Back to Journals » Risk Management and Healthcare Policy » Volume 13
Ethical Questions Linked to Rare Diseases and Orphan Drugs – A Systematic Review
Authors Kacetl J , Marešová P , Maskuriy R, Selamat A
Received 30 April 2020
Accepted for publication 20 August 2020
Published 13 October 2020 Volume 2020:13 Pages 2125—2148
DOI https://doi.org/10.2147/RMHP.S260641
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Marco Carotenuto
Jaroslav Kacetl,1 Petra Marešová,2 Raihan Maskuriy,3,4 Ali Selamat5,6
1Department of Linguistics, Faculty of Informatics and Management, University of Hradec Kralove, Hradec Kralove, Czech Republic; 2Department of Economics, Faculty of Informatics and Management, University of Hradec Kralove, Hradec Kralove, Czech Republic; 3Malaysia Japan International Institute of Technology (MJIIT), Universiti Teknologi Malaysia Kuala Lumpur, Kuala Lumpur, Malaysia; 4Department of Architecture, Faculty of Design and Architecture, Universiti Putra Malaysia (UPM), Serdang, Malaysia; 5Media and Games Center of Excellence (MagicX), Universiti Teknologi Malaysia, Skudai, Malaysia; 6School of Computing, Faculty of Engineering, Universiti Teknologi Malaysia (UTM), Skudai, Malaysia
Correspondence: Petra Marešová Email [email protected]
Background: Rare or orphan diseases have become an important target of healthcare activities all over the world. The study aims to identify ethical questions linked to rare diseases and orphan drugs and ethical principles or approaches applied to solve them.
Methods: Relevant peer-reviewed articles were identified by means of a systematic review. The literature was searched from 20 May 2020 to 20 June 2020. The search included the databases PubMed, Scopus and Web of Science (2010 – April 2020). A total of 4,139 papers related to rare diseases were identified; with 1,205 papers obtained from Scopus; 2,476 papers from PubMed; and 458 from Web of Science with keyword search “ethics” AND “rare” AND “disease”, “ethical” AND “orphan”, “ethical” AND “orphan” AND “drug”, and “ethical” AND “rare“ AND “disease”. Finally, XX studies were chosen for further analysis.
Results: The main findings reveal five main ethical issues. The most essential one shows that funding research and development in the field of orphan drugs poses an almost impossible dilemma. Other issues include the significance of non-economic values like compassion and beneficence in decision-making related to orphan drugs and rare diseases; the identification of limits to labelling diseases as rare; barriers to global, supranational and international cooperation; and last but not least, determining and establishing panels of decision-makers.
Conclusions: A strictly global approach would be the most appropriate way to deal with rare diseases. Nonetheless, international, let alone global, cooperation seems to be completely beyond the reach of the current international community, although the EU, for instance, has a centralized procedure for labelling orphan drugs. This deficit in international cooperation can be partly explained by the fact that the current technologically globalized world still lacks globally accepted ethical values and rules. This is further aggravated by unresolved international and intercultural conflicts. In addition, the sub-interests of various parties as well as the lack of desire to deal with other people’s problems need to be taken into account. The aforementioned problems are difficult to avoid. Nevertheless, let us be cautiously optimistic. At least, there are people who raise ethical questions about rare diseases and orphan drugs.
Keywords: ethical aspects, ethical principles, rare disease, orphan drugs
Background
Rare or orphan diseases have become an important target of healthcare activities all over the world and have been intensively discussed at national and even international levels.1,2 Currently, there are an estimated 400 million people globally suffering from about 7,000 distinct types of rare diseases.3 As many as 20% to 35% of all recognized diseases are rare and about 250,000 people in the EU suffer from them.4 According to the European Commission website, 25 countries have a national strategic plan on rare diseases (European Commission, 2016). Care for patients in this area is based on the Universal Declaration of Human Rights (proclaimed in 1948), the European Convention on Human Rights (1950),5 the Constitution of the World Health Organization (1946), the Declaration of Helsinki6 (1964, 7th revision 2013) and – not mentioning other international treaties and declarations – the Convention on the Rights of Persons with Disabilities (the UN, 2006). Gericke et al (2005)7 mention the Charter of Fundamental Rights of the European Union (section 35, 2000/C 364/01), whose Article 35, on healthcare, reads:
Everyone has the right of access to preventive health care and the right to benefit from medical treatment under the conditions established by national laws and practices. A high level of human health protection shall be ensured in the definition and implementation of all Union policies and activities.8
At the same time, rare diseases and orphan drugs open up important ethical considerations. Picavet et al (2013)9 mention a potential problem between individual and societal approaches as the principles of equity, entitlement and non-abandonment favour individuals, whereas society may strive to maximize the health of the population as a whole. McCabe et al (2005)10 examine whether or not rare diseases deserve special status and conclude that they do not, as the costs would be borne by patients with more common diseases.10 Van der Burg & Oerlemans (2018)11 call for a clarification of values and distinguish hard impacts – like health, life expectancy, absence of suffering – and soft impacts, eg, wellbeing, good care, supportive environment. The authors maintain that whereas hard impacts are rarely controversial and should be treated by policymakers, soft harms are hotly debated. From the liberal point of view, they should be decided by patients and their families.11
There is a clash between the moral duty of non-abandonment and the requirement of distributive justice, which makes the issue of funding orphan drug research an extraordinarily complex one. Gericke et al (2005)7 discuss the issue with respect to theories of utilitarianism, social justice and professional responsibility and conclude that the economic criteria considered in a standard investment evaluation cannot be indiscriminately applied in the case of medical science research funding. From the economic point of view, financing orphan drug development is not a profitable strategy because the market is too small, given the small number of patients in need of the orphan drug, and because the rare health condition may be prevalent in developing countries, which are unable to cover the drug costs.
On one hand, the utilitarian point of view on orphan disease research suggests that it is not in the interest of the most to divert resources to benefit only a small number of individuals at the expense of the society at large. From this perspective, funding orphan drug development is regarded an unethical decision. On the other hand, it can hardly be considered humane to abandon underprivileged individuals whose only crime is that they happen to suffer from a rare disease. The society has a moral obligation to protect and assist its vulnerable members as required. Eventually, there is also the professional obligation to advance scientific knowledge, which is a compelling argument supporting orphan disease research and drug development.
Gericke et al (2005)7 elaborate on the approach introduced by Beauchamp and Childress, who formulate four principles to be considered when it comes to the ethical questions of orphan drug funding. These cornerstone principles include considerations of autonomy, non-maleficence, beneficence and justice; out of which beneficence speaks for a greater involvement of the public sector in decision-making processes regarding orphan disease research.7
The aim of this paper is to identify ethical questions linked to rare diseases and orphan drugs and ethical principles or approaches applied to resolve them. We identified this area as potentially intricate from the ethical point of view. We therefore decided to find out whether the existing studies strive to solve the identified ethical questions linked to rare diseases and orphan drugs. More specifically, we asked two main questions: What ethical questions and moral issues are seen as relevant in the area of rare diseases and orphan drugs? What ethical principles are applied to resolve these questions and issues?
