Back to Journals » Drug Design, Development and Therapy » Volume 15
Establishment of Prognostic Nomograms for Predicting the Survival of HR-Positive, HER2-Negative Metastatic Breast Cancer Patients Treated with Everolimus
Authors Duan F , Song C, Ma Y, Jiang K , Xu F , Bi X, Huang J, Hong R, Huang Z, Lu Q, Yuan Z, Wang S, Xia W
Received 7 April 2021
Accepted for publication 29 July 2021
Published 10 August 2021 Volume 2021:15 Pages 3463—3473
DOI https://doi.org/10.2147/DDDT.S314723
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tuo Deng
Fangfang Duan,* Chenge Song,* Yuyu Ma, Kuikui Jiang, Fei Xu, Xiwen Bi, Jiajia Huang, Ruoxi Hong, Zhangzan Huang, Qianyi Lu, Zhongyu Yuan, Shusen Wang, Wen Xia
Departments of Medical Oncology, Sun Yat-sen University Cancer Center, The State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangzhou, Guangdong, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Wen Xia
Department of Medical Oncology, Sun Yat-sen University Cancer Center, The State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, 651 Dongfeng Road East, Guangzhou, Guangdong, 510060, People’s Republic of China
Email [email protected]
Background: There are no clinically available prognostic models for patients with hormone receptor-positive (HR+), human epidermal growth factor receptor 2-negative (HER2-) metastatic breast cancer treated with everolimus. We aimed to develop a tool to predict the progression-free survival (PFS) and overall survival (OS) of these patients and to identify optimal candidates who would benefit from everolimus-based treatment in this heterogeneous patient population.
Methods: The clinical data of patients with HR+, HER2- metastatic breast cancer receiving everolimus between May 2012 and January 2018 at Sun Yat-sen University Cancer Center were retrospectively retrieved. Based on potential prognostic factors derived from multivariate Cox analysis, we established predictive nomogram models for PFS and OS and evaluated their predictive values by means of the concordance index (C-index). Calibration curves were used to estimate the consistency between the actual observations and the nomogram-predicted probabilities.
Results: A total of 116 patients with HR+, HER2- metastatic breast cancer were enrolled in this study. Three independent prognostic factors, including the line of everolimus in the metastatic setting, everolimus clinical benefit rate and number of liver metastatic lesions, were identified from the multivariate Cox analysis. Prognostic models for individual survival prediction were established and graphically presented as nomograms. The C-index was 0.738 (95% confidence interval [CI]: 0.710– 0.767) for the PFS nomogram and 0.752 (95% CI: 0.717– 0.788) for the OS nomogram, which showed favourable discrimination. The calibration curves for the probabilities of 6-, 9-, and 12-month PFS and 1-, 2-, and 3-year OS suggested satisfactory consistency between the actual observations and the predicted probabilities.
Conclusion: We constructed convenient nomogram models for patients with HR+, HER2- metastatic breast cancer to individually predict their potential benefits from everolimus in the metastatic setting. The models showed good performance in terms of accuracy, discrimination capacity and clinical application value.
Keywords: prognostic nomogram, metastatic breast cancer, everolimus, progression-free survival, overall survival
Introduction
Breast cancer (BC) is the most common malignant neoplasm and the leading cause of cancer-related death in women.1 Approximately 70% of BCs are hormone receptor-positive (HR+) and human epidermal growth factor receptor 2-negative (HER2-).2,3 Nearly 5–10% of BC patients have de novo metastatic disease, and 25–30% of early-stage patients relapse and develop metastatic breast cancer (MBC) after initial treatment.4,5 Endocrine therapy (ET) is the preferred option and an integral part of the management of HR+, HER2- MBC,6,7 but 25% of BC patients present primary endocrine resistance, and those responding to initial ET typically develop acquired endocrine resistance.8–10
The treatment landscape of HR+, HER2- MBC has changed dramatically with the advent of new targeted therapeutic strategies in the past decade. Everolimus, an oral agent inhibiting mammalian target of rapamycin (mTOR), showed predominant progression-free survival (PFS) benefits in combination with exemestane in the BOLERO-2 trial. In 2012, the US Food and Drug Administration (FDA) approved everolimus plus exemestane for postmenopausal women with advanced breast cancer (ABC) who progressed during prior treatment with nonsteroidal aromatase inhibitors (NSAIs).11,12 The 4EVER trial and the observational BRAWO study also confirmed the superior efficacy of everolimus for HR+, HER2- ABC/MBC, especially in the first-line metastatic setting.13,14 A series of randomized clinical trials showed that compared to ET alone, the addition of a cyclin-dependent kinase (CDK) 4/6 inhibitor to ET or fulvestrant was more effective.15–21 In 2015 and 2017, these combinations were recommended as the first- or second-line therapeutic option for HR+, HER2- MBC to achieve a better PFS and overall survival (OS).22–24 However, approximately 15% of MBC patients receiving a CDK4/6 inhibitor plus an aromatase inhibitor (AI) and 30% of MBC patients treated with a CDK4/6 inhibitor plus fulvestrant developed progression within six months, and relapse occurred in all patients with MBC due to acquired resistance.25 Despite these findings, the PI3K/ATK/mTOR signal pathway, which is crucial for cell growth, differentiation and survival,26 was still intact, and BC developing resistance to CDK4/6 inhibitors or fulvestrant could restore sensitivity by means of PI3K/ATK/mTOR inhibitors. Thus, everolimus-based treatment is considered as a therapeutic option post CDK4/6 inhibitors.14,27
Sequencing therapy is a vital part of managing MBC, but the optimal sequence of ET in the metastatic setting for HR+, HER2- MBC remains unclear. Clinicians usually choose regimens according to the previously exposed agents and disease burden.4 Given the heterogeneity of MBC, identifying non-invasive and effective biomarkers to select subsets of patients who would benefit from everolimus is important.7,28 Although some clinical features, including poor performance status, visceral crisis, number of metastatic sites, prior ET exposure, and prior chemotherapy, have been found to be relevant to poor prognosis in HR+, HER2- MBC,29–31 there are no currently available validated models for predicting the survival benefit of everolimus in the metastatic setting for BC.
Nomograms are prospective tools that are widely used to predict the individual probability of a clinical event by integrating various prognostic factors presented as a graphical diagram.32–34 To our knowledge, no clinically available prognostic nomogram has been designed for selecting patients with MBC who are suitable for treatment with everolimus in the metastatic setting; therefore, we aimed to develop a predictive and practical tool to make individual survival predictions and identify optimal candidates for everolimus-based treatment among patients with HR+, HER2- MBC.
Methods
Eligible Patients
Between May 2012 and January 2018, 116 patients with HR+, HER2- MBC treated with everolimus were enrolled in this retrospective study. We obtained approval from the ethics committee of Sun Yat-sen University Cancer Center (SYSUCC, registration number 2021-FXY-093). HER2- was defined as a score of 0, 1+ by immunohistochemical (IHC) analysis or a score of 2+ by IHC without ERBB2 gene amplification on fluorescence in situ hybridization (FISH) according to the American Society of Clinical Oncology/College of American Pathologists guideline updated in 2018.35 The cut-off for HR+ status was 10% or higher staining in nuclei. The key inclusion criteria included the following: (1) histological diagnosis of ABC/MBC; (2) age ≥ 18 years; (3) Eastern Cooperative Oncology Group (ECOG) performance status < 2; (4) measurable disease according to Response Evaluation Criteria in Solid Tumours (RECIST) version 1.1;36 (5) life expectancy ≥ 12 weeks; (6) no pregnancy or births; (7) at least completing two courses of everolimus; and (8) absence of other primary cancers within five years except for curable carcinoma of the cervix or squamous or basal cell carcinoma of the skin. The exclusion criteria were as follows: (1) severe or uncontrolled infection; (2) serious metabolic disorder; and (3) uncontrolled brain metastasis.
Treatment
Patient data were collected from medical records at SYSUCC, and patients underwent a complete evaluation prior to the initiation of everolimus, including routine physical examination, haematological and laboratory tests, and breast and abdominal ultrasound. Magnetic resonance imaging (MRI), chest radiograph/computed tomography (CT), bone scans or positron emission tomography (PET)-CT were conducted if necessary. All enrolled patients received everolimus (10 mg orally once daily) in combination with ET or fulvestrant (500 mg intramuscular injection every 28 days) in the metastatic setting, dosage reduction during treatment due to stomatitis or any reasons were allowed.
Follow-Up and Endpoints
Patients were monitored from the initiation of everolimus, and routine examinations were performed in accordance with the baseline evaluation every two months during the first two years and every three months thereafter. The primary endpoint was PFS, defined as the time from the initiation of treatment to disease progression or death. The secondary endpoint was OS, defined as the time from the initiation of everolimus treatment to death due to any cause; clinical benefit rate (CBR), defined as the proportion of patients with complete response (CR), partial response (PR), and stable disease (SD) for ≥ 24 weeks according to the RECIST version 1.1.
