Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 14
Ethyl 2-[2,3,4-Trimethoxy-6-(1-Octanoyl)Phenyl] Acetate (TMPA) Ameliorates Lipid Accumulation by Disturbing the Combination of LKB1 with Nur77 and Activating the AMPK Pathway in HepG2 Cells and Mice Primary Hepatocytes
Authors Wang X , Li G , Guo C, Zhang J , Kong J, He J , Li F, Liu Y, Yang Y, Lu Z, Liu J
Received 25 May 2021
Accepted for publication 30 August 2021
Published 2 October 2021 Volume 2021:14 Pages 4165—4177
DOI https://doi.org/10.2147/DMSO.S321246
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Juei-Tang Cheng
Xiaoyu Wang,1 Guangbing Li,1,2 Changfa Guo,3 Jiayao Zhang,1 Junjie Kong,1,2 Jingyi He,1,2 Feiyu Li,1 Yong Liu,1 Yang Yang,1 Ziwen Lu,1 Jun Liu1,2
1Department of Hepatobiliary Surgery and Center of Organ Transplantation, Shandong Provincial Hospital, Cheeloo College of Medicine, Shandong University, Jinan, Shandong, People’s Republic of China; 2Department of Hepatobiliary Surgery and Center of Organ Transplantation, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, Shandong, People’s Republic of China; 3Department of Neurosurgery, Shandong Provincial Hospital Affiliated to Shandong University, Cheeloo College of Medicine, Jinan, Shandong, People’s Republic of China
Correspondence: Jun Liu
Department of Hepatobiliary Surgery and Center of Organ Transplantation, Shandong Provincial Hospital Affiliated to Shandong University, Jinan, Shandong, People’s Republic of China
Email [email protected]
Background: The AMP-activated protein kinase alpha (AMPKα) pathway has widely been considered a key factor in energy metabolism. Ethyl 2-[2,3,4-trimethoxy-6-(1-octanoyl)phenyl] acetate (TMPA) is a novel AMPK agonist, which influences the stability of Nuclear Receptor Subfamily 4, Group A, Member 1 (Nur77)-serine-threonine kinase 11 (LKB1) in the nucleus. A recent study has determined that TMPA can ameliorate the reduction of insulin resistance in type II db/db mice. However, the role of TMPA in hepatocyte lipid metabolism has not been elucidated.
Objective: To investigate whether TMPA could ameliorate liver lipid accumulation under the stimulation of free fatty acids (FFAs) in vitro.
Methods: We evaluated differences of Nur77 and AMPK pathway in mice fed a high-fat diet and those fed a normal diet. In vitro, TMPA was added to HepG2 cells and primary hepatocytes before FFAs stimulation. Oil red O staining, Nile red staining were used to evaluate lipid deposition. Western blot and immunofluorescence were used to quantify related proteins.
Results: Nur77, AMPKα, LKB1, 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR), acetyl-CoA carboxylase phosphorylation (p-ACC), and carnitine palmitoyltransferase 1 (CPT1A) showed significant differences in vivo. Under the intervention of TMPA, HepG2 cells and primary hepatocytes showed considerable amelioration of lipid deposition and improved the expression of phosphorylated (p)-AMPKα (p-AMPKα), p-LKB1, p-ACC, and CPT1A. Furthermore, Western blotting and immunofluorescence studies indicated that LKB1 dramatically increased expression in the cytoplasm but decreased in the nucleus. Further, AMPKα phosphorylation (p-AMPKα) also showed a higher expression in cytoplasm instead of the nucleus.
Conclusion: TMPA ameliorated lipid accumulation by influencing the stability of Nur77-LKB1 in vitro.
Keywords: ethyl 2-[2,3,4-trimethoxy-6-(1-octanoyl)phenyl] acetate, Nur77, AMPK pathway, HepG2 cells, primary hepatocytes
Introduction
Metabolic associated fatty liver disease (MAFLD) is an umbrella term for a comprehensive range of liver diseases, including nonalcoholic fatty liver disease (NAFLD), and the more severe nonalcoholic steatohepatitis (NASH).1 MAFLD is the most common cause of chronic liver disease in the world, and increasing evidence shows that MAFLD is a multi-systemic disease, affecting extra-hepatic organs and regulatory pathways. Many factors can generate the occurrence and progression of this disease, such as type 2 diabetes mellitus (T2DM), cardiovascular (CVD), cardiac diseases, and chronic kidney disease (CKD).2 As MAFLD progresses, many patients will progress to liver cirrhosis and end-stage hepatocellular carcinoma. The increase in prevalence of this chronic disease has caused a growing economic burden and reduced the quality of life for many elderly individuals.3
Nuclear Receptor Subfamily 4, Group A, Member 1 (Nur77, also called TR3, NGFIB, and NR4A1), is an orphan member of the nuclear receptor superfamily and plays a critical role in apoptosis, fibrosis, glucose metabolism, inflammation, lipid metabolism, and in the regeneration and injury of cells.4–9 More recently, Nur77 has been shown to be involved in glucose and lipid metabolism,10,11 especially through the serine-threonine kinase 11/AMP-activated protein kinase (LKB1/AMPK) pathway.
