Back to Journals » Drug Design, Development and Therapy » Volume 15
Establishment and Verification of UPLC-MS/MS Technique for Pharmacokinetic Drug–Drug Interactions of Selinexor with Posaconazole in Rats
Authors Zhou C, Wang H, Zhou C, Li C, Zhu MJ, Qiu X
Received 27 January 2021
Accepted for publication 18 March 2021
Published 15 April 2021 Volume 2021:15 Pages 1561—1568
DOI https://doi.org/10.2147/DDDT.S303928
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Anastasios Lymperopoulos
Chen-jian Zhou,1 Hui-jun Wang,2 Chun-yan Zhou,2 Chao-fan Li,2 Ming-Jia Zhu,2 Xiang-jun Qiu2
1Department of Pharmacy, Wenzhou Central Hospital, Wenzhou, 325027, People’s Republic of China; 2School of Basic Medical Sciences, Henan University of Science and Technology, Luoyang, 471023, People’s Republic of China
Correspondence: Xiang-jun Qiu
School of Basic Medicine, Henan University of Science and Technology, 263 Kaiyuan Avenue, Luoyang, 471023, People’s Republic of China
Tel +8613698882699
Fax +86379-64830346
Email [email protected]
Background: A method for the determination of selinexor by UPLC-MS/MS was established to study the effect of posaconazole on the pharmacokinetics of selinexor in rats.
Methods: The experiment rats were divided into group A (0.5% CMC-Na) and group B (posaconazole, 20 mg/kg), 6 rats in each group. 30 minutes after administration of 0.5% CMC-Na or posaconazole, all the rats were given selinexor (8 mg/kg), and plasma samples were collected. The plasma samples underwent acetonitrile protein precipitation, and were separated by UPLC on an Acquity UPLC BEH C18 column with gradient elution. Acetonitrile and 0.1% formic acid were used as the mobile phases. The analyte detection was used a Xevo TQ-S triple quadrupole tandem mass spectrometer and multiple reaction monitoring (MRM) for analyte monitoring. We use acetonitrile for protein precipitation.
Results: Selinexor had good linearity (1.0– 1000 ng/mL, r2= 0.996 2), and the accuracy and precision, recovery rate and matrix effects(ME) were also met the FDA approval guidelines. Compared with group A, the Cmax, AUC(0−t) and AUC(0−∞) of selinexor in group B increased by 60.33%, 48.28% and 48.27%, and Tmax increased by 53.92%, CLz/F reduced by 32.08%.
Conclusion: This bioanalysis method had been applied to the study of drug interactions in rats. It was found that posaconazole significantly increased the concentration of selinexor in rats. Therefore, when selinexor and posaconazole are combined, we should pay attention to the possible drug–drug interactions to reduce adverse reactions.
Keywords: UPLC-MS/MS, selinexor, posaconazole, pharmacokinetics, DDIs, rats
Introduction
Selinexor (KPT-330, Figure 1A) is the world’s first, oral, small molecule selective inhibition of nuclear export (SINE).1 Exportin-1 (XPO1) is a tumor suppressor protein, it can act on transcription factors, and growth regulators and oncoprotein mRNA.2–4 The overexpression of XPO-1 leads to an increase in the output of tumor suppressor and regulatory proteins, which can lead to enhanced signal transduction for abnormal cell growth and prevent apoptosis.5 XPO1 is overexpressed in most cancer types and is associate with poor prognosis of acute myeloid leukemia, glioma, pancreatic cancer, cervical cancer and ovarian cancer.6–10 Inhibition of XPO-1 can force tumor suppressor proteins and growth regulators to remain in the nucleus, leading to cancer cell apoptosis.5
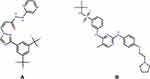 |
Figure 1 The chemical structure of selinexor (A) and fedratinib (IS, (B)). |
Selinexor works by binding and inhibiting nuclear export protein XPO1.11 It causing tumor suppressor protein to accumulate in the nucleus, thereby restarting and amplifying its function as a tumor suppressor, leading to apoptosis of cancer cells, while largely protecting normal cells from damage.2–4
