckh M, Hirsch HH, et al. Definitions of Cytomegalovirus Infection and Disease in Transplant Patients for Use in Clinical Trials. Clin Infect Dis. 2017;64(1):87–91. doi:10.1093/cid/ciw668
4. Maertens J, Cordonnier C, Jaksch P, et al. Maribavir for Preemptive Treatment of Cytomegalovirus Reactivation. N Engl J Med. 2019;381(12):1136–1147. doi:10.1056/NEJMoa1714656
5. Chemaly RF, Chou S, Einsele H, et al. Definitions of Resistant and Refractory Cytomegalovirus Infection and Disease in Transplant Recipients for Use in Clinical Trials. Clin Infect Dis. 2019;68(8):1420–1426. doi:10.1093/cid/ciy696
6. Avery RK, Alain S, Alexander BD, et al. Maribavir for Refractory Cytomegalovirus Infections With or Without Resistance Post-Transplant: results from a Phase 3 Randomized Clinical Trial. Clin Infect Dis. 2021. doi:10.1093/cid/ciab988
7. Biron KK, Harvey RJ, Chamberlain SC, et al. Potent and selective inhibition of human cytomegalovirus replication by 1263W94, a benzimidazole L-riboside with a unique mode of action. Antimicrob Agents Chemother. 2002;46(8):2365–2372. doi:10.1128/AAC.46.8.2365-2372.2002
8. Evers DL, Komazin G, Shin D, Hwang DD, Townsend LB, Drach JC. Interactions among antiviral drugs acting late in the replication cycle of human cytomegalovirus. Antiviral Res. 2002;56(1):61–72. doi:10.1016/S0166-3542(02)00094-3
9. Hakki M. Moving Past Ganciclovir and Foscarnet: advances in CMV Therapy. Curr Hematol Malig Rep. 2020;15(2):90–102. doi:10.1007/s11899-020-00557-6
10. Chou S, Ercolani RJ, Derakhchan K. Antiviral activity of maribavir in combination with other drugs active against human cytomegalovirus. Antiviral Res. 2018;157:128–133. doi:10.1016/j.antiviral.2018.07.013
11. Marschall M, Feichtinger S, Milbradt J. Regulatory roles of protein kinases in cytomegalovirus replication. Adv Virus Res. 2011;80:69–101.
12. Prichard MN. Function of human cytomegalovirus UL97 kinase in viral infection and its inhibition by maribavir. Rev Med Virol. 2009;19(4):215–229. doi:10.1002/rmv.615
13. Aoki FY. Antivirals against herpes viruses. In: Bennett JE, Dolin R, Blaser MJ editor(s). Prinicples and Practice of Infectious Diseases. Elsevier, 2015;8(1):546‐62.