Statistical Analysis
Statistical analysis was performed using R software (“rms” package, version 4.0.1; Vanderbilt University, Nashville, TN) and SPSS version 22.0 software (IBM, Corp., Armonk, NY). Categorical variables are shown as frequencies with percentages. Univariate and multivariate analyses were conducted using Cox proportional hazards regression models to select potential prognostic factors for PFS and OS. Variables that achieved a P value of < 0.05 in the univariate analysis were entered into the multivariate Cox analysis. Nomograms of 6-, 9-, and 12-month PFS and 1-, 2-, and 3-year OS were developed based on the results of the multivariable analysis. In addition, we used the concordance index (C-index), which ranges from 0.5 (random chance) to 1.0 (perfect prediction), and calibration curves to estimate the accuracy of these models. A two-sided P value of less than 0.05 was considered statistically significant in all statistical tests unless otherwise specified.
Results
Clinical Characteristics and Survival
Between May 2012 and January 2018, 116 patients with HR+, HER2- MBC receiving everolimus at SYSUCC were included in this study. As shown in Table 1, the mean age was 44.13 years (range 26 to 65). A total of 10.3% of patients had de novo metastatic disease, and the other 89.7% developed MBC after initial neo/adjuvant treatment. The mean prior lines of chemotherapy in the metastatic setting were 2.23 (range 0–8), in terms of prior lines of chemotherapy, 49.1% and 33.6% of patients had < 3 prior lines and ≥ 3 prior lines in the metastatic setting, respectively. The mean prior lines of ET in the metastatic setting were 1.43 (range 0–5), and 87.9% of patients previously received < 3 lines of ET in this setting. Tamoxifen was mostly used in prior advanced disease (68.1%), and AI, NSAI and fulvestrant accounted for 31.9%, 59.5%, and 10.3% of prior ET cases, respectively. A total of 11.2% of patients received everolimus as a first-line treatment in the metastatic setting, 70.7% and 18.1% of patients received everolimus as the 2–5 lines or ≥ 6-line treatment, respectively. A quarter of patients had primary resistance to ET, and 29.3% of patients had a heavy liver metastatic burden (≥ 6). The CBR reached 69.8%, and 56% of patients had stomatitis. During the whole follow-up process, one patient received melbine due to her diabetes, other 23 patients among these undergoing everolimus dosage reduction due to stomatitis received topical steroids washing.
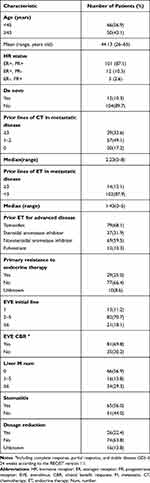 |
Table 1 Clinicopathologic Characteristics of Patients in This Study |
Independent Prognostic Factors
The results of univariate and multivariate Cox analyses are listed in Table 2. Whether primary resistance developed did not have an effect on PFS (P = 0.162) or OS (P = 0.137). Compared everolimus as the first-line treatment in the metastatic setting, everolimus as a ≥ 6-line treatment showed a higher risk for survival (PFS: hazard ratio [HR] = 3.269, 95% confidence interval [CI]: 1.236–8.647, P = 0.017; OS: HR = 4.728, 95% CI: 1.290–17.332, P = 0.019). Patients who achieved a clinical benefit experienced more favourable PFS (HR = 0.161, 95% CI: 0.094–0.277, P < 0.001) and OS (HR = 0.268, 95% CI: 0.130–0.551, P < 0.001) than those who did not. The number of liver metastases was associated with prognosis, and patients with a heavy liver metastatic burden (≥ 6) tended to have worse survival (PFS: HR = 2.079, 95% CI: 1.267–3.412, P = 0.004; OS: HR = 3.183, 95% CI: 1.676–6.044, P < 0.001).
 |
Table 2 Univariate and Multivariate Cox Analyses of Survival |
Nomogram Construction and Evaluation
Independent prognostic factors derived from the multivariate Cox analysis were integrated into nomogram models for PFS (Figure 1) and OS (Figure 2), including the line of everolimus in the metastatic setting, everolimus CBR and number of liver metastatic lesions. Every subtype of these factors had a corresponding score on the point scale. By adding the total score and locating it on the total point scale, the PFS and OS probabilities of each patient could be obtained at the time points of 6-, 9-, and 12-month and 1-, 2-, and 3-year, respectively.