The LKB1/AMPK axis is considered a crucial metabolic sensor in cellular homeostasis especially for glucose and lipid metabolism.12,13 The AMPK pathway is altered following exposure to a mixture of free fatty acids (FFAs) in HepG2 cells.14 During lipid metabolism in the liver, activated AMPKα can phosphorylate 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR) at Ser87215 and acetyl-CoA carboaxylase (ACC) at S79, and subsequently inhibits the activation of HMGCR and ACC, which is crucial for lipid production.12,16 In addition, ACC is a potent allosteric inhibitor of carnitine palmitoyltransferase 1 (CPT1A), which imports long chain fatty acyl-CoA into the mitochondria and promotes fatty acid β-oxidation. Therefore, the suppression of ACC promotes the activation of CPT1A and then reduces the deposition of FFAs.12 Hence, the AMPK pathway has been considered a useful approach to treat diseases associated with fat metabolism.
Zhan et al17 confirmed that Nur77-specific binding of liver kinase B1 (LKB1) in the nucleus prevented the translocation of LKB1 from the nucleus to the cytosol. Similar results showing that Nur77 stimulates FcεRI signaling by counteracting the LKB1/AMPK axis have been reported in anaphylactic responses.18 Altogether, this evidence implies that the inhibition of Nur77 and LKB1 binding may improve hepatic steatosis. Recent studies have shown that ethyl 2-[2,3,4-trimethoxy-6-(1-octanoyl)phenyl] acetate (TMPA), an Nur77 antagonist, prevents the binding of Nur77 and LKB1 as well as stimulates LKB1 transport to the cytosol and its phosphorylation (p-LKB1).17–19 Activation of AMPKα by phosphorylated LKB1 activates downstream molecules related to lipid metabolism. Therefore, TMPA is also considered a potential target treatment for diabetes and obesity.17
In this study, we investigated the effects of TMPA on lipid metabolism in HepG2 and primary liver cells. The results showed that TMPA facilitates LKB1 transport to the cytosol and its phosphorylation (p-LKB1). Furthermore, p-LKB1 subsequently promoted AMPKα phosphorylation (p-AMPKα) and activated downstream molecules like phosphorylated (p) ACC (p-ACC) and CPT1A. The results of the present study indicate that TMPA could prevent fatty accumulation in HepG2 cells and primary hepatocytes.
Methods
Reagents
Palmitic acid was purchased from MCE (NJ, USA). Oleic acid was purchased from Selleck Company (Shanghai, China). Antibodies against phosphorylated (p)-LKB1 (Ser428, 63473, 138386), p-ACC (S79, 68191), ACC (109368, 45174), Nur77 (109180), AMPKα (32047), HMGCR (174830), ADFP (108323), SREBP-1 (28481) and CPT1A (220789, 234111) were purchased from Abcam (UK). Antibodies against β-actin (#3700), and p-AMPKα (T172, #50081) were purchased from Cell Signaling Technology (Danvers, MA, USA). Antibodies against LKB1 (#3050)(A2122) was bought from Cell Signaling Technology (Danvers, MA, USA) and ABclonal (Wuhan, China). Antibodies against PCNA (10205-2-AP) were purchased from Proteintech (Wuhan, China). TMPA was purchased from MCE (NJ, USA), and subsequently dissolved in dimethyl sulfoxide (DMSO, Solarbio, Beijing, China) to prepare a 40mM stock solution.
Cell Culture
The HepG2 cell line was purchased from Procell Life Science & Technology Co. (China) and cultured in Dulbecco’s modified Eagle’s medium (DMEM, Gibco, China), supplemented with 10% fetal bovine serum (FBS; Gibco, New Zealand). The trypsin enzyme-digestion technique was used to passage the cells and the trypsin enzyme was produced by Gibco (USA).