Theoretical Background
Legislative Context of Rare Diseases
According to Gericke et al (2005),7 Kinney (2014)12 and Pinxten et al (2012),4 most developed countries – particularly Australia, the EU, Japan, Taiwan or the USA – have passed legislation defining criteria for designating the orphan status to particular diseases and providing tax breaks and market exclusivity to pharmaceutical companies that produce orphan drugs. Statistics prove that such legislation helps promote research in rare diseases as the number of orphan drugs usually substantially increases in most countries following the implementation of orphan drug legislation.4,5,7 This kind of legislation has been around for several decades. In the USA, the US Orphan Drug Act was passed in 1983 and the Rare Disease Act in 2002.12 In the European Union, Regulation (EC) No 141/2000 and Regulation (EC) No 847/2000 were passed in 2000. Moreover, as eg, Rodriguez-Monguio et al (2017)5 and Kinney (2014)12 mention, developed countries have established bodies responsible for the policy in the field of rare diseases. For instance, it is the US Food and Drugs Administration (FDA) – specifically the FDA Office of Orphan Products Development (OOPD) in the US – or the European Medicines Agency (EMA) in the EU. Of course, there are non-governmental organizations supporting and connecting those who want to help. For example, Kinney (2014)12 mentions the National Organization for Rare Disorders, which is an organization connecting especially patients and other stakeholders.
Although the legislation has been successful, there are still a lot of problems. For instance, Gericke et al (2005)7 point out that
there is a substantial problem with legal rights as they can apply only to the provision of existing treatment options, while in case of still non-existing treatments it is difficult to imagine the way of enforcing individual rights.13
Prefer strict legal regulation at all levels, that is, local, European and international.
Furthermore, there are duties involved in research and development, where ethics committees, biobanks and registries play a key role. Ethics committees should oversee patient registries and databases. Health information is legally protected but different countries apply different rules. In general, confidential health information in databases and registries must not be misused or abused, the practice should be transparent. Ethics committees make sure this is happening. They may also determine specific exceptions to the generally accepted rules in order to minimize potential harm to the involved parties.14 Another task of ethics committees is to make sure that researchers accessing databases have previous experience in registry-based research and comply with the requirement of personal data protection. Although this form of research does not present physical health risks for the patients, there is a risk of privacy breach and abuse of personal identification details. Care must be taken to make sure that no individual can be identified by combining the personal details made available about them for research purposes.15 Furthermore, research projects involving the use of biological samples stored in biobanks are subject to approval of the ethics committee. Finally, in cases when the researcher works with the patient in person, the ethics committee makes sure that the patient provides their informed consent beforehand.15
Ethics in Context of Rare Diseases
Although modern societies consider it to be an ethical imperative to address patients’ unmet health needs and provide them with access to drugs (eg,5), ethical aspects of orphan drug research and distribution have not yet become a major issue (eg,7). The most frequently mentioned problem linked to orphan drugs is the lack of their cost-effectiveness (eg,7,9,12), which leads to the lack of interest in drug development (eg,4). As Gericke et al (2005)7 contend, pharmaceutical companies are unwilling to invest in research on orphan diseases because there is a serious risk of failure in developing orphan drugs and in case of tropical (neglected) diseases, the condition is prevalent in developing countries unable to pay fair prices for the drug. Rodriguez-Monguio et al (2017)5 also see the risk in the development of orphan medicinal products and agree that investment in research and development is less attractive to the pharmaceutical industry. The resulting low interest of the pharmaceutical industry in developing and marketing orphan drugs is therefore understandable, and as a consequence, health inequities persist.5 On the other hand, the EU and national governments want to mitigate these problems by prioritizing orphan drug development.16
An example of global, or at least international, collaborative effort is described by Nguyen et al (2019).17 The authors inform about recently designed model consent clauses for rare disease research created by an international task force consisting of experts from different countries and covering various expertise areas. This model is meant to ensure maximizing benefits of technological advancements while bearing in mind the unique realities of rare disease research. Of course, local and regional variations to this model can be expected based on differing laws, policies and approaches.17 At the same time, Picavet et al (2013)9 maintain that it is necessary to enforce strict observance of not only legislation but also ethical and other rules, like those of Good Clinical Practice, which are meant to protect drug trial subjects. Not only do they suggest that clinical trials for orphan drugs should be scrutinized as much as those for non-orphan drugs, but also argue that databases, biobanks and registries for rare diseases should be improved.
The most frequently tackled issue in this area is the reimbursement system for research and development and distribution of orphan drugs. Nonetheless, the whole problem of rare diseases and orphan drugs is rather complex and should be viewed from different angles, as there are multifaceted regulatory, ethical, economic as well as clinical issues (eg,5). Some authors (eg,18) even emphasize that ethical issues ought to be high on the agenda and that there should be space for debate featuring medical ethicists, experts in human sciences, lawyers as well as patients.13 Farberov et el (2013)13 list various points of view that should be taken into account, namely legal, ethical, scientific, social and that of the humanities. Multidisciplinary discourse is therefore seen as vital to this area (eg,7,9,13).
Conferences like the Brocher Symposium or the workshop “Ethical aspect of exome and whole genome-sequencing studies in rare diseases” (eg,9,13) offer the much-needed space for the aforementioned multidisciplinary discourse. These and similar events provide an opportunity for specialists in ethics, law and medicine to meet and share their views on rare diseases.
Methods
Design of the Study
The systematic literature review was conducted between 20 May 2020 and 20 June 2020. In this review-based study, we adopted the bibliometric mapping study method19 and review technique20 based on PRISMA guidelines21 to provide a systematic and holistic review of the ethical questions linked to rare diseases. The combination of two methods could facilitate deeper understanding of the topic on top of able to produce intellectual mapping structure.19 We collected bibliometric studies of papers published in PubMed, Scopus and Web of Science from 2010 to June 2020. The following keywords were used for the search: “ethics” AND “rare” AND “disease”, “ethical” AND “orphan”, “ethical” AND “orphan” AND “drug” and “ethical” AND “rare” AND “disease” follow-up with a snow-balling process (Table 1). Most papers were published in the BMJ Open (22 publications), Medicine (10 publications) and European Journal of Human Genetics (10 publications). Table 2 shows the most frequently represented journals under the keyword search. Other publications are scattered in other journals with few occurrences each.
 |
Table 1 Publication Statistics in Selected Publications |
 |
Table 2 The Most Frequently Represented Journals in Relation to Keywords |
The methodological framework introduced by Arksey et al20 involves five stages of research flow. First, the research questions are outlined; then, the relevance of the study is identified; and next, the selection is processed. Subsequently, the data are charted; after which information is collated and summarized and the results are reported. The flow diagram in Figure 1 demonstrates the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)21 flow of articles from search to the final selection.
 |
Figure 1 Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram of search process. |
A total of 4,139 papers related to rare diseases were identified – with 1,205 papers obtained from Scopus; 2,476 papers from PubMed; and 458 from Web of Science – with keyword search “ethics” AND “rare” AND “disease”, “ethical” AND “orphan”, “ethical” AND “orphan” AND “drug” and “ethical” AND “rare” AND “disease”. Due to the large quest for keywords and the inability to narrow quest by particular subject areas and keywords in the database, manual scoping was needed to finalize the appropriate articles. Nearly all of the reported works were false positive: they contained the right properties; however, the material was not applicable to the subject being discussed. The exclusion of duplicated works eliminated 1399 articles. After the unrelated works were removed via the keyword screening process, there was a total of 2388 papers left for further processing. Four hundred and thirty-eight papers were qualified for complete paper review after the final manual screening of the abstracts, and only 21 full-length papers were evaluated and synthesized.
