Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 17
Etanercept Combined with Glucocorticoid and Gamma Globulin for Treating Children with Toxic Epidermal Necrolysis: A Case Report
Authors Fu Y, Xiao Y, Gao T, Zhang J, Wang T
Received 8 October 2023
Accepted for publication 29 December 2023
Published 23 January 2024 Volume 2024:17 Pages 167—171
DOI https://doi.org/10.2147/CCID.S440476
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Anne-Claire Fougerousse
Yanhua Fu,1 Yuanyuan Xiao,2 Tianji Gao,1 Junxiu Zhang,3 Tianjiao Wang1
1Department of Rheumatology, Baoding Hospital of Beijing Children’s Hospital, Baoding, 071000, People’s Republic of China; 2Department of Dermatology, National Center for Children’s Health (Beijing) Children’s Hospital Affiliated to Capital Medical University, Beijing, 100045, People’s Republic of China; 3Department of Dermatology, Baoding Hospital of Beijing Children’s Hospital, Baoding, 071000, People’s Republic of China
Correspondence: Yuanyuan Xiao, Department of Dermatology, National Center for Children’s Health (Beijing) Children’s Hospital Affiliated to Capital Medical University, No. 56 of Nanlishi Road, Xicheng District, Beijing, 100045, People’s Republic of China, Tel +86 10 59612345, Email [email protected]
Abstract: Toxic epidermal necrolysis (TEN) is a type of drug eruption in dermatology emergencies that is rare in clinical practice but has a high mortality rate. The main causes are drug and viral infections. Unfortunately, no expert consensus on treating this disease exists, and a standard therapy is absent. Up to now, glucocorticoids combined with gamma globulin are commonly used in clinical practice, but their efficacy is highly controversial. This study reports on a 7-year-old girl with TEN who did not respond to traditional therapy, such as methylprednisolone combined with gamma globulin, but was finally cured with an additional low-dose etanercept. The results showed that etanercept therapy in paediatric TEN is safe, reliable and worth recommending.
Keywords: child toxic epidermal necrolysis, etanercept, glucocorticoids, gamma globulin
Toxic epidermal necrolysis (TEN) is a potentially life-threatening delayed hypersensitivity reaction primarily triggered by medications. It exhibits rapid onset and severe progression, with a 30–35% mortality rate.1 Toxic epidermal necrolysis can affect individuals of all ages and is linked to various medications and infections. Causative medications for TEN include antibiotics, anticonvulsants, sulphonamides and allopurinol. The typical clinical manifestations of TEN are widespread erythema, blistering and severe epidermal necrosis and detachment, sometimes leading to multi-organ involvement. Despite the growing understanding of the condition, consensus regarding the optimal treatment regimen for TEN remains elusive. Clinically, conventional treatments such as cyclophosphamide, cyclosporine and glucocorticoid (GC) therapy are often employed but are accompanied by significant side effects and an increased risk of infection.2,3 Although previous literature has discussed using biological agents in treating adult patients with TEN, scant information exists regarding paediatric patients.4 To date, only 4 children with toxic epidermal necrolysis have been treated with infliximab and 2 with etanercept. Andrea et al5 reported an 8-year-old boy who was diagnosed with TEN induced by levetiracetam. He was first treated with methylprednisolone 1.5mg/kg/d and intravenous immunoglobulin 1g/kg/d for 5 consecutive days. However, significant progression and mucosal lesions were observed 2 days later, followed by etanercept at a dose of 0.8mg/kg (25mg), which stopped blistering within 24 hours after the use of etanercept, and the mucosal lesions were alleviated. Gavigan et al6 reported an 11-year-old girl whose symptoms were significantly relieved after receiving methylprednisolone for 4 days 30mg/kg/d, cyclosporine 5mg/kg/d for 3 days, and 2 doses of etanercept 25mg every 24 hours. Although it has been reported that etanercept is effective in the treatment of TEN, there are still few reports about etanercept in the treatment of TEN in children. Therefore, it is necessary to explore the treatment of etanercept in children with TEN and summarize the clinical experience. In our department, we encountered a paediatric patient with TEN and effectively treated her using a combination therapy of etanercept (Yisaipu), GCs, and intravenous immunoglobulin (IVIG). Given the favourable outcomes, a detailed account of this case is provided in this report.
Clinical Data
A 7-year-old girl was admitted with the chief complaints of fever, rash and lip swelling for 2 days. Two days before admission, the patient developed an unprovoked fever, with the highest recorded temperature reaching 39.6°C. Initial fever management with ibuprofen failed to alleviate the symptoms. The patient did not experience chills or seizures. Subsequently, erythematous patchy skin rashes appeared on her face and ears, gradually spreading throughout her body. Concurrently, the patient developed blisters on her limbs and trunk and complained of itching on her hands and feet. Pronounced lip swelling was also noted (Figure 1A). The patient denied having a cough, sputum production, excessive drowsiness or an exaggerated startle response.