Primary Hepatocytes Extraction and Culture
C57BL/6J mice were used as the source of mice primary hepatocytes. Mice livers were first perfused with Hank’s Solution (Solarbio, Beijing, China), followed by perfusion with IV collagenase via the portal vein. The digested liver tissue was then shaken with sterile forceps to exfoliate the cells on the ultra-clean table. Afterwards, the primary cells were centrifuged in a 15mL sterile centrifuge tube for 3 times, 1000 revolutions per time, 4min. After each centrifugation, discard the waste solution and wash with Hank’s solution. Finally, the primary cells were implanted in a dish covered with rat tail collagen and cultured in William’s E Medium (Gibco, England).
Induction of Fat-Overload in HepG2 Cells and Primary Hepatocytes
The cells were incubated with 0.25mM FFAs mixture (OA:PA = 2:1) for 24h. The FFAs (1.5mL, 0.5mL and 0.1mL) were added to the 6well, 24well and 96well plates, respectively. The FFAs were prepared in culture medium containing DMEM with no bovine serum albumin.
Hematoxylin-Eosin Staining and Oil Red O Staining
The tissue of liver in mice was isolated and washed with saline solution. The tissue was dehydrated and immersed in wax to be embedded. The samples were cut into sections with a thickness of 4–5μm. Subsequently, all sections underwent alcohol dehydration, xylene penetration, H&E staining, dehydration and permeabilization, and resinene sealing. Oil red O staining kit (Solarbio, Beijing, China) was used to assess lipid accumulation in cells cultured. HepG2 cells and primary hepatocytes were washed with phosphate-buffered saline (PBS) (Biosharp, China), and then fixed with cell Fixative for about 25min. We then used 60% isopropanol to wash the cells for 5min, after which the cells were subjected to oil red O staining for 20min. The nuclei were strained by hematoxylin for about 1min.
Nuclear and Cytoplasmic Separation
The Nucleoplasmic separation kit was bought from Boster company (Wuhan, China). After the cells were scraped in PBS, the RIPA Lysis Buffers in the kit were added successively. Protease inhibitor and phosphatase inhibitor were added to the lysate of each part in a ratio of 100:1:1. Each step was operated on ice. Extracted nucleoprotein and plasma protein were frozen in the −80°C refrigerator.
Protein Extraction and Western Blot Analysis
Proteins were extracted from HepG2 cells and primary liver cells using RIPA lysis buffer (Solarbio, Beijing, China) and protein phosphatase inhibitor (100:1:1) mixture. Cell lysates were centrifuged at 12,000rpm for about 20min. After centrifugation, middle-level clarified liquid was aspirated. Bicinchoninic acid (BCA) kit (Beyotime, Shanghai, China) was used to measure the protein concentration. All protein samples were stored at −80°C. Extracted protein was added to SDS-PAGE on a 10% resolving gel with 120V constant voltage and then transferred to a nitrocellulose (NC) filter membrane with 220V constant current. After blocking nonspecific binding with 5% BSA solution, the membranes were incubated with primary antibodies overnight at 4°C. The membranes were washed three times with TBST (Trisbuffered saline and 0.1% Tween 20) and then incubated with the HRP-conjugated secondary antibody which diluted to 1/5000 (Proteintech, Wuhan, China) at room temperature for 1h.
Nile Red Staining
Nile red staining was used to identify the intracellular lipid content in HepG2 cells and mice primary hepatocytes. Cells were washed three times by PBS first. They were then incubated with Nile red fluorescent stain (10μg/mL in PBS, Solarbio, Beijing, China) for 15min in 37°C. They were then incubated with DAPI (Solarbio, Beijing, China) for 7min at common temperature for nuclear localization. Inverted confocal fluorescence microscope is a tool for observing the fluorescence. Excitation wavelength of Nile red stain was 530nm.
Immunofluorescence Assay
Cells on the 24-wells were fixed in 4% paraformaldehyde for 25min at room temperature. After that, the paraformaldehyde was removed, and the cells were washed with PBS three times for 5min. Then, cells were permeabilized by 0.5% Triton X-100 for 40min at room temperature. Next, we used PBS to wash three times for 5min each. Goat serum was used to block for 1h at 37°C. First antibody (anti-LKB, ABclonal, WuHan, China) was diluted in goat serum to 1:100 and added to all wells in 300ul, incubating overnight at 4°C. The second antibody (SA00013-4 Proteintech, Wuhan, China) diluted in PBS to 1:200 and incubated for 1h at room temperature. Finally, discarding the secondary antibody and wash with PBS three times in 5min. The results were photographed by an inverted confocal fluorescence microscope.
