Back to Journals » International Journal of General Medicine » Volume 15
Establishment of Prediction Models for Venous Thromboembolism in Non-Oncological Urological Inpatients – A Single-Center Experience
Authors Li K, Yu M, Li H, Zhu Q, Wu Z, Wang Z, Tang Z
Received 16 December 2021
Accepted for publication 10 March 2022
Published 24 March 2022 Volume 2022:15 Pages 3315—3324
DOI https://doi.org/10.2147/IJGM.S354288
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Kaixuan Li,1 Meihong Yu,2,3 Haozhen Li,1 Quan Zhu,1 Ziqiang Wu,1 Zhao Wang,1,4 Zhengyan Tang1,5
1Department of Urology, Xiangya Hospital of Central South University, Changsha, 410008, People’s Republic of China; 2Department of Gastroenterology, The Second Xiangya Hospital of Central South University, Changsha, Hunan, 410011, People’s Republic of China; 3Research Center of Digestive Disease, Central South University, Changsha, Hunan, 410011, People’s Republic of China; 4National Clinical Research Center for Geriatric Disorders, Changsha, 410008, People’s Republic of China; 5Provincial Laboratory for Diagnosis and Treatment of Genitourinary System Disease, Changsha, 410000, People’s Republic of China
Correspondence: Zhao Wang, Department of Urology, Xiangya Hospital, Central South University, 87 Xiangya Road, Changsha, 410008, People’s Republic of China, Tel +86-15116358241, Email [email protected] Meihong Yu, Department of Gastroenterology, The Second Xiangya Hospital of Central South University, No. 139 Middle Renmin Road, Changsha, Hunan, 410011, People’s Republic of China, Tel +86-15243646849, Fax +86-731-85533525, Email [email protected]
Purpose: Venous thromboembolism (VTE) comprises deep venous thrombosis (DVT) and pulmonary embolism (PE), which can lead to death. VTE is an insidious disease with no specific symptoms and overlooked readily. We aimed to establish prediction models for VTE in non-oncological urological inpatients to aid urologists to better identify VTE patients.
Patients and Methods: A retrospective analysis of 1453 inpatients was carried out. The risk factors for VTE had been clarified in our previous study. A stepwise regression method was used to screen the relevant influencing factors for VTE and construct a logistic regression prediction model to predict VTE. To validate the accuracy of the model, data from 291 patients from another cohort were used for external validation.
Results: A total of 1453 inpatients were enrolled. Five potential risk factors (previous VTE; treatment with anticoagulants or anti-platelet agents before hospital admission; D-dimer ≥ 0.89 μg/mL; lower-extremity swelling; chest symptoms) were selected by multivariable analysis with p < 0.05. These five risk factors were used to build a logistic regression prediction model. When p < 0.1 in the multivariable logistic regression model, two additional risk factors were added: Caprini score ≥ 5 and complications, and all seven risk factors were used to build another prediction model. Internal verification showed the cutoff values, sensitivity, and specificity of the two models to be 0.02474, 0.941, 0.816 (model 1) and 0.03824, 0.941, and 0.820 (model 2), respectively. Both models had good predictive ability, but prediction accuracy was 43.0% for both when using the data of the additional 291 inpatients in the two models.
Conclusion: Two novel prediction models were built to predict VTE in non-oncological urological inpatients. This is a new method for VTE screening, and internal validation showed a good performance. External validation results were suboptimal but may provide clues for subsequent VTE screening.