At the same time, during the analysis of full texts of the articles, the authors decided to include in the researched ethical issues one publication7 to which many authors refer and which is also original in some ideas, so it is included in the summary Table 5 as publication 22.
 |
Table 3 Clusters of Keywords in Web of Science |
 |
Table 4 List of Leading Countries Related to the Keyword Search |
 | 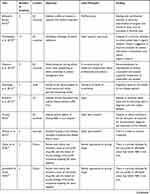 |  |
Table 5 Main Objectives and Findings of Research in the Ethical Area for Rare Diseases |
Data Extraction, Study Quality Evaluation and Eligibility Criteria
Eligible works were sorted by researchers who had independently reviewed the studies. Every paper was examined in terms of the following elements: title, author(s), publication type and language. For a study to be eligible for further review, it had to meet the following specific set of criteria:
- Articles were published/written between 2010 and April 2020
- English-written, peer-reviewed, full-text articles in scientific journals
- The aim of research described in these articles is to analyse ethical questions and principles linked to rare diseases and orphan drugs, more specifically, they discuss the ethical principles of justice and beneficence, health disparities and inequalities
- The output of these articles suggests how to support ethical principles and find ways to better develop, diagnose and treat orphan diseases in order to eliminate this health disparity
- The selected articles mention an ethical principle or an idea closely related to it
- The selected articles mention an issue related to ethics committees, biobanks and patient registries in the area of rare diseases
- Considerations within the ethical dilemmas
Articles were excluded from the analysis if they had the following features:
- Articles written in languages other than English
- Articles focusing on medicine and the treatment of particular rare diseases
- Chemistry articles focusing on rare diseases
- Paediatric articles on rare diseases, where ethical issues are only marginally mentioned in the introduction
- Articles related to the management of particular rare diseases which remained in the selection after it was narrowed down to the humanities
- Ethical questions addressed in clinical trials which are connected with a selected disease
- European policies on orphan diseases and drugs stimulating drug development where the ethical issue is solved only in terms of set rules of the legislation
- Articles on pricing strategies for pharmaceutical companies where ethics is only marginally mentioned and is not the point of the discussion
- Articles treating other ethical aspects (related to medical research)
Results
Cluster Analysis of Search results
The search results were analysed in each database using cluster analysis. The VOSviewer is able to show the most commonly co-occurring keywords related to the keyword search of “ethics” AND “rare” AND “disease”, “ethical” AND “orphan”, “ethical” AND “orphan” AND “drug” and “ethical” AND “rare” AND “disease”. In PubMed, from 45,686 co-occurring keywords, 1,432 keywords met the threshold set by default with 10 minimum occurrences. Figure 2 shows the top 50 keywords that have a minimum of 10 instances of co-occurrence and a maximum of 168 instances of co-occurrence to the keyword search in PubMed. Metastasis (168), breast cancer (85), receptor (68) and biobank (57) were found to be the most frequently co-occurring keywords to the keyword search.
 |
Figure 2 Co-occurring keywords in PubMed. |
In Scopus, from 20,460 co-occurring keywords, 640 keywords met the threshold set by default with 10 minimum occurrences. Figure 3 shows the top 50 keywords that have a minimum of 10 instances of co-occurrence and a maximum of 68 instances of co-occurrence to the keyword search. Biobank (68), sequencing (63), infant (50) and genome (42) were found to be the most frequently co-occurring keywords to the keyword search.
 |
Figure 3 Co-occurring keywords in Scopus. |
In Web of Science, from 18,630 co-occurring keywords, 517 keywords met the threshold set by default with 10 minimum occurrences. Figure 4 shows the top keywords that have a minimum of 10 instances of co-occurrence and a maximum of 159 instances of co-occurrence to the keyword search. Rare disease (159), diagnosis (142), child (118) and disorder (107) were found to be the most frequently co-occurring keywords to the keyword search.
 |
Figure 4 Co-occurring keywords in Web of Science. |
Other than the number of co-occurring keywords and the relevance score, the VOSviewer also generated the most recent keywords used and the most cited keywords that have significant occurrences with the keyword search (Figure 5). The most recent keywords that co-occur together with the keyword search were acute exacerbation, brain metastasis colchicine, hbv and igg4 with a minimum of 10 co-occurrences in 2018. The most cited keywords were next-generation sequencing (38 citations), genome (36 citations), whole exome sequencing (35 citations), male breast cancer (33 citations) and genomic medicine (32 citations).
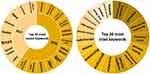 |
Figure 5 Top 20 most recent keywords and most cited keywords to the keyword search. |
Table 3 shows the available clusters of the co-occurring keywords from the selected keyword search in Web of Science. Note that these keywords have a strong significant connection that makes it possible to create sets of clusters. At the same time, it is clear from the keywords in the individual clusters that only some of them are related to an ethical problem, specifically Cluster 1 and Cluster 3. Cluster 2 deals with questions related to the issue in clinical trials, Cluster 4 and 5 address research issues generally. Thus, many results are not directly related to ethical dilemmas in rare disease research.
For the above-mentioned reasons, relating to broadly focused search topics, special attention was paid to links with the phrase “ethical issues”. Figures 6 and 7 show the relationship of ethical issues with other linked keywords and the relationship of rare diseases with other keywords, respectively. The result indicates that the phrase ethical issue has 80 linked keywords altogether. The most prominent linked keywords were disorder (43 link strength), trial (35 link strength) and diagnosis (30 link strength). Meanwhile, the phrase rare disease has 92 linked keywords altogether, while the most significant linked keywords were trial (113 link strength), orphan drug (87 link strength) and review (64 link strength). The phrase ethical issue was not listed among the top significant linked keywords.
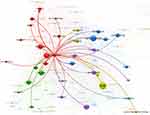 |
Figure 6 The relationship between the phrase ethical issue and linked keywords in Web of Science. |
 |
Figure 7 The relationship between the phrase rare disease and linked keywords in Web of Science. |
To get a holistic view, Figure 8 shows the co-authorship of countries with each country having published a minimum of 25 papers related to the keyword search of “ethics” AND “rare” AND “disease”, “ethical” AND “orphan”, “ethical” AND “orphan” AND “drug” and “ethical” AND “rare” AND “disease”. In Scopus, from 88 countries that had a relationship with the keyword search, 32 countries met the threshold. Table 4 reveals the list of leading countries.
 |
Figure 8 Co-authorship of countries. |
The contents of the keyword search of “ethics” AND “rare” AND “disease”, “ethical” AND “orphan”, “ethical” AND “orphan” AND “drug” and “ethical” AND “rare” AND “disease” were analysed after the initial keyword and abstract inspection. Then, we examined the selected papers and their citations to further evaluate the particular author’s and publication’s contributions. The number of citations increased dramatically in 2017 and continued increasing in 2018 and 2019, which indicates the increase of the interest in and the value of this research topic (Figure 9).
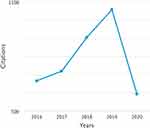 |
Figure 9 Sum of citations per year of the keyword search of “ethics” AND “rare” AND “disease”, “ethical” AND “orphan”, “ethical” AND “orphan” AND “drug” and “ethical” AND “rare” AND “disease”. |
The papers most frequently cited are listed in Appendix Table 1. Even from the group of the most cited papers, only a few of them were relevant to ethical issues. As mentioned above, articles on these topics are not collected in one journal, are published across the fields of health and medicine and in comparison with articles directly focused on medicine, the number of citations corresponds more to social sciences, where the values are generally lower. Most of the contributions included below (Table 5) are therefore mainly based on a selection according to the established search criteria in the chapter “Methods”, and not on the basis of the highest number of citations only.