Experimental Animals and Diet
C57BL/6J male mice were purchased from the Charles River Laboratories (Beijing, China) at 6–7 weeks of age. All mice were fed adaptively for one week. Animal experiments were conducted according to the Principles of Laboratory Animal Care established by the National Institutes of Health. All experiments were approved by the Animal Research committee of Shandong Provincial Hospital. All mice were randomly divided into two groups: a normal diet group (NC) and a high-fat diet group (HFD). NC group was fed a standard mice chow diet (10% calories from fat, XieTong Organism Inc.) and the HFD group was fed a high-fat diet (60% calories from fat, XieTong Organism) over 8 weeks.
Biochemical Evaluation
Levels of alanine transaminase (ALT), aspartate transaminase (AST), triglyceride, total cholesterol, Total bile acid in serum were determined to evaluate liver injury using RAYTO Chemray 800 automatic biochemical analyzer (Shenzhen, China) and commercial reagent kit (RAYTO, Shenzhen, China, S03030, S03040, S03074, S03027, S03042).
Statistical Analysis
All assays were performed on 3 separate occasions. Data are expressed as means ± SD. The Image J software (NIH) was used to quantify oil red O staining and Western blotting findings. The statistical data obtained from Image J quantitative data were processed using GraphPad Prism 6.0. Data were analyzed using one-way analysis of variance. A P-value <0.05 was considered statistically significant.
Results
Expression of Nur77 and Related Proteins Differed Between MAFLD Mice and Normal Mice
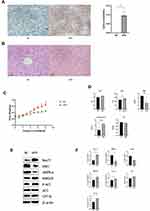
Significant differences in weight were observed between the two groups after 4 weeks of feeding. These differences became more pronounced as time progressed. Oil red O staining revealed many fat droplets, which indicated severe fat deposition in the HFD group (Figure 1A) compared to the NC group. Hematoxylin and eosin (H&E) staining showed that the HFD group had more fat vacuoles with irregular hepatocyte structure compared with NC groups (Figure 1B). The NAS score indicated that mice fed with HFD could be defined as NASH (Table 1). HFD group exhibited significant changes in aspartate transaminase (AST), total bile acid (TBA), and cholesterol levels, but no obvious differences in glutamic-pyruvic transaminase (ALT) and triglyceride (TG) (Figure 1D). Next, we investigated differences in the expression of lipid-related proteins between the two groups. Western blotting analysis showed high expression of Nur77, p-ACC, CPT1A, and HMGCR and low expression of LKB1 and AMPKα in the HFD group. Interestingly, ACC expression showed no significant differences in the NC group (Figure 1E and F).
 |
Table 1 Nonalcoholic Fatty Liver Disease (NAFLD) Activity Score (NAS) |
TMPA Reduced Lipid Accumulation Following FFAs Stimulation in HepG2 Cells and Primary Hepatocytes
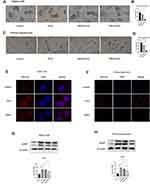
TMPA (Figure 2A) can interfere with LKB1 and Nur77 binding. Oil red O staining was used to evaluate lipid accumulation induced by FFAs (0.25mM) in HepG2 cells and primary hepatocytes. Oil red O stain showed that under stimulation of FFAs (oleic acid and palmitic acid in a 2:1 ratio) (0.25mM), HepG2 cells (Figure 3A and B) and primary hepatocytes (Figure 3C and D) showed apparent lipid accumulation and fat vacuoles. However, the DMSO control group showed no significant differences compared with the FFAs-stimulated group. Furthermore, we attenuated TMPA in FBS to 10µm and added the solution to HepG2 cells and primary hepatocytes, which were incubated for about 6h before stimulation with FFAs. Lipid accumulation and fat vacuoles were both reduced by TMPA (Figure 3A and B). We also used Nile red staining to evaluate the lipid content in these cells. The cells in the FFAs group showed stronger fluorescence intensity and more lipid droplets. However, the TMPA treatment group had the lower fluorescence intensity compared with the FFAs-treated group (Figure 3E and F). Furthermore, the expression of ADFP (also known as Plin2) which was associated with hepatic lipid accumulation20 also increased under FFAs and the intervention of TMPA ameliorated the content of ADFP, compared with the FFAs-treated group and FFAs+DMSO group (Figure 3G and 3H). All the data indicated that TMPA ameliorated hepatic steatosis by FFAs.