To evaluate the predictive value of the constructed nomograms, a bootstrap validation method was performed. The C-index after bootstrap correction of the nomogram was 0.738 (95% CI: 0.710–0.767) for PFS and 0.752 (95% CI: 0.717–0.788) for OS, which suggested that the nomograms had good accuracy in predicting the survival of patients with HR+, HER2- MBC who received everolimus in the metastatic setting. The Y-axis of the calibration curves represents the actual observation of the survival rate, and the X-axis represents the survival rate predicted by the established nomograms. The calibration plot for the probability of 6-, 9-, and 12-month PFS (Figure 3) and 1-, 2-, and 3-year OS (Figure 4) indicated satisfactory consistency between the actual observations and the predicted probabilities.
Discussion
Due to the clinical and molecular heterogeneity of HR+, HER2- MBC, the survival outcomes and mechanisms of ET resistance vary largely among patients.28 Endocrine-based treatment remains a core part of treatment in the metastatic setting, but its optimal sequence is unclear.4,7,37 With the advent of new targeted therapeutic options, the value of everolimus in the metastatic setting needs to be re-evaluated, and better selection of patients who may benefit from it also needs to be explored.28 In this study, we retrospectively collected the medical data of 116 patients with HR+, HER2- MBC and analysed their clinical characteristics using multivariate Cox proportional hazards analysis. Three independent variables were associated with the survival of HR+, HER2- MBC patients treated with everolimus, including the line of everolimus in the metastatic setting, everolimus CBR and number of liver metastatic lesions. Furthermore, we integrated these prognostic factors to establish predictive nomogram models for PFS and OS, which were shown to have good accuracy and predictive capacity.
The observational BRAWO study achieved a median PFS of 8.0 months for postmenopausal patients with HR+, HER2- ABC/MBC receiving everolimus as first- and second-line therapy in the metastatic setting, which was consistent with the primary endpoint of the BOLERO-2 trial.12,14 However, for subsets of patients receiving everolimus plus exemestane as the first-line treatment, a 2.1-month increase in the median PFS was achieved compared with that in all intention-to-treat patients, which suggested that an everolimus-based regimen as an early therapeutic option for HR+, HER2- MBC should be recommended.14 Consistently, the multivariate Cox analysis in this retrospective study showed that the initial line of everolimus in the metastatic setting was an independent prognostic factor for survival. Compared to those receiving everolimus as first-line therapy, patients receiving everolimus as later-line therapy had a worse PFS (P = 0.017) and OS (P = 0.019). Therefore, we included this variable in our predictive nomogram models.
It was demonstrated in the BOLERO-2 trial that patients with HR+, HER2- MBC progressing during prior NSAI treatment achieved a longer PFS from everolimus plus ET than from ET alone (7.8 months vs 3.2 months, P < 0.0001). Subgroup analysis further suggested that the combination was an effective option in all patients, including those with visceral burdens, those receiving prior chemotherapy in the metastatic setting, those with prior use of hormonal treatment other than NSAIs, and those with sensitivity to previous hormonal therapy.12 However, the expanded, phase IIIb, single-arm 4EVER study, which was conducted in postmenopausal women with HR+, HER2- ABC/MBC, found that everolimus plus exemestane resulted in a median PFS of only 5.6 months. One possible explanation for the discrepancy between the findings of this study and the BOLERO-2 study might be the differences in baseline patient characteristics. The 4EVER study recruited more heavily pre-treated patients, and over 30% underwent > 3 lines of prior regimens for metastatic disease. Subgroup analysis confirmed that prior chemotherapy in the metastatic setting had a negative effect on obtaining benefits from everolimus in the metastatic setting, which suggested that everolimus-based therapy should be conducted prior to chemotherapy.13 Javier Puente et al found that prior chemotherapy in metastatic disease likely selected resistant tumour cells, which could induce de novo mutations and result in a more aggressive metastatic disease behavior, so it was associated with a worse survival (P < 0.001),31 similar to the findings of a study in Spain including 297 patients with MBC.38 However, in this study, we did not find a prognostic value of lines of prior chemotherapy in the metastatic setting for patient survival from subsequent everolimus treatment. One possible reason might be that the majority of patients (82.8%) in this study had been similarly treated with chemotherapy in the metastatic setting before everolimus, with a median number of previous chemotherapy lines of 2.23, which is a very common phenomenon in real-world clinical practice,37 so baseline differences among patients were not apparent.