Keywords: non-oncological surgery, prediction model, urology, venous thromboembolism
Introduction
Venous thromboembolism (VTE) includes deep venous thrombosis (DVT) and pulmonary embolism (PE). VTE can cause preventable morbidity and mortality in patients undergoing pelvic surgery, which increases the burden of care for patients and society.1 VTE risk is particularly high in hospitalized patients because most of them have multiple risk factors for developing VTE. VTE prevalence for hospital inpatients can be as high as 34.7% (15–40% for major urological surgery), with fatal PE being documented in 9.4% according to autopsy studies.2
In recent years, many urologists have paid increasing attention to VTE development, especially in patients undergoing surgical procedures for urological tumors because VTE is thought to be associated with different cancer types.1,3–5 According to the literature, insufficient attention has been paid to non-oncological surgical procedures by urologists. High-quality evidence is lacking, but VTE prevalence in non-oncological urologic surgery is not as low as postulated. For major urological surgical procedures, the VTE prevalence varies from 0.3% to 10.8%; it is particularly high in open recipient nephrectomy and open simple prostatectomy, with a prevalence of 1.3–5.3% and 2.7–10.8%, respectively.6 Therefore, more attention should be paid to non-oncological patients in surgical departments.
We aimed to build prediction models for VTE according to our previous research on risk factors.7 We also aimed to help urologists identify more precisely patients with VTE and make appropriate clinical choices for non-oncological urological inpatients because VTE is an occult disease without specific symptoms and can be overlooked readily in urological clinics. We made use of statistical methods to build prediction models for VTE. A similar prediction model has not been proposed to predict VTE in urological inpatients undergoing non-oncological surgical procedures. In addition, we verified the accuracy of these two prediction models using internal and external data.
Materials and Methods
Study Population
This retrospective single-center study involved 1453 consecutive individuals admitted to the non-oncological urological ward in Xiangya Hospital within Central South University (Changsha, China) from 1 January 2018 to 31 December 2018. Data were collected from the Electronic Medical Record System with approval of the Ethics Committee of Xiangya Hospital (2019030078). All patients provided written informed consent to participate in the study. In-hospital VTE events were defined as any episode of DVT or PE that appeared at any time from hospital admission to hospital discharge and determined by appropriate imaging procedures (ultrasound, computed tomography, pulmonary angiography).3
Exclusion Criteria
Patients were excluded if: (a) aged <18 years; (b) they were admitted for surgery to remove a malignant tumor; (c) postoperative pathological findings showed a malignant tumor; (d) medical records were incomplete.
Procedures
The demographic details and clinical data of all 1453 patients at baseline had been collected and the risk factors for VTE clarified during our previous research.7 The main types of surgical procedures were percutaneous nephrostolithotomy, ureteroscopic lithotripsy, retrograde intrarenal stone surgery (RIRS), transurethral resection of the prostate gland, urethroplasty, and other types of non-oncological urological surgical procedures. We included all the items we are interested in (including risk factors from published guidelines and the literature) to make this research more persuasive. Once patients had been admitted to hospital, our hospital VTE team evaluated the patient comprehensively and took the corresponding measures: stockings, mechanical compression, and pharmacological agents.
Each independent risk factor was given a specific score according to its odds ratio (OR) as a regression coefficient of the equation. Then, the regression coefficient and the variable (X) were used to build models. A prediction model was established for predicting VTE of a non-oncological urological surgical procedure by calculating a comprehensive score for each patient.
Evaluations
To verify the accuracy of the models, another 291 inpatients admitted to the non-oncological urological ward from 1 January 2019 to 30 June 2019 were enrolled. Their related data had been collected. These data were used for verification of these novel prediction models.
Statistical Analyses
Statistical analyses were undertaken using SAS 9.3 (SAS Institute, Cary, NC, USA). If p < 0.05 in the univariate analyses, the related factors were inputted into the multivariable logistic regression model. A stepwise regression method was employed to screen the relevant influencing factors for VTE and construct a logistic regression prediction model. A receiver operator characteristic (ROC) curve was constructed to evaluate the accuracy and efficiency of the prediction model. Statistical tests were deemed significant for p < 0.05 (two-tailed) but another prediction model was created using p < 0.1 in the multivariable logistic regression model.