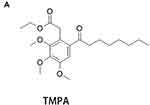 |
Figure 2 (A) The molecular formula of TMPA. |
TMPA Increased the Phosphorylation of LKB1 and Induced the Phosphorylation of AMPKα
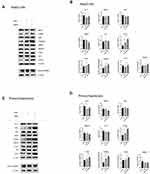
Several studies have suggested that the phosphorylation of LKB1 can activate the phosphorylation of AMPK at Thr172.17,18 Therefore, we investigated whether the decline in lipid levels effected by TMPA in HepG2 cells and primary hepatocytes, occurred via the AMPK pathway. Subsequently, TMPA 10µm was added to HepG2 cells and primary hepatocytes. We observed that under the stimulation of FFAs, there was a marked change in Nur77, p-AMPKα and CPT1A expression in HepG2 cells (Figure 4A and B). Primary hepatocytes exhibited a similar change in Nur77, p-AMPKα as well as CPT1A expression (Figure 4C and D). Both in HepG2 cells (Figure 4A and B) and primary hepatocytes (Figure 4C and D), the effects of TMPA included an increase in the phosphorylation of LKB1 (Ser428) and AMPKα (Thr172), rather than of LKB1, Nur77, AMPKα, cleaved-SREBP-1 or HMGCR downstream of AMPK. We also observed increased expression of downstream molecular p-ACC and CPT1A, which are reduced FFAs in the liver, but no similar changes in ACC (Figure 4A–D). All data showed that the reduction of FFAs accumulation in HepG2 cells and primary hepatocytes occurred via the over-expression of CPT1A in the LKB1-AMPK pathway.
LKB1 Translocated from the Nucleus to the Cytosol Under the Influence of TMPA
The binding of TMPA has been reported to interfere with the binding stability between Nur77 and LKB1 in the nucleus, and thereby facilitated the interruption of the interface region, based on atomistic molecular simulations.19 Therefore, we investigated whether Nur77 was involved in the subcellular localization of LKB1 following treatment with TMPA (10µm), and whether TMPA could destabilize the translocation of LKB1 from the nucleus to the cytosol, following AMPKα phosphorylation in HepG2 cells and primary hepatocytes under the stimulation of FFAs.
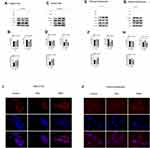
LKB1 exhibited marked translocation between cytosol and nucleus in HepG2 cells and primary hepatocytes (Figure 5A–H) under FFAs stimulation. Next, we compared cells subjected to FFAs stimulation alone with those in the TMPA treatment group. As expected, LKB1 levels were reduced in the nucleus (Figure 5C, D, G, H) and were increased in the cytosol (Figure 5A, B, E, F). Furthermore, p-AMPKα showed a significant increase in cytosolic levels (Figure 5A, B, E and F), but not in the nucleus (Figure 4C, D, G and H) in HepG2 cells and primary hepatocytes. In addition, AMPKα in both the nucleus and cytosol showed no apparent changes. Immunofluorescence staining of LKB1 indicated that the treatment of TMPA leads to a fluorescence intensity decline in the nucleus and an increased intensity in the cytoplasm of HepG2 cells and mice primary hepatocytes (Figure 5I and J). Our results confirmed that the phosphorylation of AMPKα in the TMPA group was influenced by the interaction between LKB1 and Nur77. Therefore, intervention by TMPA could decrease the stability of LKB1 and Nur77 in the nucleus and in turn, lead to the increased phosphorylation of LKB1 in the cytosol and activation of AMPKα.
Discussion
The orphan nuclear receptor 4 subfamily 1 (NR4A1, Nur77) is considered a significant metabolic regulatory protein that can directly combine with LKB1 in the nucleus.17 In addition, Nur77 inhibition by TMPA affects glucose metabolism and the progression of diabetes.17 However, whether TMPA is involved in hepatic lipid metabolism or is associated with AMPK-related downstream proteins of lipid metabolism has not been confirmed. A lipid accumulation model in vitro was constructed by adding saturated FFAs and unsaturated FFAs.21 The AMPK pathway was closely associated with lipid metabolism in the liver.12 Thus, we investigated differences in protein expression between mice fed a regular chow diet and those fed a HFD. It has been demonstrated that male mice are often used to establish the model of MAFLD.22 The results showed remarkable differences in expression of proteins involved in the AMPK signaling pathway in livers with marked steatosis. Furthermore, marked increases in the expression of p-ACC, and CPT1A also indicated the existence of some compensatory mechanisms in these mice.
The Nur77-LKB1 interaction is complex and attenuate the phosphorylation of LKB1.17,19 Under stimulation of FFAs, both HepG2 cells and primary hepatocytes exhibited changes in Nur77, p-AMPKα, and CPT1A levels, which showed that hepatic steatosis was associated with the AMPK signaling pathway. Following the addition of TMPA, lipid deposition was considerably improved under FFAs stimulation. A previous study confirmed that LKB1 is a major kinase that activates phosphorylation of AMPKα at Thr172, while the phosphorylation of LKB1 at Ser428 is essential for the activation of AMPKα by metformin.23 Thus, we hypothesized that the mechanism of TMPA activity in liver lipid metabolism is associated with AMPK and activation of relevant signaling proteins.