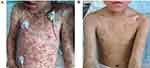 |
Figure 1 (A) The clinical image of the patient before treatment. (B) The clinical image of the patient after treatment. |
Medical History
The patient had a history of a respiratory tract infection approximately 2 weeks prior to admission and was orally administered naproxen and spiramycin; however, the dosages were not specified. There was no reported history of drug allergies.
Physical Examination on Admission
Upon admission, the patient presented with a body temperature of 38.6°C, a heart rate of 136 beats per minute, a respiratory rate of 36 breaths per minute, a blood pressure of 93/70 mmHg and a weight of 28 kg. She appeared alert, with suboptimal mental status, but her responses were still acceptable Her superficial lymph nodes were within normal size. Notably, there were reddish patches on her face and ears, some exhibiting signs of epidermal necrosis and exudation. Reddish papules and erythematous patches were also evident on her limbs and trunk, with some displaying vesicles. No obvious ulcerations or scaling were observed. The perianal and vulvar skin appeared moist with increased secretions. Her pupils were equal in size and reacted to light. Both eyes displayed conjunctival congestion with yellowish secretions. The oral mucosa was red, swollen and ulcerated. The posterior pharyngeal wall exhibited redness, and bilateral tonsils had grade II enlargement without secretions. No abnormalities were detected in her cardiovascular, pulmonary, abdominal or neurological systems.
Key Auxiliary Examination Findings
The routine complete blood count revealed a white blood cell count of 13.00 × 10^9/L, comprising 71.3% neutrophils and 15.6% lymphocytes. The C-reactive protein (CRP) level was elevated at 26.39 mg/L. The patient’s cardiac enzymes showed elevated levels of lactate dehydrogenase (LDH) at 302 U/L and creatine kinase isoenzymes (CK-MB) at 56 U/L, both exceeding normal ranges. The procalcitonin (PCT) level was elevated at 1.15 ng/mL, also above normal. The patient’s liver and kidney function, electrolytes, blood glucose, blood lipids, alpha-amylase (α-amylase) and lipase were all within normal limits. The erythrocyte sedimentation rate (ESR) was elevated at 60 mm/H. The pre-transfusion examination showed no abnormalities, and the chest computed tomography and electrocardiogram revealed no abnormalities.
Diagnosis
The patient was diagnosed with erythema multiforme exudative/TEN, possibly induced by naproxen. The severity of the patient’s TEN was evaluated according to the SCOTEN score standard,7 and the results showed that the SCOTEN score was 2. The maximum detachment area of the child was calculated by nine-point method to be 63%.
Treatment
Treatment commenced after admission, with IVIG administered at a dose of 1 g/kg per day for 2 days and intravenous methylprednisolone at a dose of 10 mg/kg per day. A topical iodine tincture was applied for facial rashes, and mupirocin ointment was used locally on the trunk and limbs. Eye care included administering gatifloxacin eye gel and pranoprofen eye drops 3 times daily. Daily bed linen changes and high-temperature disinfection were implemented.
Progress
After 3 days, the patient’s temperature remained unstable, peaking at 38.3°C. New rashes continued to emerge, characterised by patchy erythema across the body, central purpuric lesions and vesicles. Some lesions had progressed to ulceration and erosion. The patient’s eyes retained congestion with slight discharge, and her lips displayed erosion with a significant exudate. The perianal and vulvar skin maintained erythema without discharge.
Follow-Up Examination Results
The patient’s cardiac enzyme levels showed a notable improvement, with LDH decreasing to 197 U/L, falling within the normal range. However, CK-MB levels remained elevated at 159 U/L. The patient’s liver and kidney function, electrolytes, blood glucose, blood lipids, PCT and α-amylase all remained within normal limits, except for elevated lipase levels at 38 U/L, surpassing the normal range. The extent of skin involvement was quantified at 30%, prompting a revised diagnosis of TEN. Given the potential cross reaction between pranoprofen eye drops (a non-steroidal anti-inflammatory drug) and naproxen, pranoprofen was discontinued, and sodium hyaluronate eye drops were initiated, administered 3 times daily. The methylprednisolone dosage was escalated to 20 mg/kg per day for pulse therapy.