14. Lalezari JP, Aberg JA, Wang LH, et al. Phase I dose escalation trial evaluating the pharmacokinetics, anti-human cytomegalovirus (HCMV) activity, and safety of 1263W94 in human immunodeficiency virus-infected men with asymptomatic HCMV shedding. Antimicrob Agents Chemother. 2002;46(9):2969–2976. doi:10.1128/AAC.46.9.2969-2976.2002
15. Wang LH, Peck RW, Yin Y, Allanson J, Wiggs R, Wire MB. Phase I safety and pharmacokinetic trials of 1263W94, a novel oral anti-human cytomegalovirus agent, in healthy and human immunodeficiency virus-infected subjects. Antimicrob Agents Chemother. 2003;47(4):1334–1342. doi:10.1128/AAC.47.4.1334-1342.2003
16. Marty FM, Boeckh M. Maribavir and human cytomegalovirus-what happened in the clinical trials and why might the drug have failed? Curr Opin Virol. 2011;1(6):555–562. doi:10.1016/j.coviro.2011.10.011
17. Winston DJ, Young JA, Pullarkat V, et al. Maribavir prophylaxis for prevention of cytomegalovirus infection in allogeneic stem cell transplant recipients: a multicenter, randomized, double-blind, placebo-controlled, dose-ranging study. Blood. 2008;111(11):5403–5410. doi:10.1182/blood-2007-11-121558
18. Marty FM, Ljungman P, Papanicolaou GA, et al. Maribavir prophylaxis for prevention of cytomegalovirus disease in recipients of allogeneic stem-cell transplants: a phase 3, double-blind, placebo-controlled, randomised trial. Lancet Infect Dis. 2011;11(4):284–292. doi:10.1016/S1473-3099(11)70024-X
19. Winston DJ, Saliba F, Blumberg E, et al. Efficacy and safety of maribavir dosed at 100 mg orally twice daily for the prevention of cytomegalovirus disease in liver transplant recipients: a randomized, double-blind, multicenter controlled trial. Am J Transplant. 2012;12(11):3021–3030. doi:10.1111/j.1600-6143.2012.04231.x
20. Avery RK, Marty FM, Strasfeld L, et al. Oral maribavir for treatment of refractory or resistant cytomegalovirus infections in transplant recipients. Transpl Infect Dis. 2010;12(6):489–496. doi:10.1111/j.1399-3062.2010.00550.x
21. Papanicolaou GA, Silveira FP, Langston AA, et al. Maribavir for refractory or resistant cytomegalovirus infections in hematopoietic-cell or solid-organ transplant recipients: a randomized, dose-ranging, double-blind, phase 2 study. Clin Infect Dis. 2019;68(8):1255–1264. doi:10.1093/cid/ciy706
22. Duarte R, Alain S, Chemaly R, et al. TMaribavir Versus Investigator-Assigned Therapy for the Treatment of Transplant Recipients With Refractory/Resistant Cytomegalovirus Infection: efficacy Data From a Randomized Phase 3 Open-Label Study
23. Pereira M, Cervera C, Kotton C Efficacy and Safety of Maribavir as a Rescue Treatment for Investigator Assigned Therapy in Transplant Recipients With Refractory or Resistant Cytomegalovirus Infections in the SOLSTICE Study: phase 3 Trial Results
24. Wang FZ, Roy D, Gershburg E, Whitehurst CB, Dittmer DP, Pagano JS. Maribavir inhibits Epstein-Barr virus transcription in addition to viral DNA replication. J Virol. 2009;83(23):12108–12117. doi:10.1128/JVI.01575-09
25. Goldwater DR, Dougherty C, Schumacher M, Villano SA. Effect of ketoconazole on the pharmacokinetics of maribavir in healthy adults. Antimicrob Agents Chemother. 2008;52(5):1794–1798. doi:10.1128/AAC.00951-07
26. Pescovitz MD, Bloom R, Pirsch J, Johnson J, Gelone S, Villano SA. A randomized, double-blind, pharmacokinetic study of oral maribavir with tacrolimus in stable renal transplant recipients. Am J Transplant. 2009;9(10):2324–2330. doi:10.1111/j.1600-6143.2009.02768.x
27. Song I, Sun K, Ilic K, Martin P. Summary of Maribavir (SHP620) Drug-Drug Interactions Based on Accumulated Clinical and Nonclinical Data. Biol Blood Marrow Transplant. 2019;25(S):S370–S371. doi:10.1016/j.bbmt.2018.12.600
28. Song IH, Ilic K, Murphy J, Lasseter K, Martin P. Effects of Maribavir on P-Glycoprotein and CYP2D6 in Healthy Volunteers. J Clin Pharmacol. 2020;60(1):96–106. doi:10.1002/jcph.1504
29. Song IH, Ilic K, Wu J Lack of drug–drug interaction between maribavir and voriconazole.
30. Chetty M, Neuhoff S, Barter Z, Maribavir dose considerations for treatment of cytomegalovirus infections during co-administration of CYP3A4 inducers and inhibitors, based on physiologically based pharmacokinetic modeling. Poster presented at.