As we expect, the intervention of TMPA increased the expression of p-LKB1 and p-AMPKα in these cells. Furthermore, the downstream proteins of the AMPK pathway were also increased, such as phosphorylated ACC and CPT1A. But no significant changes in HMGCR or SREBP-1 levels, which are strongly associated with cholesterol metabolism, were observed. It is generally accepted that ACC is a central enzyme that is crucial as source of fatty acids from nonlipid precursors by de novo lipogenesis.16 CPT1A is an essential rate-limiting enzyme involved in the breakdown of fatty acids (β-oxidation), and effectively translocates fatty acids into the mitochondria. The regulation of CPT1A is closely associated with malonyl-CoA, downstream of ACC, which negatively regulates CPT1A expression and may be positively regulated by ACC.24,25 Based on the present findings, the reduced levels of FFAs in HepG2 cells and primary hepatocytes under the influence of TMPA were due to the activity of the LKB1/AMPK pathway. The fact that no significant changes were observed in HMGCR and SREBP-1 levels indicates that TMPA may have a limited influence on cholesterol metabolism but augments ACC phosphorylation and increases levels of downstream CPT1A, and subsequently activates fatty acid oxidation. Therefore, TMPA interferes with hepatic steatosis via phosphorylation of ACC to inhibit its activity, in combination with increased levels of CPT1A.
Lipophagy regulates the stability of lipid metabolism in cells. Therefore, liver fat metabolism is closely associated with lipophagy.26,27 Lipophagy is a type of autophagy, and its activation can reduce lipid deposition by phagocytosis of lipid droplets in the cytoplasm and lysozyme formation. There is a negative regulatory relationship between m-TOR and lipophagy, and the increased expression of m-TOR will increase the deposition of fatty acids.28 Activation of AMPK inhibits m-TOR through the phosphorylation pathway.27 Therefore, we consider that TMPA may have the function that inhibits m-TOR activity through phosphorylation by activating the LKB1-AMPK axis, and in turn activate lipophagy to increase fatty acid decomposition.
Moreover, high expression of ACC and SREBP-1 will accelerate the production of fatty acids.16,29 The increase in SREBP-1 levels can also positively regulate the expression of ACC. Our results showed no significant differences in SREBP-1 or ACC, but we observed an increase in p-ACC. These data indicated that TMPA had no influence on fatty acid synthesis in the SREBP-1 pathway but limited the activation of ACC by phosphorylation, which means that TMPA inhibits de novo fatty acid synthesis by inhibiting ACC activity and accelerates the degradation of fatty acids to alleviate the liver fat deposition of cells.
In bone marrow mononuclear cells, LO2 cells, and Hela cells, TMPA antagonizes the formation of Nur77-LKB1 binding in the nucleus and enhances the stability of LKB1-AMPK formation in the cytosol.17,18 However, whether TMPA exerts the same function under the stimulation of FFAs in HepG2 cells and primary hepatocytes has not been verified. We observed that LKB1 was influenced by FFAs, and may influence the process of hepatic steatosis. As expected, TMPA also antagonized LKB1-Nur77 interactions in the nucleus and promoted the translocation of additional LKB1 into the cytosol. Further study elucidated the mechanisms through which TMPA ameliorated hepatic steatosis involved the phosphorylation of the excitatory AMPK pathway through the nucleoplasmic shuttle LKB1. Our findings were consistent with previous reports that showed no significant changes in the levels of AMPKα, but instead we observed a marked increase in AMPKα phosphorylation after exposure to TMPA. Differences in nucleoprotein and cytoplasmic protein expression indicated that the activation of LKB1 and AMPKα may also be a consequence of reduced Nur77 and LKB1 binding, and translocation of LKB1 from the nucleus to the cytoplasm.
Zhan et al17 confirmed that TMPA could reduce blood glucose levels in mice, and predicted that this could represent a potential target treatment for diabetes. Importantly, MAFLD is also associated with diabetes and fat intake.30 Based on the results of the present study, TMPA influenced FFAs stimulation by affecting the formation of LKB1-Nur77 in the nucleus, and phosphorylating LKB1 and AMPKα in the cytosol. Consequently, the effects of CPT1A, the rate-limiting enzyme of fatty acid oxidation, improved following activation of AMPK (Figure 6). Therefore, we propose TMPA as a potential target drug for use in the treatment of MAFLD, as it can inhibit the nuclear stability of LKB1 and Nur77.