Results
Characteristics of Patients
A total of 1453 inpatients were enrolled in this study. The VTE prevalence in non-oncological urological inpatients was 2.3%, and comprised 32 DVT (2.2%), 4 PE (0.3%), 12 (35.3%) proximal, 23 (67.6%) distal, 6 (17.6%) symptomatic, and 25 (73.5%) asymptomatic cases. The following types of patients were more susceptible to VTE: older age; previous VTE; history of varicose veins in the lower extremities; received any type of surgical procedure within 1 month; taking anticoagulants or anti-platelet agents before hospital admission; had suffered preoperative bleeding; had preoperative sepsis; high level of D-dimer; high Caprini Score; complications (any complications after urological surgical procedures were considered, such as bleeding, sepsis, atelectasis, or incision infection); lower-extremity swelling; lower-extremity pain; chest symptoms (swelling and pain in lower limbs, pain in lower limbs, decreased oxygen saturation (SpO2), dyspnea, chest pain, or electrocardiography performance with unstable circulation).7
Prediction Model 1 for VTE
To establish a prediction model, we incorporated the above-mentioned risk factors into a multivariable logistic regression model with p < 0.05, and used a stepwise regression method to screen the relevant influencing factors for VTE. Previous VTE (X1), treatment with anticoagulants or anti-platelet agents before hospital admission (X2), D-dimer ≥0.89 μg/mL (X3), lower-extremity swelling (X4), and chest symptoms (X5) were considered to be independent risk factors for VTE (Table 1). The cutoff value for D-dimer was derived from our previous research.8 The logistic regression model was built as 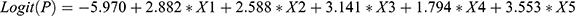 . The probability model for predicting VTE was
. The probability model for predicting VTE was 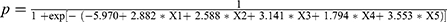 .
.
 |
Table 1 Logistic Regression Prediction Model for Influencing Factors of VTE (p <0.05) |
Evaluation for Prediction Model 1
Next, the data for 1453 patients were inputted into this equation to evaluate the accuracy of this prediction model (Table 2). Among the 1453 patients, 32 patients were predicted to have VTE, and they did; 1158 patients were predicted not to have VTE, and they did not have VTE.
 |
Table 2 Comparison of Actual VTE and Predicted VTE (model 1) |
To further evaluate this model for discriminating VTE, first a ROC curve was created. Then, the area under the ROC curve (AUC) was calculated and the cutoff point was determined (Figure 1). After calculation, the AUC curve was 0.915 (95% CI: 0.864–0.967). With regard to the cutoff point, the maximum value of sensitivity and specificity was 1.761 when the sensitivity and specificity were 0.941 and 0.820, respectively. The corresponding cutoff value was 0.03824, which meant that if the predicted value ≥0.03824, then the patient would be considered to be more susceptible to VTE.
 |
Figure 1 ROC curve of multivariable logistic regression prediction model 1. ROC curve to distinguish the cut-off value of the prediction model 1 |
Also, 291 additional inpatients admitted to the non-oncological urological ward from 1 January 2019 to 30 June 2019 were enrolled to verify the accuracy and efficiency of the prediction model. The baseline characteristics of patients used for external verification are shown in Table 3. The results for external verification are shown in Table 4.
 |
Table 3 Baseline Characteristics of Patients Used for External Verification |
 |
Table 4 Comparison of Actual VTE and Predicted VTE Using Additional Data (model 1) |
Prediction Model 2 for VTE
To assess more potential risk factors for VTE, we let p < 0.1 and built a new prediction model. Compared with the first prediction model, the new model included two novel variables: Caprini Score ≥5 (X6) and complications (X7) (Table 5). The cutoff value of the Caprini score was derived from our previous research.8 The new model turned became 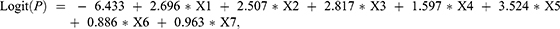
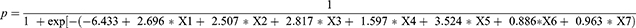
 |
Table 5 Logistic Regression Prediction Model for Influencing Factors of VTE (p <0.1) |
Evaluation for Prediction Model 2
Similar internal verification and ROC curves were used to evaluate the new prediction model. Also, a ROC curve was created to evaluate the new model (Figure 2), (Table 6). The AUC was 0.941 (95% CI: 0.910–0.972). When the sensitivity was 0.941 and specificity was 0.816, the sum of the sensitivity and specificity had a maximum value of 1.757, and the corresponding cutoff value was 0.02474.