On July 3, 2019, the US FDA approved Selinexor in combination with low-dose dexamethasone for the treatment of relapsed or refractory multiple myeloma (RRMM).1,12 Jan 29, 2021, Karyopharm receives positive CHMP opinion for NEXPOVIO® (selinexor) for the treatment of patients with refractory multiple myeloma.13 In clinic, the recommended starting dose of Selinexor is 80 mg, and the effect of food on its pharmacokinetics is not significant.1,2 In cancer patients, tmax is about 2–4 h; plasma protein binding rate is about 95%; t1/2 is 6–8 h; CLz/F is 17.9 L/h. Selinexor is mainly metabolized by CYP3A4.1
The most common adverse reactions of Selinexor (≥20%) were thrombocytopenia, fatigue, nausea, anemia, decreased appetite, decreased weight, diarrhea, vomiting, hyponatremia, neutropenia, leukopenia, constipation, dyspnea and upper respiratory tract infection. In the STORM trial, fatal adverse reactions occurred in 9% of patients. Serious adverse reactions occurred in 58% of patients. Treatment discontinuation rate due to adverse reactions was 27%.13
Drug–drug interactions (DDIs) often occur, especially for patients with multiple underlying diseases who use various kinds of drugs. In clinical practice, polypharmacy is a common problem and results in the increased risks of DDIs.14 Due to fungal infection in patients with multiple myeloma, antifungal drugs are often used in clinic. Therefore, selinexor and posaconazole may be used at the same time in clinic. Because selinexor and posaconazole were both metabolized by the CYP3A4 enzyme, so when the two drugs were used at the same time, the exposure of selinexor would increase due to the inhibition of CYP3A4 enzyme by posaconazole. Thereby enhancing drug concentrations and DDIs.
UPLC-MS/MS had been widely used for the determination of drug concentrations in various biological matrices, which had many advantages such as short time, high sensitivity and strong specificity.15,16 This paper presented a new, rapid and accurate UPLC-MS/MS method for determining the concentration of selinexor and investigated the effects of posaconazole on the pharmacokinetics of selinexor using fedratinib as the internal standard (IS, Figure 1B).
Methods
Chemicals Materials
Selinexor and posaconazole (purity over 98%) was provided by Jiangsu Aikang Biomedicine R & D Co., Ltd, and fedratinib (purity over 98%, IS) was obtained from Beijing sunflower and technology development CO., LTD (Beijing, China). Acetonitrile and formic acid were analytical grade, and water was deionized water.
Chromatographic Conditions
The Waters ACQUITY UPLC I-Class (Milford, MA, USA) used in this experiment and the Acquity BEH C18 column (2.1 mm × 100 mm, 1.7 μm) were used as the separation column. The mobile phase used 0.1% formic acid (A) and acetonitrile (B), and the gradient elution is shown in Table 1.
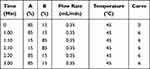 |
Table 1 The Gradient of Mobile Phase |
MS Conditions
Mass spectrometry analysis was performed on a Waters Xevo TQ-S triple quadrupole mass spectrometer. Multiple reaction monitoring (MRM) conditions are shown in Table 2.
 |
Table 2 The MS Parameters Parameters of Selinexor and IS |
Animal Experiments
The Laboratory Animal Center of Henan University of Science and Technology (Luoyang, China) provides male Sprague-Dawley rats (200 ± 20 g), and the animal certification was SCXK (Hubei) 2007–0001. Necessary approval from the Animal Ethics Committee of the animal laboratory of Henan university of science and technology was obtained to carry out the experiments, and the experimental content was approved according to the Laboratory animals-guidelines for ethical review of welfare (GB/T 35892–2018). The institutional approval number for the preclinical study of this experiment was 2019100003. All authors have read the NC3Rs ARRIVE guidelines.