31. Chou S, Marousek GI. Accelerated evolution of maribavir resistance in a cytomegalovirus exonuclease domain II mutant. J Virol. 2008;82(1):246–253. doi:10.1128/JVI.01787-07
32. Chou S, Wechel LC, Marousek GI. Cytomegalovirus UL97 kinase mutations that confer maribavir resistance. J Infect Dis. 2007;196(1):91–94. doi:10.1086/518514
33. Chou S, Marousek GI, Senters AE, Davis MG, Biron KK. Mutations in the human cytomegalovirus UL27 gene that confer resistance to maribavir. J Virol. 2004;78(13):7124–7130. doi:10.1128/JVI.78.13.7124-7130.2004
34. Chou S, Wu J, Song K, Bo T. Novel UL97 drug resistance mutations identified at baseline in a clinical trial of maribavir for resistant or refractory cytomegalovirus infection. Antiviral Res. 2019;172:104616. doi:10.1016/j.antiviral.2019.104616
35. Chou S, Song K, Wu J, Bo T, Crumpacker C. Drug resistance mutations and associated phenotypes detected in clinical trials of maribavir for treatment of cytomegalovirus infection. J Infect Dis. 2020. doi:10.1093/infdis/jiaa462
36. Chou S. Cytomegalovirus UL97 mutations in the era of ganciclovir and maribavir. Rev Med Virol. 2008;18(4):233–246. doi:10.1002/rmv.574
37. Komazin G, Ptak RG, Emmer BT, Townsend LB, Drach JC. Resistance of human cytomegalovirus to the benzimidazole L-ribonucleoside maribavir maps to UL27. J Virol. 2003;77(21):11499–11506. doi:10.1128/JVI.77.21.11499-11506.2003
38. Drew WL, Miner RC, Marousek GI, Chou S. Maribavir sensitivity of cytomegalovirus isolates resistant to ganciclovir, cidofovir or foscarnet. J Clin Virol. 2006;37(2):124–127. doi:10.1016/j.jcv.2006.07.010
39. Strasfeld L, Lee I, Tatarowicz W, Villano S, Chou S. Virologic characterization of multidrug-resistant cytomegalovirus infection in 2 transplant recipients treated with maribavir. J Infect Dis. 2010;202(1):104–108. doi:10.1086/653122
40. Piret J, Boivin G. Clinical development of letermovir and maribavir: overview of human cytomegalovirus drug resistance. Antiviral Res. 2019;163:91–105. doi:10.1016/j.antiviral.2019.01.011
41. Chung RT, Ghany MG, Kim AY, Panel A-IHG. Hepatitis C Guidance 2018 Update: AASLD-IDSA Recommendations for Testing, Managing, and Treating Hepatitis C Virus Infection. Clin Infect Dis. 2018;67(10):1477–1492. doi:10.1093/cid/ciy585
42. Saag MS, Benson CA, Gandhi RT, et al. Antiretroviral Drugs for Treatment and Prevention of HIV Infection in Adults: 2018 Recommendations of the International Antiviral Society-USA Panel. JAMA. 2018;320(4):379–396. doi:10.1001/jama.2018.8431
43. Takeda Pharmaceuticals. Livtencity (Maribavir) [Package Insert]. Lexington, MA: Takeda Pharmaceuticals; 2021.
44. Turner N, Strand A, Grewal DS, et al. Use of Letermovir as Salvage Therapy for Drug-Resistant Cytomegalovirus Retinitis. Antimicrob Agents Chemother. 2019;63:3. doi:10.1128/AAC.02337-18
45. Upadhyayula S, Michaels MG. Ganciclovir, Foscarnet, and Cidofovir: antiviral Drugs Not Just for Cytomegalovirus. J Pediatric Infect Dis Soc. 2013;2(3):286–290. doi:10.1093/jpids/pit048
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