 |
Table 6 Comparison of Actual VTE and Predicted VTE (model 2) |
 |
Figure 2 ROC curve of multivariable logistic regression prediction model 2. ROC curve to distinguish the cut-off value of the prediction model 2 |
Not only were the internal verification and ROC curves of the two models similar, but also the external verification results were similar. The same 291 inpatients were included to verify the accuracy and efficiency of the new prediction model (Table 7).
 |
Table 7 Comparison of Actual VTE and Predicted VTE Using Additional Data (model 2) |
Discussion
Although VTE is a rare event, it can be life-threatening or cause a series of health problems. Patients with VTE have a greater mortality rate than those without VTE.9 Also, hospitalization is a major risk factor for VTE.10 In the 1990s, when anticoagulation treatment for inpatients was not as sophisticated as it is today, some studies showed VTE prevalence to be 10–30% for hospitalized patients but, interestingly, more non-surgical inpatients died than surgical inpatients.11,12 Our previous research and other studies have demonstrated that a VTE event is not rare in non-oncological urology, and can be associated with a significantly higher rate of transfer to the intensive care unit, longer recovery, greater medical costs, and higher risk of death.6,13 Hence, early diagnosis of VTE can circumvent many problems because it is preventable The risk of VTE development should be distinguished by a reliable scoring system to avoid the threat of health issues and financial burden caused by VTE.
Various risk-assessment models (RAMs) are available to stratify risk degree in Western countries (eg, Rogers,14 Padua,15 Khorana,16 Caprini).17 Although RAMs for VTE in Western countries have been verified by large-scale, multicenter studies in Asia and the Caprini Score may be suitable for Chinese people,18,19 our previous study indicated that the Caprini Score over-evaluates VTE risk, and that more efforts are required to focus on building a preferable and validated Asian model. In addition, the current RAMs for VTE show a limited ability to predict VTE development in many common types of cancer.12
Models are used commonly to diagnose a disease. Such models can rapidly and effectively identify a high-risk group from a large group of patients, and appropriate medical treatment can be undertaken. However, only a few prediction models for VTE are available in urology. Shi et al found that D-dimer ≥1 μg/mL on postoperative day (POD) 1 and Charlson Comorbidity Index ≥2 were associated independently with VTE in patients who underwent a surgical procedure for a urological tumor. Also, the plasma level of D-dimer on POD1 can predict VTE development.20 Bezan et al established a stratification model for VTE for patients with testicular germ-cell tumors. They used clinical stage (cS) and retroperitoneal lymphadenopathy to divide patients into four groups: cS IA-B, cS IS-IIB, cS IIC, and cS IIIA-C. Each group corresponded to a related specific prevalence of VTE, and this model was validated closely with an external cohort.21 A prediction model for VTE of non-oncological urological patients was not found upon searching commonly used databases (PubMed, EMBASE, China National Knowledge Infrastructure, Wanfang database, OVID, Springer) until 2 December 2022, which indicates a lack of attention in this important matter.
Consensus guidelines for VTE in urology have been updated and risk has been stratified, but they are not fully developed and are underutilized. More appropriate guidelines should be considered.22 The European Association of Urology and Canadian Urological Association have published guidelines on perioperative thrombosis and thromboprophylaxis in the urology setting in recent years; they provide thromboprophylaxis guidelines for oncological and non-oncological surgical procedures. However, the proof of recommendations is weak.23,24 To reduce the potential waste of medical resources and to identify VTE inpatients in non-oncological urology, we established two similar prediction models. These models could provide some information for physicians to distinguish VTE inpatients initially and formulate appropriate strategies.