Progress
Four days after these adjustments, the patient’s temperature had normalised, yet the rash persisted and continued to spread. Upon examination, both eyes remained congested with slight discharge, while her lips exhibited erosion with a substantial exudate. Her face and ears displayed large red patches, some with signs of epidermal necrosis and exudation. The patient’s limbs and trunk presented with patchy erythema, with certain areas exhibiting vesicles. Although there were no significant ulcerations, the vulvar and perianal regions remained erythematous with minimal discharge. Bacterial cultures of blister fluid and discharge yielded negative results. On the 11th day of onset, that is, the 9th day of treatment with methylprednisolone, the patient received a subcutaneous injection of a single dose of etanercept (Yisaipu) at a dose of 0.8 mg/kg (total 21 mg). The condition improved after 2 days of applying etanercept. Subsequently, the patient experienced rapid improvement in the skin rash, marked by vesicle drying and scab formation. Subsequent follow-up examinations revealed a CRP level lower than 0.5 mg/L and an ESR of 20 mm/H. Consequently, the methylprednisolone dosage was gradually tapered and ultimately discontinued (Figure 1B).
Discharge Instructions
Upon discharge, the patient was provided with the following guidelines: (1) avoid consuming spicy foods; (2) avoid direct sunlight for 3 months to prevent sunburn and minimise skin irritation; (3) maintain meticulous skin hygiene, ensuring cleanliness and proper moisturisation, employing sunscreen as needed; (4) exercise caution when considering non-steroidal anti-inflammatory drugs (NSAIDs) because of the potential for cross reactions; (5) return to the hospital outpatient clinic for weekly check-ups during the initial month, including blood analysis and CRP monitoring. Subsequently, monitor liver and kidney function every 2 weeks. Fortunately, all follow-up examinations yielded no abnormalities.
Discussion
Medications serve as the predominant triggers of TEN in paediatric cases, with commonly sensitising drugs encompassing antibiotics, anticonvulsants and NSAIDs.8 The TEN cases managed in our department were precipitated by a specific NSAID, naproxen, which potentially led to exacerbation of the disorder due to the simultaneous use of another NSAID, pranoprofen. This highlights the critical importance of avoiding medications within the same category or those that may entail cross reactions when addressing TEN. Early interventions involving methylprednisolone in conjunction with IVIG have demonstrated favourable efficacy in paediatric patients with TEN and play a pivotal role in prognosis. However, for the patient in this study, despite administering IVIG for 2 days and methylprednisolone at a dose of 10 mg/kg for 3 days, followed by an escalation to 20 mg/kg for 4 days, the skin lesions persisted and worsened. Subsequently, the introduction of Yisaipu resulted in a rapid resolution of the skin rash.
The 2008 European EuroSCAR drug monitoring study illuminated a heightened pathogenic risk associated with NSAIDs in patients with TEN.9 The currently recognised pathological mechanism of TEN revolves around the immune-mediated apoptosis of keratinocytes, leading to epidermal necrosis and detachment. Moreover, there is a genetic component to this condition. Research has shown substantial infiltration of immune cells, including CD8+ T cells (CTL), natural killer (NK) cells and neutrophils, within the blister fluid and superficial dermal blood vessels of patients with TEN.2,10 Both CTL and NK cells produce Fas ligands that bind to Fas receptors on keratinocytes, triggering a cascade reaction resulting in keratinocyte apoptosis. Upon activation, CTL or NK cells release cytotoxic granules containing perforin, which compromises the cell membrane of keratinocytes, causing mitochondrial damage and cell apoptosis. Additionally, the cytokine tumour necrosis factor-alpha (TNF-α) activates TNF-R1 death receptors and stimulates nitric oxide production, intensifying intracellular reactive oxygen species, disrupting the cell membrane and culminating in keratinocyte apoptosis.11
In recent years, there has been renewed optimism in treating severe drug eruptions, particularly with the use of TNF-α antagonists. Etanercept, owing to its capacity to neutralise TNF-α and block its receptor binding, has demonstrated promising therapeutic results. As reported,12 etanercept has substantially reduced the healing time for skin and mucous membrane issues in patients with Stevens–Johnson syndrome and TEN, concurrently diminishing the risk of gastrointestinal bleeding.13 Notably, domestic researchers, including Lu et al,14 have affirmed the efficacy and safety of recombinant human type II TNF receptor-antibody fusion protein as a treatment for drug-induced TEN. Furthermore, Zhang et al15 proposed that in cases where the efficacy of treatment with either injected recombinant human type II TNF receptor-antibody fusion protein or methylprednisolone alone is insufficient, early combination therapy may enhance treatment outcomes. It is worth noting that the reports mentioned earlier are predominantly based on experiences with adult patients, and there is currently limited treatment data available for paediatric patients. Within our department, the treatment approach involving Yisaipu in conjunction with GCs and IVIG for children with TEN marks the inaugural reported instance of such treatment in China.
The conventional treatment for TEN has centred on administering GCs and IVIG.16 Given the absence of a consensus among experts and the scarcity of large-scale multicentre studies, we elected to pursue a combination therapy strategy involving Yisaipu, GCs and IVIG for managing paediatric TEN. This novel approach yielded favourable outcomes without any discernible adverse reactions.
Data Sharing Statement
All data generated or analyzed during this study are included in this published article.