Analysis of Ethical Problems Linked to Rare Diseases
We have come across several ethical problems linked to rare diseases and orphan drugs, which occurred in the final selection of 21 articles. A detailed description of the targeted articles is provided in Table 5, which lists the main objective, methodology used, findings and country of origin of each paper.
The content and subject of research in the above-mentioned studies focus on three areas. One of them, which is connected with an ethical problem, is the allocation of financial resources with respect to the lack of cost-effectiveness. Another area is the cooperation across nations and various groups of stakeholders in data collection, data sharing and preparation of legislation. The third area covers the functioning, involvement and competence of ethics committees. The above areas are then addressed and discussed in the context of various moral principles.
The problem areas include, for instance, the acceptable treatment of financial resources, as it remains unclear how to allocate money in the best way, which is not necessarily the “just”, “equitable” or “right” way. Another issue is to determine whether and to what extent to take into account the potential non-financial justifications for producing and distributing orphan drugs. Moreover, there is no globally accepted definition of rare diseases based on their incidence. Inevitably, this is linked to the lack of coordinated international action to improve global cooperation in treating rare diseases. Last but not least, the place of ethics and ethics committees in making the aforementioned decisions is far from clear. All in all, this chapter helps to show how complicated the decision-making process in the area of rare diseases and orphan drugs might be.
Key Ethical Questions
We have identified five ethical questions that are currently relevant in connection with rare diseases and orphan drugs (Table 6).
 |
Table 6 Key Questions Related to Rare Diseases and Orphan Drugs |
This question is closely related to one of the basic ethical issues, namely the value of an individual and their life in comparison with the value of the human society as a whole. The area of rare diseases and orphan drugs puts a price tag on human life. Thus, the issue of allocating financial resources goes down to economic views on tackling rare diseases. When it comes to allocating financial resources, it is by no means ethically clear-cut where the money should go. Therefore, there is a need for negotiating a compromise.
Authors like Pinxten et al (2012) and Juth (2017)4,22 pose questions about the fair share of resources for orphan drugs. On the one hand, they realize how challenging it is to balance allocating a substantial share of meagre resources to an insignificant number of individuals, and on the other hand, they consider the ethical dimensions of the abandonment of others affected by serious but rare diseases.22 Asks whether or not it is justifiable to provide rare diseases with economically favourable conditions. Taylor et al (2018)23 discuss the ethical dilemma in balancing the considerations of equity and opportunity cost. They argue that there are conflicting moral arguments, namely the utilitarian one, the one of equity in access to treatment, the imperative to save individuals regardless the cost, as well as the striving to advance knowledge.
Pinxten et al (2012)4 suggest a way to allocate sufficient resources to orphan drug research and development in an equitable way. There should be two tracks of research allocation, namely one group of patients should have guaranteed access to these funds based on rational priorities, and all the other patients would have a random opportunity to access the money.4 It may be seen as an effort to balance targeting versus universalism, which24 view as the main conflict that concerns orphan drugs.
Gericke et al (2005)7 agree that resource allocation for research in rare diseases has extremely uncertain results and there are also conflicting ethical views. The costs of developing a new drug vary widely and investing much money in rare conditions may be deemed unjust and unethical from the utilitarian perspective. On the other hand, there are moral obligations of non-abandonment and beneficence.
Hew Girard et al (2020)25 ask whether scientific advancement in common diseases should enjoy priority over that in rare diseases. In the same vein, Taylor et al (2018, p. 1)23 pose questions: “Why fund rare disease therapies?” and “How to achieve access to rare disease therapies in an equitable manner?” Gericke et al (2005, p. 164–165, 167)7 ask the following questions: “How much a society should spend on orphan diseases?”; “How many resources should be devoted to orphan diseases overall?”; “How many resources should be allocated to each individual orphan disease?”; “What should determine the boundaries of moral obligations of beneficence and distributive justice?”; “Should these be determined by national boundaries, political constituencies, or economic influence or industrial profitability?”
A strictly global approach seems to be appropriate. Nonetheless, global cooperation appears to be unreachable. It is not viable for various reasons. First, the technologically globalized world lacks globally accepted ethical values and rules. Second, it is politically unfeasible to disregard national interests. Third, there are unresolved international and intercultural conflicts. Fourth, the sub-interests of various parties as well as the lack of desire to deal with other people’s problems need to be taken into account. All of these issues are difficult to deal with. Therefore, individual countries apply their own varied approaches to evaluating and funding rare disease therapies, ranging from less rigorous ones in countries such as Germany and France, to stricter ones, such as in the UK, Scotland and Australia.23
Whereas the previous question goes down to economic values, there are nonetheless other, non-economic views, too. The second question, albeit also based on economic factors, relates to the problem of rules and equality. Should all rules apply to all (people) to the same extent or is there in some particular cases, including rare diseases and orphan drugs, any ethically defendable space for “adjusting” or even “breaking” them? For instance, Hew-Girard et al (2020)25 suggest that researchers in rare diseases should consider what circumstances justify breaking the rules.
Jarosławski et al (2018)26 show various pricing models with their corresponding ethical considerations and discuss ethical challenges linked to orphan non-profit and for-profit drug research and development, respectively. According to them, the former is funded by donations from the public and charities, or by governments. Consequently, non-profit research and development have a moral obligation to provide orphan drugs at an affordable price. On the other hand, the for-profit companies seek profit-maximizing pricing. Jarosławski et al (2018)26 also claim that for-profit pharmaceutical industry used to ignore rare diseases. Nonetheless, pro-orphan drug policies – like expedited drug approval or extended market exclusivity – have reversed the situation. Recently, as Wellmann et al (2010)27 maintain, pharmaceutical companies have started getting significant revenues from producing and selling orphan drugs. The problem is the resulting high price of these products, and Jarosławski and Toumi (2018)26 on this account look for a way to set a model for an affordable pricing of orphan drugs.
Authors like Hyry et al (2015)28 remind us that there is more to tackling rare diseases than the financial side by asking questions like: “Are there any ethical reasons for offering orphan drugs for free?”; “Is it only sentiment or also a coherent ethical theory?”; “Why should a profit-making enterprise donate a drug?” Hyry et al (2015, p. 1)28 also mention that there are compassionate use programmes in both the United States and European countries and add that the EU is testing an adaptive pathways approach, which is a collection of evidence aimed to facilitate fast access to medications. The accelerated approval of orphan drugs is not necessarily a good thing, though. Hyry et al (2015)28 and Kesselheim (2012)29 also warn that some drugs are not properly tested in comparison with testing conducted for non-orphan drugs, which leads to higher risk and lower efficacy. According to Kesselheim (2012),29 accelerated approval should be limited to the most innovative products intended for those with no other alternatives.
On the whole, we need to distinguish non-profit and for-profit research and development. Whereas the former have moral obligations to those who help to fund them, for-profit orphan drug research and development do not seem to act compassionately and they do not have any obligation to do so. Moreover, for-profit companies use opportunities presented by pro-orphan drug policies to increase their profits. Last but not least, rule-breaking policies like the aforementioned accelerated approval bring about pitfalls like higher risk and lower efficacy.