Variables which showed a significant difference between VTE inpatients and non-VTE inpatients in non-oncological urology were identified.7 Then, we these variables were incorporated into multivariable logistic regression models to build a prediction model for VTE. The variables which were screened out eventually were previous VTE (X1), treatment with anticoagulants or anti-platelet agents before hospital admission (X2), D-dimer ≥0.89 μg/mL (X3), lower-extremity swelling (X4), and chest symptoms (X5). Most of the variables have been demonstrated to be independent risk factors for VTE, such as previous VTE,25 high D-dimer value,20 and chest symptoms.26 The prediction model was 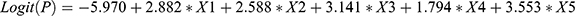 . Unlike other prediction models, we first tried to use logistic regression to build a VTE prediction model. This approach enables the risk of VTE to be calculated directly using a formula. It is a simple procedure and could be automated.
. Unlike other prediction models, we first tried to use logistic regression to build a VTE prediction model. This approach enables the risk of VTE to be calculated directly using a formula. It is a simple procedure and could be automated.
We focused mainly on hospitalized patients to increase the awareness of non-oncological urologists to VTE during a patient’s hospitalization and ignored patients who had been discharged from hospital. However, more than three-quarters of all thrombosis episodes occur after hospital discharge and occur in approximately half of patients when anticoagulation prophylaxis has been discontinued, so these are important factors that merit attention.27
ROC curves were created to evaluate the accuracy and efficiency of the prediction model to predict VTE events in non-oncological urological inpatients. The cutoff point of this model was 0.03824, with an AUC of 0.915, a sensitivity of 0.941, and specificity of 0.820. This model is considered to have a high degree of accuracy of predicting VTE in non-oncological urological inpatients according to our statistical data. The score is correlated positively with VTE risk. Hence, the higher the score, the greater is the VTE risk. Furthermore, the data of 291 additional inpatients were used to verify this prediction model, and the accuracy was 43.0%: the clinical-application value of this value was not sufficient. However, the sensitivity and specificity were 96.43% and 37.2%, respectively, so this model could be used in VTE screening. Despite the limited clinical-application value of this model, it could be employed to exclude VTE and could greatly help VTE screening in non-oncological urological inpatients.
A new prediction model was created using p < 0.1 in the multivariable logistic regression model. There were three main reasons why the p-value was widened. First, the sample size was too small and some potential risk factors would be eliminated, so we wanted to evaluate more potential risk factors for VTE. Second, the new variables proved to be important independent risk factors, especially the Caprini Score.19,28 Third, widening of the p-value can provide clues for follow-up studies of VTE. The new prediction model became 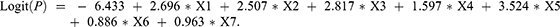
Overall, VTE is a potentially life-threatening disease and merits greater attention from non-oncological urologists. A few prediction models have been established for VTE and achieved good results. We built two similar prediction models using previous research data. This is the first time that logistic regression prediction models for VTE screening using p < 0.05 and p < 0.1, respectively, have been built. The internal verification achieved very good results, with high specificity and sensitivity. However, the external verification was inferior and may have been caused by: (i) the total amount of validation data being small, which might have caused a bias; (ii) changes in hospital policies, such as VTE education for patients. Nevertheless, the new VTE prediction models described here could have a certain role in VTE screening in the urology setting.
The present study had four main limitations. First, the models were based only on the whole-year case data of 2018 in a tertiary hospital, so the sample size was relatively small. The number of clinical cases will be expanded in the future to optimize the model. Second, some patients with VTE were missed due to unclear screening methods, which resulted in a bias. Third, the data used to validate the model came from patients who had a complete set of diagnostic images, so there may have been some limitations and biases. Fourth, this was a single-center, retrospective study; multi-center, large-scale studies are needed to establish more accurate models. Nevertheless, these are the first two prediction models of VTE for urological non-oncological inpatients. As such, they will provide intuitive assessment of VTE development before, during, and after urological non-oncological surgical procedures.