Abbreviations
TMPA, Ethyl 2-[2,3,4-trimethoxy-6-(1-octanoyl)phenyl] acetate; MAFLD, Metabolic associated fatty liver disease; NASH, nonalcoholic steatohepatitis; NAS, MAFLD activity scorn; H&E stain, hematoxylin-eosin staining; AST, Glutamic oxalacetic transaminase; ALT, Glutamic-pyruvic transaminase; TBA, Total bile acid; TG, Triglyceride; Nur77, Orphan nuclear receptor 4 subfamily 1; LKB1, liver kinase B1; AMPKα, AMP-activated protein kinase alpha; FFAs, free fatty acids; ACC, acetyl-CoA carboxylase; CPT1A, carnitine palmitoyltransferase 1; HMGCR, 3-hydroxy-3methylglutaryl coenzyme A reductase; ADFP, adipose differentiation-related protein; SREBP-1, Sterol Regulatory Element Binding Protein 1.
Funding
This study was supported by grants from the National Natural Science Foundation of China (No. 81373172 and No. 81770646).
Disclosure
The authors declare that the study was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Cobbina E, Akhlaghi F. Non-alcoholic fatty liver disease (MAFLD) - pathogenesis, classification, and effect on drug metabolizing enzymes and transporters. Drug Metab Rev. 2017;49(2):197–211. doi:10.1080/03602532.2017.1293683
2. Byrne CD, Targher G. MAFLD: a multisystem disease. J Hepatol. 2015;62(1 Suppl):S47–S64. doi:10.1016/j.jhep.2014.12.012
3. Friedman SL, Neuschwander-Tetri BA, Rinella M, Sanyal AJ. Mechanisms of MAFLD development and therapeutic strategies. Nat Med. 2018;24(7):908–922. doi:10.1038/s41591-018-0104-9
4. Chao LC, Wroblewski K, Zhang Z, et al. Insulin resistance and altered systemic glucose metabolism in mice lacking Nur77. Diabetes. 2009;58(12):2788–2796. doi:10.2337/db09-0763
5. Palumbo-Zerr K, Zerr P, Distler A, et al. Orphan nuclear receptor NR4A1 regulates transforming growth factor-β signaling and fibrosis. Nat Med. 2015;21(2):150–158. doi:10.1038/nm.3777
6. Tan Y, Li Y. HCV core protein promotes hepatocyte proliferation and chemoresistance by inhibiting NR4A1. Biochem Biophys Res Commun. 2015;466(3):592–598. doi:10.1016/j.bbrc.2015.09.091
7. Hu M, Luo Q, Alitongbieke G, et al. Celastrol-induced Nur77 interaction with TRAF2 alleviates inflammation by promoting mitochondrial ubiquitination and autophagy. Mol Cell. 2017;66(1):141–153.e6. doi:10.1016/j.molcel.2017.03.008
8. Zhou H, Du W, Li YE, et al. Effects of melatonin on fatty liver disease: the role of NR4A1/DNA-PKcs/p53 pathway, mitochondrial fission, and mitophagy. J Pineal Res. 2008;64(1):e12450.