Previous studies confirmed that visceral metastases were associated with a worse prognosis, MBC patients with visceral metastases showed a worse median survival in first-line NSAI treatment,39,40 and their therapeutic options after progression during/after previous NSAI therapy were restricted.41 Clinical treatment guidelines recommend chemotherapy instead of ET for HR+, HER2- MBC with extensive visceral metastases to control their rapid symptoms.42 However, some patients with visceral metastases do not show visceral crisis and have a low disease burden, they might have the potential to postpone chemotherapy, avoid treatment-related myelosuppression and benefit from ET with more manageable adverse events (AEs).42,43 A subset analysis of the BOLERO-2 study found that the addition of everolimus to exemestane yielded a significant 4-month increase in the median PFS for patients with HR+, HER2- MBC despite visceral metastases, which suggested that adding everolimus to ET could enhance their endocrine sensitivity and everolimus-based treatment was an effective option for patients with visceral metastases from HR+, HER2- MBC beyond progression during/after prior NSAI treatment.41 Javier Puente et al found that liver metastasis was a predictive marker of worse outcome even if the liver was the only site of metastatic disease (P < 0.001) and that the median survival of patients with visceral metastases (mainly in the liver) was 1.15 years, which was worse than that of patients with other locations of metastatic disease.31 The findings of our study were fully consistent with the findings of these studies. We found that liver metastatic lesions were an independent prognostic factor for survival and that the number of liver metastases was associated with the survival prediction of benefits from everolimus. Patients with a heavy liver metastatic burden (≥ 6) showed the worst prognosis in terms of PFS (HR = 2.079, 95% CI: 1.267–3.412, P = 0.004) and OS (HR = 3.183, 95% CI: 1.676–6.044, P < 0.001). Thus, the number of liver metastatic lesions was included in our nomogram models.
The main objective of the treatment for HR+, HER2- MBC patients is palliative care to maintain disease control and preserve quality of life.4,6 Therefore, it is meaningful for patients to achieve a clinical benefit, including CR, PR, and SD ≥ 24 weeks. We hypothesized that the CBR in everolimus-based therapy would have an effect on patients’ benefits from treatment, and this hypothesis was finally confirmed in the multivariate Cox analysis. Compared with those who failed to achieve a clinical benefit, patients who achieved a clinical benefit showed a better prognosis in terms of PFS (HR = 0.161, 95% CI: 0.094–0.277, P < 0.001) and OS (HR = 0.268, 95% CI: 0.130–0.551, P < 0.001). Therefore, we included the everolimus CBR in our predictive nomogram models.
Moreover, our nomogram models provided significant predictive value in identifying candidates with a greater possibility of benefiting from sequencing everolimus-based treatment. The bootstrap-corrected C-indexes of the models reached 0.738 for PFS and 0.752 for OS. Through our nomogram models, clinicians and patients could predict the prognosis of individuals and guide their individual therapeutic and care options. For example, patients with high scores on the total point scale tended to have a poor prognosis, additional care and more frequent monitoring were essential for them to control distant lesions and identify tumour progression early. As the number of liver metastases was confirmed to be associated with a worse prognosis for survival, a more precise imaging diagnosis and monitoring, such as PET-CT, is necessary to accurately evaluate the tumour burden while personalizing therapy for patients with HR+, HER2- MBC.28 In addition, the line of everolimus in the metastatic setting was found to be significant in predicting the survival of patients, and it would be sensible to apply an everolimus-based regimen as the front-line treatment option.
Nevertheless, there were some limitations of our study. First, selection bias was unavoidable in retrospective studies, and patient characteristic data were limited according to the retrospective medical records, making it difficult to collect all information on patient characteristics. Therefore, we failed to consider several clinicopathological factors that might affect prognosis, including histological grade, TNM classification, family history of breast cancer and so on. Second, although the nomograms established in our study achieved satisfactory C-indexes and good consistency according to the calibration curves, we did not validate them in other external cohorts because it was difficult to acquire data from other medical centers. Third, this study just enrolled limited samples in one hospital in China, results may not be generalizable to patients from other racial/ethnic backgrounds. Therefore, the practical applicability of our nomograms should be interpreted with caution.