Conclusions
Based on analyses of our previous studies of risk factors, we established, for the first time, two similar prediction models for assessing the development and risk of VTE for non-oncological urological inpatients. These prediction models exhibited excellent accuracy during internal verification, but had poor clinical-application value during external verification. Therefore, to build better prediction models for VTE, large-scale, multicenter studies are required. Nevertheless, these two prediction models can be used as screening tools for VTE by urologists.
Data Sharing Statement
The authors intend to share personally unidentified participant data, including model establishment database and validation database. All the data and further inquiries can be directed to the corresponding author from the day the article was published.
Ethics Approval and Informed Consent
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The trial was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Ethical approval was given by the ethics committee of Xiangya Hospital, Central South University (2019030078). Trial registration number: ChiCTR1900027180. Date of registration: 3 November 2019 (registered retrospectively).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Krimphove MJ, Reese S, Chen X, et al. Minimally invasive cancer surgery is associated with a lower risk of venous thromboembolic events. J Surg Oncol. 2020;121:578–583. doi:10.1002/jso.25832
2. Agarwal S, Lee AD, Raju RS, Stephen E. Venous thromboembolism: a problem in the Indian/Asian population? Indian J Urol. 2009;25:11–16. doi:10.4103/0970-1591.45531
3. Chen EC, Papa N, Lawrentschuk N, Bolton D, Sengupta S. Incidence and risk factors of venous thromboembolism after pelvic uro-oncologic surgery–a single center experience. BJU Int. 2016;117 Suppl 4:50–53. doi:10.1111/bju.13238
4. Laymon M, Harraz A, Elshal A, et al. Venous thromboembolism after radical cystectomy and urinary diversion: a single-center experience with 1737 consecutive patients. Scand J Urol. 2019;53(6):392–397. doi:10.1080/21681805.2019.1698652
5. Klaassen Z, Wallis CJD, Lavallee LT, Violette PD. Perioperative venous thromboembolism prophylaxis in prostate cancer surgery. World J Urol. 2020;38(2):593–600. doi:10.1007/s00345-019-02705-x
6. Tikkinen KAO, Craigie S, Agarwal A, et al. Procedure-specific risks of thrombosis and bleeding in urological non-cancer surgery: systematic review and meta-analysis. Eur Urol. 2018;73(236–241). doi:10.1016/j.eururo.2017.02.025
7. Wang Z, Li K, Zhu Q, et al. Incidence and risk factors of in-hospital venous thromboembolism in non-oncological urological inpatients: a single center experience. Asian J Urol. 2021. doi:10.1016/j.ajur.2021.11.007
8. Wu ZQ, Li KX, Zhu Q, et al. Application value of D-dimer testing and Caprini risk assessment model (RAM) to predict venous thromboembolism (VTE) in Chinese non-oncological urological inpatients: a retrospective study from a tertiary hospital. Transl Androl Urol. 2020;9(5):1904–1911. doi:10.21037/tau-20-320
9. Sorensen HT, Mellemkjaer L, Olsen JH, Baron JA. Prognosis of cancers associated with venous thromboembolism. N Engl J Med. 2000;343(25):1846–1850. doi:10.1056/NEJM200012213432504
10. Samama MM. An epidemiologic study of risk factors for deep vein thrombosis in medical outpatients: the Sirius study. Arch Intern Med. 2000;160:3415–3420. doi:10.1001/archinte.160.22.3415