9. Jung YS, Lee HS, Cho HR, et al. Dual targeting of Nur77 and AMPKα by isoalantolactone inhibits adipogenesis in vitro and decreases body fat mass in vivo. Int J Obes. 2019;43(5):952–962. doi:10.1038/s41366-018-0276-x
10. Pei L, Waki H, Vaitheesvaran B, Wilpitz DC, Kurland IJ, Tontonoz P. NR4A orphan nuclear receptors are transcriptional regulators of hepatic glucose metabolism. Nat Med. 2006;12(9):1048–1055. doi:10.1038/nm1471
11. Perez-Sieira S, Martinez G, Porteiro B, et al. Female Nur77-deficient mice show increased susceptibility to diet-induced obesity. PLoS One. 2013;8(1):e53836. doi:10.1371/journal.pone.0053836
12. Lage R, Diéguez C, Vidal-Puig A, López M. AMPK: a metabolic gauge regulating whole-body energy homeostasis. Trends Mol Med. 2008;14(12):539–549. doi:10.1016/j.molmed.2008.09.007
13. Hardie DG. AMP-activated protein kinase: an energy sensor that regulates all aspects of cell function. Genes Dev. 2011;25(18):1895–1908. doi:10.1101/gad.17420111
14. Kim MH, Seong JB, Huh JW, Bae YC, Lee HS, Lee DS. Peroxiredoxin 5 ameliorates obesity-induced non-alcoholic fatty liver disease through the regulation of oxidative stress and AMP-activated protein kinase signaling. Redox Biol. 2020;28:101315. doi:10.1016/j.redox.2019.101315
15. Clarke PR, Hardie D, GClarke PR, Hardie DG. Regulation of HMG-CoA reductase: identification of the site phosphorylated by the AMP-activated protein kinase in vitro and in intact rat liver. EMBO J. 1998;9(8):2439–2446. doi:10.1002/j.1460-2075.1990.tb07420.x
16. Park M, Kang C, Lee H-J. Effect of bombyx mori on the liver protection of non-alcoholic fatty liver disease based on in vitro and in vivo models. Curr Issues Mol Biol. 2021;43(1):21–35. doi:10.3390/cimb43010003
17. Zhan YY, Chen Y, Zhang Q, et al. The orphan nuclear receptor Nur77 regulates LKB1 localization and activates AMPK. Nat Chem Biol. 2012;8(11):897–904. doi:10.1038/nchembio.1069
18. Jin F, Li X, Deng Y, et al. The orphan nuclear receptor NR4A1 promotes FcεRI-stimulated mast cell activation and anaphylaxis by counteracting the inhibitory LKB1/AMPK axis. Allergy. 2019;74(6):1145–1156. doi:10.1111/all.13702
19. Rungsung I, Rajagopalan M, Ramaswamy A. Molecular dynamics study of TMPA mediated dissociation of Nur77-LKB1 complex. Comput Biol Chem. 2018;76:67–78. doi:10.1016/j.compbiolchem.2018.06.002
20. Jin Y, Tan Y, Chen L, Liu Y, Ren Z. Reactive oxygen species induces lipid droplet accumulation in HepG2 Cells by increasing perilipin 2 expression. Int J Mol Sci. 2018;19(11):3445. doi:10.3390/ijms19113445
21. Gomez-Lechon MJ, Donato MT, Martínez-Romero A, Jiménez N, Castell JV, O’Connor JE. A human hepatocellular in vitro model to investigate steatosis. Chem Biol Interact. 2007;165(2):106–116. doi:10.1016/j.cbi.2006.11.004
22. Jiang C, Li P, Ruan X, et al. Comparative transcriptomics analyses in livers of mice, humans, and humanized mice define human-specific gene networks. Cells. 2020;9(12):2566. doi:10.3390/cells9122566
23. Xie Z, Dong Y, Scholz R, Neumann D, Zou MH. Phosphorylation of LKB1 at serine 428 by protein kinase C-zeta is required for metformin-enhanced activation of the AMP-activated protein kinase in endothelial cells. Circulation. 2008;117(7):952–962. doi:10.1161/CIRCULATIONAHA.107.744490
24. Ronnett GV, Kim EK, Landree LE, Tu Y. Fatty acid metabolism as a target for obesity treatment. Physiol Behav. 2005;85(1):25–35. doi:10.1016/j.physbeh.2005.04.014
25. Schlaepfer IR, Joshi M. CPT1A-mediated fat oxidation, mechanisms, and therapeutic potential. Endocrinology. 2020;161(2):bqz046. doi:10.1210/endocr/bqz046
26. González-Rodríguez A, Mayoral R, Agra N, et al. Impaired autophagic flux is associated with increased endoplasmic reticulum stress during the development of NAFLD. Cell Death Dis. 2014;5(4):e1179. doi:10.1038/cddis.2014.162
27. Liang Y, Zhang Z, Tu J, et al. γ-linolenic acid prevents lipid metabolism disorder in palmitic acid-treated alpha mouse liver-12 cells by balancing autophagy and apoptosis via the LKB1-AMPK-mTOR pathway. J Agric Food Chem. 2021;69(29):8257–8267. doi:10.1021/acs.jafc.1c02596
28. Garcia-Macia M, Santos-Ledo A, Leslie J, et al. An mTORC1-Plin3 pathway is essential to activate lipophagy and protects against hepatosteatosis. Hepatology. Epub 2021 Jul 7. doi:10.1002/hep.32048
29. Bakan I, Laplante M. Connecting mTORC1 signaling to SREBP-1 activation. Curr Opin Lipidol. 2012;23(3):226–234. doi:10.1097/MOL.0b013e328352dd03
30. Tilg H, Moschen AR, Roden M. NAFLD and diabetes mellitus. Nat Rev Gastroenterol Hepatol. 2017;14(1):32–42. doi:10.1038/nrgastro.2016.147
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.