In conclusion, we established nomogram models for patients with HR+, HER2- MBC by integrating three independent predictive factors, including the line of everolimus in the metastatic setting, everolimus CBR and number of liver metastatic lesions, which showed satisfactory accuracy and discrimination capacity for predicting the probabilities of 6-, 9-, and 12-month PFS and 1-, 2-, and 3-year OS. To extend the practical use of these nomograms, a validation cohort from other medical centres is needed.
Abbreviations
BC, breast cancer; ABC, advanced breast cancer; MBC, metastatic breast cancer; HR, hormone receptor; HER2, human epidermal growth factor receptor type 2; PFS, progression-free survival; OS, overall survival; C-index, concordance index; HR, hazard ratio; CI, confidence interval; ET, endocrine therapy; NSAI, nonsteroidal aromatase inhibitor; AI, aromatase inhibitor; mTOR, mammalian Target of rapamycin; CDK, cyclin-dependent kinase; FDA, Food and Drug Administration; SYSUCC, Sun Yat-sen University Cancer Center; CBR, clinical benefit rate; AEs, adverse events; IHC, immunohistochemical; FISH, fluorescence in situ hybridization; ECOG, Eastern Cooperative Oncology Group; RECIST, Response Evaluation Criteria in Solid Tumors; CT, computerized tomography; MRI, magnetic resonance imaging; PET, positron emission tomography; CR, complete response; PR, partial response; SD, stable disease.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding authors on reasonable requests.
Ethics Approval and Consent to Participate
This study was proved by the ethics committee of Sun Yat-sen University Cancer Center (Registration number 2021-FXY-093) with a waiver of requirement of patient consent because we just reviewed their medical records without impairing their health. We covered patient data confidentiality and conducted this study in compliance with the Declaration of Helsinki.
Acknowledgments
We gratefully acknowledge patients and their families for all their help in enabling the successful completion of this study. Fangfang Duan and Chenge Song are co-first authors in this study.
Author Contributions
FFD, CGS, YYM, KKJ, FX, XWB, JJH, RXH, ZZH, QYL, ZYY, SSW and WX contributed conception, study design, execution, acquisition of data, analysis and interpretation, and have written and substantially revised the manuscript. All authors reviewed and agreed on all versions of this manuscript, agreed on the journal to which the article has been submitted, took responsibility and were accountable for the contents of this article.
Funding
There is no funding to report.
Disclosure
All authors declare that they have no competing interests to declare.
References
1. Heer E, Harper A, Escandor N, Sung H, McCormack V, Fidler-Benaoudia M. Global burden and trends in premenopausal and postmenopausal breast cancer: a population-based study. Lancet Global Health. 2020;8(8):e1027–e37. doi:10.1016/S2214-109X(20)30215-1
2. Howlader N, Altekruse S, Li C, et al. US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst. 2014;106(5):548. doi:10.1093/jnci/dju055
3. Du Rusquec P, Blonz C, Frenel J, Campone M. Targeting the PI3K/Akt/mTOR pathway in estrogen-receptor positive HER2 negative advanced breast cancer. Ther Adv Med Oncol. 2020;12:1758835920940939. doi:10.1177/1758835920940939
4. Ballinger T, Meier J, Jansen V. Current landscape of targeted therapies for hormone-receptor positive, HER2 negative metastatic breast cancer. Front Oncol. 2018;8:308. doi:10.3389/fonc.2018.00308
5. Mariotto A, Etzioni R, Hurlbert M, Penberthy L, Mayer M. Estimation of the number of women living with metastatic breast cancer in the United States. Cancer Epidemiol Biomarkers Prevent. 2017;26(6):809–815. doi:10.1158/1055-9965.EPI-16-0889
6. Rugo H, Rumble R, Macrae E, et al. Endocrine therapy for hormone receptor-positive metastatic breast cancer: American society of clinical oncology guideline. J clin oncol. 2016;34(25):3069–3103. doi:10.1200/JCO.2016.67.1487