11. Stein PD, Henry JW. Prevalence of acute pulmonary embolism among patients in a general hospital and at autopsy. Chest. 1995;108:978–981. doi:10.1378/chest.108.4.978
12. Spyropoulos AC. Emerging strategies in the prevention of venous thromboembolism in hospitalized medical patients. Chest. 2005;128:958–969. doi:10.1378/chest.128.2.958
13. Pinto SM, Galang G. Venous thromboembolism as predictor of acute care hospital transfer and inpatient rehabilitation length of stay. Am J Phys Med Rehabil. 2017;96:367–373. doi:10.1097/PHM.0000000000000643
14. Rogers SO
15. Barbar S, NOVENTA F, ROSSETTO V, et al. A risk assessment model for the identification of hospitalized medical patients at risk for venous thromboembolism: the Padua Prediction Score. J Thromb Haemost. 2010;8:2450–2457. doi:10.1111/j.1538-7836.2010.04044.x
16. Khorana AA, Dalal M, Lin J, Connolly GC. Incidence and predictors of venous thromboembolism (VTE) among ambulatory high-risk cancer patients undergoing chemotherapy in the United States. Cancer. 2013;119:648–655. doi:10.1002/cncr.27772
17. Hachey KJ, Hewes PD, Porter LP, et al. Caprini venous thromboembolism risk assessment permits selection for postdischarge prophylactic anticoagulation in patients with resectable lung cancer. J Thorac Cardiovasc Surg. 2016;151:37–44 e31. doi:10.1016/j.jtcvs.2015.08.039
18. Liew NC, Alemany GV, Angchaisuksiri P, et al. Asian venous thromboembolism guidelines: updated recommendations for the prevention of venous thromboembolism. Int Angiol. 2017;36:1–20. doi:10.23736/S0392-9590.16.03765-2
19. Zhou HX, Peng L-Q, Yan Y, et al. Validation of the Caprini risk assessment model in Chinese hospitalized patients with venous thromboembolism. Thromb Res. 2012;130(5):735–740. doi:10.1016/j.thromres.2012.08.001
20. Shi A, Kitajima I, Kabata T, et al. Postoperative D-dimer predicts venous thromboembolism in patients undergoing urologic tumor surgery. Urol Oncol. 2018;36:307 e315–307 e321. doi:10.1016/j.urolonc.2018.03.003
21. Bezan A, Posch F, Ploner F, et al. Risk stratification for venous thromboembolism in patients with testicular germ cell tumors. PLoS One. 2017;12:e0176283. doi:10.1371/journal.pone.0176283
22. Saluja M, Gilling P. Venous thromboembolism prophylaxis in urology: a review. Int J Urol. 2017;24(8):589–593. doi:10.1111/iju.13399
23. Tikkinen KA, Cartwright R, Gould MK. EAU Guidelines on thromboprophylaxis in Urological Surgery. Eur Assoc Urol. 2017;3:45.
24. Violette PD, Lavallee LT, Kassouf W, Gross PL, Shayegan B. Canadian Urological Association guideline: perioperative thromboprophylaxis and management of anticoagulation. Can Urol Assoc J. 2019;13:105–114. doi:10.5489/cuaj.5828
25. Pedersen MH, Wahlsten LR, Grønborg H, et al. Symptomatic venous thromboembolism after achilles tendon rupture: a nationwide Danish cohort study of 28,546 patients with achilles tendon rupture. Am J Sports Med. 2019;47(13):3229–3237. doi:10.1177/0363546519876054
26. Tyson MD, Castle EP, Humphreys MR, Andrews PE. Venous thromboembolism after urological surgery. J Urol. 2014;192(3):793–797. doi:10.1016/j.juro.2014.02.092
27. Heit J, Crusan D, Ashrani A, Petterson T, Bailey K. Effect of a near-universal hospitalization-based prophylaxis regimen on annual number of venous thromboembolism events in the US. Blood. 2017;130(2):109–114. doi:10.1182/blood-2016-12-758995
28. Gould MK, Garcia DA, Wren SM, et al. Prevention of VTE in nonorthopedic surgical patients: antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2):e227S–e277S. doi:10.1378/chest.11-2297
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.