The third identified question points to applying the same or different rules to all people, and thus to equality. There is currently no consensus whether or not the prevalence of a disease or the size of the patient population is justifiable for orphan drugs to be approved and reimbursed (eg,5). Limits for defining of what already is and what still is not a rare disease may vary from one political unit to another. For example, Rodriguez-Monguio et al (2017)5 maintain that the US and the EU use different methods to classify an orphan disease. They add5 that whereas the FDA uses disease prevalence (ie, the number of people suffering from the disease), the EMA uses the prevalence proportion (that is the proportion of people in the population that have the disease). As a result, a disease of the same prevalence may be labelled in some countries as a rare disease, whereas in others it may not qualify as one. Moreover, what happens if the prevalence is just slightly above the limit? According to the Regulation (EC) No 141/2000, rare diseases affect up to five people per 10,000 in the EU. Nevertheless, the Regulation enables to extend the threshold in case of “life threatening, seriously debilitating, or serious and chronic diseases”. In other words, what makes a disease eligible to become a rare disease is an in-some-way-justified exception from commonly applied rules.
This question reminds us of the discrepancy between the actual (real) and the ideal situation. There is a tension between what is and what should be the case.
As30 contend, due to the fact that patients suffering from rare diseases are often dispersed across the world, it might be difficult to find the required number of patients for clinical trials, which may cause lower relevance of findings and hinder determining differences between alternative options of treatment. Pinxten et al (2012)4 also mention that the lack of eligible research subjects and their geographical dispersal may cause problems. It is therefore advisable, as16 note, that data should be concentrated and standardized in order to accelerate research and development in the area of rare diseases. On the other hand, as Gelinas et al (2019)31 argue, in research, remote access is sometimes impossible and in order to be able to participate in research, dispersed patients have to be relocated to trial sites. According to the authors, such relocation poses ethical problems for sponsors as to how reimbursement applies, especially when participation requires lengthy travel and accommodation for research participants.
There is, furthermore, a dilemma whether national resources can be used to deal with global health problems. It is usually viewed as next to impossible to implement global measures, as governments are responsible to their countries’ citizens. As Afroze et Brown (2012)32 point out, most low- and middle-income countries face far more acute ethical challenges in allocating their insufficient financial resources to treat rare diseases than high-income countries. Gericke et al (2005)7 ask whether the moral obligation to distribute resources extends outside the society to other countries and answer that it is not politically acceptable to spend national resources in order to maximize global health. On the other hand, Gericke et al (2005)7 suggest that international funding agencies could take responsibility for it. Still, as Boy et al (2011)24 contend, the universal healthcare system is not only deficient in meeting demands, but also prone to problems with the management of available resources.
In spite of all hindrances, there exist internationally coordinated approaches. McCormack et al (2013)16 praise as crucial the formation of the International Rare Diseases Research Consortium, which links the efforts of the EU, Canada and the USA, and view such joint effort as a way to reduce the cost of developing orphan drugs.
Schmiedeke et al (2019)33 point out that in Europe there is a platform created by the European Commission and patient organisations whose key aim is centralising the treatment of rare conditions – The European Reference Networks for patients with rare diseases (ERN). Centralisation helps to pool patient data and share knowledge in order to improve treatment results. At the same time, Schmiedeke et al (2019)33 warn that centralisation should be conducted carefully and point out several potential pitfalls to avoid in this process, for instance, more difficult access to healthcare, or the question of place and responsibility for the follow-up treatment.33
The need for cooperation is viewed as inevitable in Latin America, too. For instance, Encina et al (2019)34 offer recommendations that include, among other things, compiling a patient registry or setting up a Latin American cooperation network. Developing countries get some support, too. According to Jarosławski et al (2018),26 developing countries have been provided assistance by product partnerships, bodies facilitating cooperation among research agencies, donors, biotech and pharmaceutical companies since the early 2000s.
By and large, despite multiple efforts to establish international, supranational or even global cooperation, much remains to be done. An ideal approach to fighting rare diseases would be global, since patients suffering from rare diseases are often dispersed across the world, which makes a lot of things, including drug or treatment trials, difficult to organize. Such a global approach requires cooperation, and that not only international or supranational but also across different groups of stakeholders, including medical staff, the pharmaceutical industry, various experts, political bodies, patients and their families. According to,23 there is currently no internationally accepted model to evaluate and fund rare disease therapies, although there are common features, such as the opportunity for early stakeholder engagement, flexibility concerning evidence requirements, criteria of cost-effectiveness and transparency of decision-making processes.
Ethics committees for rare diseases should consist of specialists in various relevant fields. These committees should assess risks and benefits of actions related to research and development in rare diseases. It includes the way in which the sensitive information from databases, biobanks and registries for rare diseases is used and treated.
As Barrera & Galindo (2010)35 assert, ethics committees should consist of lay people as well as experts. These bodies ought to approve research proposals and clinical trials and closely follow different activities, especially those potentially risky for participants. Ethics committees are also supposed to oversee by using the same standards for trials in both developed and developing countries.
Some authors, however, question the necessity of establishing ethics committees. For instance, Hansson et al (2012)36 suggest that a bureaucratic ethics system is a hindrance as it sometimes takes years to get the committee’s approval, which delays development in treating rare diseases. On the other hand,16 welcome the work of advisory groups drawing upon the expertise of their interdisciplinary membership and suggest that all registries should have their own ethics committee to monitor the inflow and outflow of data.
There is an implicit agreement among authors like Farberov et al (2013), Kesselheim (2012) and McCormack et al (2013)13,16,29 that making decisions on rare diseases should be the responsibility of experts and stakeholders. McCormack et al (2013)16 propose interdisciplinary advisory groups. Farberov et al (2013)13 inform about ideas springing from a workshop attended by top-level specialists in ethics, law and medicine.29 Kesselheim (2012)29 points out that people suffering from rare diseases perceive their illness in a different way in comparison to those whose diseases are more common, which makes it necessary to find out their actual needs and requirements.
Several authors believe that it is vital to improve existing patient registries and databases in the field of rare diseases (eg,9,16,18). Duchange et al (2014)18 mention that databases and registries collecting data for rare diseases from all over the world are seen as inevitable, although varying legal requirements in different countries hinder their development. They contend that gathering information on a large scale is very important and add that ethical rules must be elaborated and strictly followed, as there are concerns about data privacy, access to data, sharing of data, informed consent and more.18 Rodriguez-Monguio et al (2017)5 emphasize the importance of patient privacy protection as registries contain not only valuable but also highly sensitive information.13 Farberov et al (2013)13 discuss the issue of informed consent about samples and data archived in biobanks.
All in all, the decision-making should ideally be based on informed discussions of interdisciplinary panels of experts that would also take into account views of other stakeholders. Ethics committees might not only approve and follow procedures involved in orphan drug research and development, but also spot, formulate, and interpret new ethical rules and then monitor whether or not the involved parties should adhere to them.