7. Cardoso F, Costa A, Senkus E, et al. 3rd ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 3). Ann Oncol. 2017;28(12):3111. doi:10.1093/annonc/mdx036
8. Ma C, Reinert T, Chmielewska I, Ellis M. Mechanisms of aromatase inhibitor resistance. Nat Rev Cancer. 2015;15(5):261–275. doi:10.1038/nrc3920
9. Tomlinson D, Knowles M, Speirs V. Mechanisms of FGFR3 actions in endocrine resistant breast cancer. Int j Cancer. 2012;130(12):2857–2866. doi:10.1002/ijc.26304
10. Johnston S. Enhancing endocrine therapy for hormone receptor-positive advanced breast cancer: cotargeting signaling pathways. J Natl Cancer Inst. 2015;107(10):djv212. doi:10.1093/jnci/djv212
11. Yardley D, Noguchi S, Pritchard K, et al. Everolimus plus exemestane in postmenopausal patients with HR(+) breast cancer: BOLERO-2 final progression-free survival analysis. Adv Ther. 2013;30(10):870–884. doi:10.1007/s12325-013-0060-1
12. Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366(6):520–529. doi:10.1056/NEJMoa1109653
13. Tesch H, Stoetzer O, Decker T, et al. Efficacy and safety of everolimus plus exemestane in postmenopausal women with hormone receptor-positive, human epidermal growth factor receptor 2-negative locally advanced or metastatic breast cancer: results of the single-arm, phase IIIB 4EVER trial. Int j Cancer. 2019;144(4):877–885. doi:10.1002/ijc.31738
14. O’Shaughnessy J, Thaddeus Beck J, Royce M. Everolimus-based combination therapies for HR+, HER2- metastatic breast cancer. Cancer Treat Rev. 2018;69:204–214. doi:10.1016/j.ctrv.2018.07.013
15. Finn R, Crown J, Lang I, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised Phase 2 study. Lancet Oncol. 2015;16(1):25–35. doi:10.1016/S1470-2045(14)71159-3
16. Finn R, Martin M, Rugo H, et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med. 2016;375(20):1925–1936. doi:10.1056/NEJMoa1607303
17. Goetz M, Toi M, Campone M, et al. MONARCH 3: abemaciclib as initial therapy for advanced breast cancer. J clin oncol. 2017;35(32):3638–3646. doi:10.1200/JCO.2017.75.6155
18. Hortobagyi G, Stemmer S, Burris H, et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med. 2016;375(18):1738–1748. doi:10.1056/NEJMoa1609709
19. Turner N, Ro J, André F, et al. Palbociclib in hormone-receptor-positive advanced breast cancer. N Engl J Med. 2015;373(3):209–219. doi:10.1056/NEJMoa1505270
20. Sledge G, Toi M, Neven P, et al. MONARCH 2: abemaciclib in combination with fulvestrant in women with HR+/HER2- advanced breast cancer who had progressed while receiving endocrine therapy. J clin oncol. 2017;35(25):2875–2884. doi:10.1200/JCO.2017.73.7585
21. Slamon D, Neven P, Chia S, et al. Phase III randomized study of ribociclib and fulvestrant in hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: MONALEESA-3. J clin oncol. 2018;36(24):2465–2472. doi:10.1200/JCO.2018.78.9909
22. Sledge G, Toi M, Neven P, et al. The effect of abemaciclib plus fulvestrant on overall survival in hormone receptor-positive, ERBB2-negative breast cancer that progressed on endocrine therapy-MONARCH 2: a randomized clinical trial. JAMA oncol. 2020;6(1):116–124. doi:10.1001/jamaoncol.2019.4782
23. Im S, Lu Y, Bardia A, et al. Overall survival with ribociclib plus endocrine therapy in breast cancer. N Engl J Med. 2019;381(4):307–316. doi:10.1056/NEJMoa1903765
24. Slamon D, Neven P, Chia S, et al. Overall survival with ribociclib plus fulvestrant in advanced breast cancer. N Engl J Med. 2020;382(6):514–524. doi:10.1056/NEJMoa1911149
25. Migliaccio I, Bonechi M, McCartney A, et al. CDK4/6 inhibitors: a focus on biomarkers of response and post-treatment therapeutic strategies in hormone receptor-positive HER2-negative breast cancer. Cancer Treat Rev. 2020;93:102136. doi:10.1016/j.ctrv.2020.102136
26. Katso R, Okkenhaug K, Ahmadi K, White S, Timms J, Waterfield M. Cellular function of phosphoinositide 3-kinases: implications for development, homeostasis, and cancer. Annu Rev Cell Dev Biol. 2001;17:615–675. doi:10.1146/annurev.cellbio