Twelve rats were randomly divided into two groups and administered orally according to body weight. Group A was orally given with 0.5% CMC-Na and group B was orally given with 20 mg/kg posaconazole. Thirty minutes later, 8 mg/kg selinexor was orally administered to each rat. At the time point of 0.33, 0.67, 1, 1.5, 2, 3, 4, 6, 9, 12, 24 and 48 hours, approximately 0.2 mL of blood sample was collected from the tail vein of each rat. The blood samples were centrifuged at 10000 rmp * 10 min, 50 µL plasma was obtained and stored at −20°C until analysis. All rats were sacrificed by CO2 inhalation.
Solutions Ready
10.0 mg of Selinexor and IS standard product were weighed out and dissolved in methanol to prepare the stock solution with a concentration of 1.00 mg/mL. Then, the standard stock solution was gradient diluted to obtain the calibration curve and the working solution of the quality control (QC) samples. The final concentrations obtained by mixing blank plasma with the corresponding standard working solutions were: 1, 5, 10, 50, 100, 200, 500, 1000 ng/mL. The concentrations of QC samples were 2.5, 250, and 750 ng/mL, respectively. All working solutions were stored at 4°C.
Plasma Sample Processing
50 μL of rat plasma was placed in an Eppendorf (EP) tube, and 10 μL of IS (200 ng/mL) and 150 μL acetonitrile were added for protein precipitation. The mixture was vortexed for 1.0 minute, and then 10 minutes at 10,000 rmp was centrifuged. 50 µL of the supernatant was moved and detected, and the injection volume was 2 µL.
Method Validation
According to the main regulatory principles of the US FDA, we have verified the integral validation of the bioanalytical approach, including: calibration curve, selectivity, LLOQ, accuracy, precision, matrix effect, recovery, and stability.17
Statistical Analysis
In this experiment, the pharmacokinetic parameters of Selinexor were calculated using DAS 2.0 software. SPSS 18.0 software was used for data processing, and the main pharmacokinetic parameters between different groups were compared. P <0.05 was considered to be of statistical significance.
Results and Discussion
Method Development and Optimization
The UPLC-MS/MS has been widely used in the analysis and determination of samples in various biological matrices because of its faster analysis time and high sensitivity. As we all know, the choice of column was crucial. We evaluated the retention time of the analyte and the state of the peak shape, and finally selected the Acquity BEH C18 column (2.1 mm × 50 mm, 1.7 μm). The composition of the mobile phase affects sensitivity and peak shape, as well as the retention time and background noise of the analyte. We evaluated different combinations of acetonitrile, methanol, and acetic acid, respectively. Through verification, it was found that formic acid can enhance the chromatographic signal of the analyte and IS. The gradient elution mobile phase can achieve better peak-out effect. The temperature of the mobile phase was 45°C.
In this study, higher response values were obtained by improving the conditions set by the mass spectrometer. Mass spectrometry analysis was performed on the positive ionization interface of electrospray ionization (ESI) using XEVO TQ-S triple quadrupole mass spectrometer to optimize the main parameters, such as air pressure, fragmentation energy and ion spray voltage. The internal standard is fedratinib because it is stable and easy to obtain, and it has good separation from selinexor, good peak shape, and similar retention time. Endogenous substances in rat plasma samples do not affect the determination of selinexor and IS concentrations.
After screening, plasma samples were processed using acetonitrile. This method was suitable for processing a large number of samples in DDIs studies. Through gradient elution, the interference of endogenous substances was effectively removed. In addition, the acetonitrile protein precipitation method could meet the requirements of high-throughput testing.
Specificity
Observe the interference of endogenous substances and chromatographic integrity by comparing the blank plasma samples, the blank plasma samples containing the target analytes of selinexor and IS, and the samples from oral selinexor 3 h in rats were processed. The results showed that the method has good selectivity, no interference peaks of endogenous substances were observed, the peak out times of analyte and IS peaks were 1.76 and 1.59 min, respectively (Figure 2). Figure 2 Contiued.