Ethical Thought and Ethical Principles Applicable in the Area of Rare Diseases
Ethics is practical philosophy trying to help to solve the basic question: What is proper to do? Philosophers propose various moral principles indicating what is morally right to do. As Gershon and Alliey-Rodriguez (2013)37 put it, ethics is a system of moral principles that help to guide moral judgements as well as behaviour. In the area of rare diseases, these moral principles apply, too (see Table 7). The most frequently mentioned moral principles are justice (eg,4,5,7,12,30) and the utilitarian approach (eg,4,5,7,12).
 |
Table 7 The Moral Dilemma of Funding Orphan Drug Research and Development |
Justice can be seen as the most appropriate term, as any approach essentially strives to be just. Gericke et al (2005, p. 165)7 define justice as “fair, equitable, and appropriate treatment in the light of what is due or owed to individuals”. The most common interpretation of justice in healthcare is the provision of basic care for everyone.12 However, justice was further divided into several subcategories in the reviewed texts, namely into procedural, distributive and social justice. Procedural justice is favoured by Gericke et al (2005),7 who call for the transparency of decision-making and participation of civil society organizations. Distributive justice is, according to,12 concerned with the fair way of allocating resources but it does not cover orphan diseases and their treatments, which are issues of social justice, whose goal is to treat each individual with dignity and respect that they deserve as human beings. As Rosselli et al (2012)30 suggests, orphan diseases should be treated with utmost cautiousness as it is not possible to apply the usual standards of cost-effectiveness here.
The utilitarian approach can be viewed as the only one that goes strictly against supporting those affected by rare diseases. This approach demands to bring the greatest good to the greatest number or maximize the overall good (eg,5,7,12). On the other hand, these authors are aware of the pitfalls of the utilitarian approach in the area of rare diseases. Kinney (2014)12 warns that utilitarian principles leave out those affected by orphan diseases, and Rodriguez-Monguio et al (2017)5 proclaim that the utilitarian approach would not be favourable to funding rare disease research and treatment. On the other hand, Hews-Girard et al (2020)25 suggest a specific interpretation of utilitarianism in which rules can be broken if it is possible to demonstrate a benefit resulting from it.
Nevertheless, the reviewed texts feature other moral principles, like rights-based approach or egalitarian principles. This array of various principles only supports the view proposed by Rodriguez-Monguio et al (2017),5 who point out that although distributive justice or egalitarian principles of equitable healthcare form some basis for supporting the treatment of orphan diseases, there is no clear ethical mandate regarding how to address it.
Rights-based approach is based on human rights as the individual’s or a group’s justified claims.7 We should distinguish positive and negative rights. Positive rights require others to do something beneficial for right bearers, whereas negative rights require others to refrain from doing something harmful to right bearers.7 In the area of rare diseases, the right to a decent minimum of healthcare is applicable. Article 35, on healthcare, of the Charter of Fundamental Rights of the European Union says:
Everyone has the right of access to preventive health care and the right to benefit from medical treatment under the conditions established by national laws and practices. A high level of human health protection shall be ensured in the definition and implementation of all Union policies and activities.38
Nonetheless, Gericke et al (2005)7 can see several problems here. First, the main problem of the rights-based approach is that the scope of the right to healthcare can be understood in various ways. Second, legal rights can apply to existing options, not to research funding for non-existing treatments. Last but not least, even though there is a societal moral obligation of solidarity, priority setting for orphan drug research and development belongs to the pharmaceutical industry.
Several authors7,25,31 mention beneficence. Beneficence requires the agent not only to refrain from harmful acts but also to take positive steps to help others.7 The basic moral commitment to non-abandonment belongs here. As Gelinas et al (2019)31 have it, beneficence requires sponsors of research in the field of rare diseases to refrain from abandoning research participants if there is an unmet medical need.12 Kinney (2014)12 assumes that non-abandonment shows that the society recognizes its obligation to take care of patients with orphan diseases. It can be exemplified by the list of economic incentives meant to support orphan drugs in the US – grant funding for academic researchers or companies, tax credits for expenditures or a seven-year market exclusivity.5 Gericke et al (2005, p. 166) maintain that
the laws and regulations passed in recent years to provide incentive for orphan drug research could be interpreted as attempts of democratic society to pursue the principle of non-abandonment and to counteract distributive injustice caused by market incentives.
On the other hand, both the utilitarian and economic theories disagree with the aforementioned principle of non-abandonment,7 and some authors26,27 implicitly suggest that for-profit pharmaceutical companies become active in this area only if it increases their revenues, which has nothing to do with beneficence.
Rosselli et al (2012)30 as well as Rodriguez-Monguio et al (2017)5 mention the “rule of rescue”, which is another possible justification of using public resources for funding rare disease treatment. Rosselli et al (2012)30 specify that this rule was originally used by Jonsen in 1986 and refers to the imperative that the society should rescue people who face avoidable death or severe disability. For example, rescuing trapped miners does not fit the definition of cost-effectiveness but hardly anybody questions saving people in similar situations, although it may cost a lot of money.
Gericke et al (2005)7 and Kinney (2014)12 mention scientific advancement as another rule to be followed. Professional ethics of medicine poses an obligation to advance scientific knowledge and come up with new therapies, which clearly goes beyond mere utility. Gericke et al (2005, p. 166) emphasize: “Many rare diseases, however, merit scientific study for reasons other than prevalence.” In other words, the study of rare diseases may generate manifold returns as it might bring new medical insights and drugs for rare as well as common conditions, which fulfils the commitment of society towards future generations.7,28 Mention not only ethical approaches or principles but also philosophers (or ethicists), namely Rawls, Kant and Aristotle. John Rawls’ Theory of Justice provides a convincing reason for individual countries to invest in developing orphan drugs but his theory of international justice prefers justice for societies to the well-being of individuals.28 Also Boy et al (2011)24 mention Rawls’ “veil of ignorance” (if one did not know one’s own position in society, he or she would support the principles of justice), in which the principles of justice are reduced to favouring those worse off if inequality occurs. Another philosopher, Immanuel Kant, argues that people do morally right actions for intrinsically right reasons, and adds that humans should be helped not out of compassion but for their independent value.28 Aristotle contends that people should do what makes them happy – happiness means to Aristotle “acting virtuously” – and strive for the golden mean or golden middle way. For instance, in financial matters, one should be neither profligate, nor miserly, but generous. Consequently, pharmaceutical companies should run compassionate use programmes because it is generous and virtuous.28
From among other ethicists, Tom L. Beauchamp and James F. Childress and their four-principle approach to biomedical ethics or bioethics, which applies general ethical principles of non-maleficence, beneficence, justice and autonomy to medical care and practice, are sometimes mentioned (eg,7,24,25). Hews-Girard et al (2020)25 explore objectivity in rare disease research and mention philosophers including Adam Smith, Immanuel Kant, Thomas Kuhn, Karl Popper or Rudolf Carnap.
To sum up, few authors focus on discussing various ethical principles that could be employed in decision-making processes on rare diseases and orphan drugs. Even fewer of them, with the exception of eg,25 discuss philosophers and ethicists who came up with ethical principles that could be applied in this area. By far the most detailed information on various ethical principles can be found in Gericke et al (2005),7 who discuss two conflicting theories of justice, namely a utilitarian and a rights-based approach to the dilemma of orphan drug research funding. Gericke et al (2005)7 also mention beneficence, which is the basis for the societal moral obligation of non-abandonment and for medicine’s professional moral obligation of scientific advancement. Rodriguez-Monguio et al (2017)5 ponder the societal value of orphan drugs and list various approaches to the ethics of resource allocation. They mention the utilitarian doctrine, egalitarian approach and the rule of rescue, which is also discussed by.30
According to Gericke et al (2005),7 the most pressing problem is the moral dilemma of funding orphan drug research and development, as there are conflicting moral obligations of beneficence (based on social and moral obligations) and distributive justice (based on utilitarian or legal rights), which seem to demand different approaches to allocating financial resources.