Ethics Approval and Consent to Participate
This study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Baoding Hospital of Beijing Children’s Hospital. Written informed consent was obtained from parents/local guardians.
Consent for Publication
Written informed consent was obtained from parents/local guardians.
Funding
The authors have received no financial support for the research, authorship, or publication of this manuscript.
Disclosure
None of the authors have any personal, financial, commercial, or academic conflicts of interest.
References
1. Riano I, Cristancho C, Treadwell T. Stevens-Johnson syndrome-like reaction after exposure to pembrolizumab and recombinant zoster vaccine in a patient with metastatic lung cancer. J Investig Med High Impact Case Rep. 2020;8:2324709620914796. doi:10.1177/2324709620914796
2. Frantz R, Huang S, Are A, Motaparthi K. Stevens-Johnson syndrome and toxic epidermal necrolysis: a review of diagnosis and management. Medicina. 2021;57(9):895. doi:10.3390/medicina57090895
3. Surowiecka A, Barańska-Rybak W, Strużyna J. Multidisciplinary treatment in toxic epidermal necrolysis. Int J Environ Res Public Health. 2023;20(3):2217. doi:10.3390/ijerph20032217
4. Coulombe J, Belzile E, Duhamel A, et al. Pediatric SJS/TEN subdued by a combination of dexamethasone, cyclosporine, and etanercept. J Cutan Med Surg. 2019;23(5):547–550. doi:10.1177/1203475419861078
5. Estébanez A, Sáez-Martín LC, Muñoz JI, et al. Levetiracetam-induced pediatric toxic epidermal necrolysis successfully treated with etanercept. Pediatr Dermatol. 2020;37(4):701–705. doi:10.1111/pde.14179
6. Gavigan GM, Kanigsberg ND, Ramien ML. Pediatric Stevens-Johnson syndrome/toxic epidermal necrolysis halted by etanercept. J Cutan Med Surg. 2018;22(5):514–515. doi:10.1177/1203475418758989
7. Bastuji-Garin S, Fouchard N, Bertocchi M, Roujeau JC, Revuz J, Wolkenstein P. SCORTEN: a severity-of-illness score for toxic epidermal necrolysis. J Invest Dermatol. 2000;115(2):149–153. doi:10.1046/j.1523-1747.2000.00061.x
8. Yang L, Shou YH, Li F, Zhu XH, Yang YS, Xu JH. Retrospective study of 213 cases of Stevens-Johnson syndrome and toxic epidermal necrolysis from China. Burns. 2020;46(4):959–969. doi:10.1016/j.burns.2019.10.008
9. Mockenhaupt M, Viboud C, Dunant A, et al. Stevens-Johnson syndrome and toxic epidermal necrolysis: assessment of medication risks with emphasis on recently marketed drugs. The EuroSCAR-study. J Invest Dermatol. 2008;128(1):35–44. doi:10.1038/sj.jid.5701033
10. Hasegawa A, Abe R. Recent advances in managing and understanding Stevens-Johnson syndrome and toxic epidermal necrolysis. F1000Res. 2020;9. doi:10.12688/f1000research.24748.1
11. Jacobsen A, Olabi B, Langley A, et al. Systemic interventions for treatment of Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and SJS/TEN overlap syndrome. Cochrane Database Syst Rev. 2022;3(3):CD013130. doi:10.1002/14651858.CD013130.pub2
12. Wang CW, Yang LY, Chen CB, et al. The Taiwan Severe Cutaneous Adverse Reaction (TSCAR) Consortium. Randomized, controlled trial of TNF-α antagonist in CTL-mediated severe cutaneous adverse reactions. J Clin Invest. 2018;128(3):985–996. doi:10.1172/JCI93349
13. Singh N, Phillips M. Toxic epidermal necrolysis: a review of past and present therapeutic approaches. Skin Therapy Lett. 2022;27(5):7–13.
14. Lu XJ, Jing J, Shi X, et al. Recombinant human tumor necrosis factor-α receptor II: igG Fc fusion protein for the treatment of drug-induced toxic epidermal necrolysis: a multicenter clinical observation. Chin Jl of Dermatology. 2020;53(6):428–434. doi:10.35541/cjd.20200068
15. Zhang X, Li B, Wang L, Wang G, Fu M. Combination of recombinant human tumor necrosis factor-α receptor II fusion protein with methylprednisolone for treatment of five patients with toxic epidermal necrolysis. Chin J Dermatovenereol. 2020;34(9):1018–1022. doi:10.13735/j.cjdv.1001-7089.202002017
16. Rao Y, Tao CR. Combination of intravenous glucocorticoid and immunoglobulin in treatment of toxic epidermal necrolysis: a comparative study in twenty-five cases. Chin J Dermatovenereol. 2017;31(2):165–166. doi:10.13735/j.cjdv.1001-7089.201605112
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