27. Herrera-Abreu MT, Palafox M, Asghar U, et al. Correction: early adaptation and acquired resistance to CDK4/6 inhibition in estrogen receptor-positive breast cancer. Cancer Res. 2016;76(19):5907. doi:10.1158/0008-5472.CAN-16-1853
28. Sirico M, Bernocchi O, Sobhani N, et al. Early changes of the standardized uptake values (SUV) predict the efficacy of everolimus-exemestane in patients with hormone receptor-positive metastatic breast cancer. Cancers. 2020;12(11):3314. doi:10.3390/cancers12113314
29. Marshall E, Bertaut A, Desmoulins I, et al. Prognostic factors of survival among women with metastatic breast cancer and impact of primary or secondary nature of disease on survival: a French population-based study. Breast J. 2017;23(2):138–145. doi:10.1111/tbj.12717
30. Largillier R, Ferrero J, Doyen J, et al. Prognostic factors in 1038 women with metastatic breast cancer. Ann Oncol. 2008;19(12):2012–2019. doi:10.1093/annonc/mdn424
31. Puente J, López-Tarruella S, Ruiz A, et al. Practical prognostic index for patients with metastatic recurrent breast cancer: retrospective analysis of 2322 patients from the GEICAM Spanish El Alamo Register. Breast Cancer Res Treat. 2010;122(2):591–600. doi:10.1007/s10549-009-0687-4
32. Iasonos A, Schrag D, Raj G, Panageas K. How to build and interpret a nomogram for cancer prognosis. J clin oncol. 2008;26(8):1364–1370. doi:10.1200/JCO.2007.12.9791
33. Feuer E, Lee M, Mariotto A, et al. The cancer survival query system: making survival estimates from the surveillance, epidemiology, and end results program more timely and relevant for recently diagnosed patients. Cancer. 2012;118(22):5652–5662. doi:10.1002/cncr.27615
34. Balachandran V, Gonen M, Smith J, DeMatteo R. Nomograms in oncology: more than meets the eye. Lancet Oncol. 2015;16(4):e173–80. doi:10.1016/S1470-2045(14)71116-7
35. Woo JW, Lee K, Chung YR, Jang MH, Ahn S, Park SY. The updated 2018 American Society of Clinical Oncology/College of American Pathologists guideline on human epidermal growth factor receptor 2 interpretation in breast cancer: comparison with previous guidelines and clinical significance of the proposed in situ hybridization groups. Hum Pathol. 2020;98:10–21. doi:10.1016/j.humpath
36. Eisenhauer E, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. doi:10.1016/j.ejca.2008.10.026
37. Giuliano M, Schettini F, Rognoni C, et al. Endocrine treatment versus chemotherapy in postmenopausal women with hormone receptor-positive, HER2-negative, metastatic breast cancer: a systematic review and network meta-analysis. Lancet Oncol. 2019;20(10):1360–1369. doi:10.1016/S1470-2045(19)30420-6
38. Alba E, Ribelles N, Sevilla I, et al. Adjuvant anthracycline therapy as a prognostic factor in metastatic breast cancer. Breast Cancer Res Treat. 2001;66(1):33–39. doi:10.1023/a:1010616532332
39. Howell A, Robertson J, Vergote I. A review of the efficacy of anastrozole in postmenopausal women with advanced breast cancer with visceral metastases. Breast Cancer Res Treat. 2003;82(3):215–222. doi:10.1023/B:BREA.0000004375.17920.0b
40. Mouridsen H, Gershanovich M, Sun Y, et al. Superior efficacy of letrozole versus tamoxifen as first-line therapy for postmenopausal women with advanced breast cancer: results of a phase III study of the International Letrozole Breast Cancer Group. J clin oncol. 2001;19(10):2596–2606. doi:10.1200/JCO.2001.19.10.2596
41. Campone M, Bachelot T, Gnant M, et al. Effect of visceral metastases on the efficacy and safety of everolimus in postmenopausal women with advanced breast cancer: subgroup analysis from the BOLERO-2 study. Eur j Cancer. 2013;49(12):2621–2632. doi:10.1016/j.ejca.2013.04.011
42. Cardoso F, Costa A, Norton L, et al. 1st International consensus guidelines for advanced breast cancer (ABC 1). Breast. 2012;21(3):242–252. doi:10.1016/j.breast.2012.03.003
43. Wilcken N, Hornbuckle J, Ghersi D. Chemotherapy alone versus endocrine therapy alone for metastatic breast cancer. Cochrane Database Sys Rev. 2003;1(2):CD002747. doi:10.1002/14651858.CD002747
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