Gericke et al (2005, p. 167) can see
the conflict between principles of distributive justice based on utilitarian principles or legal rights and principles of beneficence based on social or moral obligations.
Pinxten et al (2012)4 highlight another moral dilemma, based on the concept of opportunity cost, which means that the share of health resources spent on orphan drug research and development cannot be used to finance something else. Therefore, the utilitarian and rights-based approaches to justice that are frequently used in determining resource allocation cannot be readily used for orphan diseases.4 As eg, Gericke et al (2005)7 maintain, from the utilitarian point of view, few resources would be allocated to individuals suffering from rare diseases. On the other hand, if taken together, they represent a substantial number of people. In the EU, there are some 25–30 million of these individuals if taken together. Two further questions arise here: “What level of resources should be devoted to orphan diseases overall?”; “What level of resources should be allocated to each individual orphan disease?” (Gericke et al, 2005, p. 165).7 Unlike the utilitarian doctrine, the egalitarian view would favour public funding for orphan drug development, as egalitarian principles favour resource distribution that minimizes inequality.5
Inevitably, there is a clash between economic and non-economic values. Whereas utilitarian approach, distributive justice or the concept of opportunity cost are based on economic values, some others, like beneficence, non-abandonment, solidarity, rule of rescue or scientific advancement derive from non-economic values. And as Hyry et al (2015)28 add, the orphan drug legislation stems from non-economic societal values. On the other hand,28 say that it would be naive to advocate charity, as profit is key, and therefore the law introduces tax exemptions, even though there are other gains for biopharmaceutical companies engaged in compassionate provision of treatments, including favourable publicity, financial approval, fulfilled employees, a demonstration of strength and an assertion of marketing confidence.
Discussion
All in all, rare diseases and research and development of orphan drugs bring up a lot of difficult questions. They are further aggravated by the fact that there is no clear ethical mandate to decide them either way. Moreover, ethical consideration remains to be overshadowed by economic reasoning. Inevitably, this approach puts a price tag on human life. Even ethics considers the value of an individual. Ethical thinking ranges from favouring the utilitarian approach of the highest benefit to the highest number of people to beneficence and non-abandonment, requiring taking steps to actively help others. All ethical approaches aim at justice, which can be further divided into procedural, distributive and social justice, all of them emphasizing their own values. It is hardly surprising that authors like25 look for compromises, suggesting that the best approach would be utilitarianism, but one that allows breaking the rules if there is a clear benefit. Still, the omnipresent tension between the ideal and the reality is innate to ethics in all areas, not only that of rare diseases.
Of course, the ideal approach to treating rare diseases would be global. This would help, for instance, in solving issues linked to information sharing in this area, as a tool for the support of the industry, development of orphan drug research, assessment of drug treatment effectiveness and long term follow-up after such treatment.39 As far as rare diseases are concerned, biobanks and patient registries usually yield information on a very limited number of patients and the number of samples is low, too. Moreover, some patients are reluctant to enter a database due to privacy concerns. Despite legislation hindering discrimination, private information sometimes negatively influences the patient’s insurance and/or employment. On the other hand, patients should take into account that the necessity to collect data may be of higher importance than privacy concerns. As orphan drug databases are vital to orphan drug development, both the society and the pharmaceutical industry should financially support their establishment and maintenance.9
Nevertheless, as the global approach is still unfeasible, the allocation policy usually depends on the value that particular societies attach to orphan drug research and development. When utilitarian values prevail, then there will be few resources available. If, on the other hand, egalitarian or rights-based approaches, including beneficence and non-abandonment, prove stronger, then orphan drug research and development may be funded to a considerable extent, albeit some will use it for their own profit. In reality, however, there will always be a debate leading to compromises. As eg, Boy et al (2011)24 contend, the best approach would be based on deliberative democracy – that is, negotiating well-founded rational decisions. This may lead to creating ethics committees consisting of experts in ethics, law and medicine, as well as stakeholders. Such bodies would necessarily employ the aforementioned multidisciplinary discourse (eg,7,9,13), which refers to Habermas and Apel’s discourse ethics. Surprisingly, these two ethicists have not been mentioned by any author writing about the ethics of orphan drug resource allocation system. Not even Bentham and Mill, founders of utilitarianism, or Herbert Spencer, who reflected on negative and positive beneficence, have been mentioned. Neither has Schopenhauer’s ethics of compassion been mentioned, although it teaches that moral acting consists in helping other people in need and not making them suffer,40 which befits the debate on rare diseases and orphan drugs.
Even though this study strives to provide a comprehensive survey of current ethical issues in this area, the reader should be aware of certain limiting factors. This study took into account only texts published in the English language. Rare diseases and orphan drugs should be perceived from the global point of view and there are a lot of countries and regions where the lingua franca is not English, but French, Spanish, Chinese or other. Moreover, philosophy and ethical thought do not necessarily work well in English, either. Essential works on ethics were written in other languages, like Greek, Latin, German and others, which makes it difficult to fully understand if translated into other languages. The reviewed articles do not offer any decisive answers to the identified questions. Neither do they provide clear arguments in favour of any ethical principle to be applied.
Conclusion
Although this study does not give a full list of ethical problems and principles, it attempts to highlight and emphasize an area that is often neglected in both literature and the public sphere, namely ethical issues in patient care and treatment. The most desirable way of tackling rare diseases would be a strictly global approach. Nonetheless, international, let alone global cooperation seems to be beyond the reach of the current international community. This deficit in international cooperation can be partly explained by the fact that the current technologically globalized world still lacks globally accepted ethical values and rules. This is further aggravated by unresolved international and intercultural conflicts. In addition, the sub-interests of various parties as well as the lack of desire to deal with other people’s problems need to be taken into account. The aforementioned problems are difficult to avoid. Nevertheless, let us be cautiously optimistic. At least, there are people who raise ethical questions about rare diseases and orphan drugs.
Abbreviations
EC, European Union, Regulation; OOPD, Office of Orphan Products Development; FDA, Food and Drugs Administration; EMA, European Medicines Agency; CAVOD, Clinical Added Value of Orphan Drugs.
Acknowledgments
This research was supported by the project Excellence 2020 (University of Hradec Kralove, Faculty of Informatics and management), Long term development plan UHK.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest for this work.
References
1. European Commision. Rare diseases | health - research and innovation - European commission. Published 2016. Available from: http://ec.europa.eu/research/health/index.cfm?pg=area&areaname=rare.
2. European Union of Private Hospitals. Access to orphan drugs: a comprehensive review of legislations, regulations and policies in 35 countries. Published 2016. Available from: http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0140002.
3. RARE Facts. Global genes. Available from: https://globalgenes.org/rare-facts/.
4. Pinxten W, Denier Y, Dooms M, Cassiman -J-J, Dierickx K. A fair share for the orphans: ethical guidelines for a fair distribution of resources within the bounds of the 10-year-old European Orphan Drug Regulation. J Med Ethics. 2012;38(3):148–153. doi:10.1136/medethics-2011-100094
5. Rodriguez-Monguio R, Spargo T, Seoane-Vazquez E. Ethical imperatives of timely access to orphan drugs: is possible to reconcile economic incentives and patients’ health needs? Orphanet J Rare Dis. 2017;12(1):1–8. doi:10.1186/s13023-016-0551-7
6. WMA - The World Medical Association. WMA declaration of Helsinki – ethical principles for medical research involving human subjects. Available from: https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/.
7. Gericke CA, Riesberg A, Busse R. Ethical issues in funding orphan drug research and development. J Med Ethics. 2005;31(3):164–168. doi:10.1136/jme.2003.007138
8. Europeen Comission. EU charter of fundamental rights. European Commission - European Commission. Published 2018. Available from: https://ec.europa.eu/info/aid-development-cooperation-fundamental-rights/your-rights-eu/eu-charter-fundamental-rights_en.
9. Picavet E, Cassiman D, Pinxten W, Simoens S. Ethical, legal and social implications of rare diseases and orphan drugs in Europe: meeting report of a Brocher symposium. Expert Rev Pharmacoecon Outcomes Res. 2013;13(5):571–573. doi:10.1586/14737167.2013.832626
10. McCabe C, Claxton K, Tsuchiya A. Orphan drugs and the NHS: should we value rarity? BMJ. 2005;331(7523):1016–1019. doi:10.1136/bmj.331.7523.1016
11. van der Burg S, Oerlemans A. Fostering caring relationships: suggestions to rethink liberal perspectives on the ethics of newborn screening. Bioethics. 2018;32(3):171–183. doi:10.1111/bioe.12425
12. Kinney J. Health disparities: exploring the ethics of orphan drugs. Am J Health Syst Pharm. 2014;71(9):692–693. doi:10.2146/ajhp130348
13. Farberov L, Gilam A, Isakov O, Shomron N. Meeting summary: ethical aspects of whole exome and whole genome sequencing studies (WES/WGS) in rare diseases, Tel Aviv, Israel, January 2013. Genet Res. 2013;95(2–3):53–56. doi:10.1017/S0016672313000104
14. Gliklich RE, Dreyer NA, Leavy MB. Principles of Registry Ethics, Data Ownership, and Privacy. Agency for Healthcare Research and Quality (US); 2014. Available from: https://www.ncbi.nlm.nih.gov/books/NBK208620/.
15. Ludvigsson JF, Håberg SE, Knudsen GP, et al. Ethical aspects of registry-based research in the Nordic countries. Clin Epidemiol. 2015;7:491. doi:10.2147/CLEP.S90589
16. McCormack P, Woods S, Aartsma-Rus A, et al. Guidance in social and ethical issues related to clinical, diagnostic care and novel therapies for hereditary neuromuscular rare diseases: “translating” the translational. PLoS Curr. 2013;5. doi:10.1371/currents.md.f90b49429fa814bd26c5b22b13d773ec
17. Nguyen MT, Goldblatt J, Isasi R, et al. Model consent clauses for rare disease research. BMC Med Ethics. 2019;20(1):55. doi:10.1186/s12910-019-0390-x
18. Duchange N, Darquy S, d’Audiffret D, et al. Ethical management in the constitution of a European database for leukodystrophies rare diseases. Eur J Paediatr Neurol. 2014;18(5):597–603. doi:10.1016/j.ejpn.2014.04.002
19. Leung XY, Sun J, Bai B. Bibliometrics of social media research: a co-citation and co-word analysis. Int J Hosp Manag. 2017;66:35–45. doi:10.1016/j.ijhm.2017.06.012
20. Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8(1):19–32. doi:10.1080/1364557032000119616
21. Moher D, Liberati A, Tetzlaff J, Altman DG; Group TP. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi:10.1371/journal.pmed.1000097
22. Juth N. For the sake of justice: should we prioritize rare diseases? Health Care Anal. 2017;25(1):1–20. doi:10.1007/s10728-014-0284-5
23. Taylor C, Jan S, Thompson K. Funding therapies for rare diseases: an ethical dilemma with a potential solution. Aust Health Rev. 2018;42(1):117–119. doi:10.1071/AH16194
24. Boy R, Schwartz IVD, Krug BC, et al. Ethical issues related to the access to orphan drugs in Brazil: the case of mucopolysaccharidosis type I. J Med Ethics. 2011;37(4):233–239. doi:10.1136/jme.2010.037150
25. Hews‐Girard J, Obilar HN, Plazas PC. Objectivity in rare disease research: a philosophical approach. Nurs Inq. 2020;27(1):e12323. doi:10.1111/nin.12323
26. Jarosławski S, Toumi M. Non-profit drug research and development: the case study of Genethon. J Mark Access Health Policy. 2018;7(1). doi:10.1080/20016689.2018.1545514
27. Wellman-Labadie O, Zhou Y. The US orphan drug act: rare disease research stimulator or commercial opportunity? Health Policy Amst Neth. 2010;95(2–3):216–228. doi:10.1016/j.healthpol.2009.12.001
28. Hyry HI, Manuel J, Cox TM, Roos JCP. Compassionate use of orphan drugs. Orphanet J Rare Dis. 2015;10. doi:10.1186/s13023-015-0306-x
29. Kesselheim AS. Ethical considerations in orphan drug approval and use. Clin Pharmacol Ther. 2012;92(2):153–155. doi:10.1038/clpt.2012.92
30. Rosselli D, Rueda J-D, Solano M. Ethical and economic considerations of rare diseases in ethnic minorities: the case of mucopolysaccharidosis VI in Colombia. J Med Ethics. 2012;38(11):699–700. doi:10.1136/medethics-2011-100204
31. Gelinas L, Crawford B, Kelman A, Bierer BE. Relocation of study participants for rare and ultra-rare disease trials: ethics and operations. Contemp Clin Trials. 2019;84:105812. doi:10.1016/j.cct.2019.105812
32. Afroze B, Brown N. Ethical issues in managing Lysosomal storage disorders in children in low and middle income countries. Pak J Med Sci. 2017;33(4):1036–1041. doi:10.12669/pjms.334.12975
33. Schmiedeke E, Schaefer S, Aminoff D, Schwarzer N, Jenetzky E. Non-financial conflicts of interest: contribution to a surgical dilemma by the European reference networks for rare diseases. Pediatr Surg Int. 2019;35(9):999–1004. doi:10.1007/s00383-019-04516-y
34. Encina G, Castillo-Laborde C, Lecaros JA, et al. Rare diseases in Chile: challenges and recommendations in universal health coverage context. Orphanet J Rare Dis. 2019;14(1):289. doi:10.1186/s13023-019-1261-8
35. Barrera LA, Galindo GC. Ethical aspects on rare diseases. Adv Exp Med Biol. 2010;686:493–511. doi:10.1007/978-90-481-9485-8_27
36. Hansson MG, Gattorno M, Forsberg JS, Feltelius N, Martini A, Ruperto N. Ethics bureaucracy: a significant hurdle for collaborative follow-up of drug effectiveness in rare childhood diseases. Arch Dis Child. 2012;97(6):561–563. doi:10.1136/archdischild-2011-301175
37. Gershon ES, Alliey-Rodriguez N. New ethical issues for genetic counseling in common mental disorders. Am J Psychiatry. 2013;170(9):968–976. doi:10.1176/appi.ajp.2013.12121558
38. European Comission. The charter of fundamental rights of the European Union. Published online 2008.
39. Hollak CE, Aerts JM, Aymé S, Manuel J. Limitations of drug registries to evaluate orphan medicinal products for the treatment of lysosomal storage disorders. Orphanet J Rare Dis. 2011;6:16. doi:10.1186/1750-1172-6-16
40. Wolf U. Com l’ètica de la compassió de Schopenhauer pot contribuir al debat ètic d’avui. Enrahonar Int J Theor Pract Reason. 2015;55:41–49. doi:10.5565/rev/enrahonar.207
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