Linearity and Carryover
The standards were added to the blank plasma of rats, and samples with concentrations of 1, 5, 10, 50, 100, 200, 500, 1000 ng/mL were prepared, the calibration curve was evaluated at three consecutive days by UPLC-MS/MS. The calibration curve was plotted against the theoretical concentration (x) of each analyte and using the weighting of the peak area ratio of selinexor to IS (1/x2). The standard curve of selinexor was: y = 0.6674*x + 1.1795 (r2 = 0.996 2). The lower limit of quantitation was 1 ng/mL. The carryover test was based on the fact that after the sample concentration was 1000 ng/mL, the analyzer does not detect any residual analyte the next time a blank plasma sample was injected. The experimental results showed that the method has a good linear relationship and the carryover does not affect the determination of selinexor.
Precision, Accuracy, Recovery and Matrix Effect (ME)
Three different concentration levels of 6 QC samples were used to evaluate the inter-day precision (RSD%) and accuracy (RE%) at three consecutive days, while the intra-day accuracy and precision were calculated in a day. The results are shown in Table 3. The recovery rate was expressed by the ratio of the chromatographic peak area of selinexor in the QC sample repeated six times to the chromatographic peak area of the analyte in the blank plasma sample processed at the corresponding concentration level. The ME was evaluated by comparing the peak area ratio of selinexor incorporated into the extracted blank matrix to the pure reference standard solution. The recovery and ME results are shown in Table 4. The results show that the precision, accuracy, recovery and ME of selinexor met the relevant regulations. In this experiment, the accuracy and precision of selinexor was good, the recovery rate of the experimental method was high.
 |
Table 3 Precision and Accuracy of Selinexor in Rat Plasma (n=6, Mean ± SD) |
 |
Table 4 The Recoveries and ME of Selinexor and IS in Rat Plasma (n=6, Mean ± SD) |
Stability
Under the three concentration conditions, and 6 replicates were obtained for each concentration, and the stability of QC samples under different treatment conditions was studied (Table 4). The QC samples were placed at room temperature for 4 hours, 4°C for 24 hours, 3 freeze-thaw cycles (−20~25°C), and −20°C for 4 weeks. The results are shown in Table 5, and passed the required accuracy and precision and met the requirements.
 |
Table 5 The Stability of Selinexor and IS in Rat Plasma (n=6, Mean ± SD) |
Pharmacokinetic DDIs Study
The newly established UPLC-MS/MS method was used to detect selinexor in rat plasma and observe the interaction between posaconazole and selinexor. The relationship between the mean concentration levels of selinexor in rat plasma and the time in group A (8 mg/kg selinexor), and group B (20 mg/kg posaconazole and 8 mg/kg selinexor) are shown in Figure 3. The main pharmacokinetic parameters of selinexor are also shown in Table 6.
 |
Table 6 Pharmacokinetic Parameters of Selinexor After Oral Administration of 8 mg/kg Selinexor in Group A and B (n=6, Mean ± SD) |
 |
Figure 3 The mean plasma concentration-time curve of selinexor in group A and B after oral administration 8 mg/kg selinexor (zoomed 1 to 6 h pharmacokinetic profile). |
The experimental results showed that after the rats were given posaconazole and selinexor at the same time, the Cmax, AUC(0−t) and AUC(0−∞) of selinexor in group B were 60.33%, 48.28% and 48.27% higher than group A, respectively. The Tmax of the experimental group were also extended, and CLz/F were also significantly reduced. This shows that posaconazole can increase the exposure of selinexor in the plasma of rats, and the pharmacokinetic process of selinexor was significantly affected by posaconazole.
Previous research results show that isavuconazole, itraconazole, and fluconazole have significant inhibitory effects on selinexor pharmacokinetics and increased selinexor plasma exposure in rats.18 Due to certain differences in metabolism in different species, the DDIs between posaconazole and selinexor should be further studied. We will continue to investigate the effect of other CYP3A4 inhibitors (for example, some anti-tumor Chinese medicine) on selinexor in subsequent experiments.
Conclusion
In summary, this experiment established a new, simple, fast, and accurate UPLC-MS/MS method, which can be applied to the detection of selinexor in rat plasma. In addition, we found that posaconazole can increase selinexor exposure and inhibit its metabolism in rats. Therefore, when selinexor and posaconazole were used in combination, the dosage should be adjusted to avoid adverse reactions in clinic.
Data Sharing Statement
The data used to support the findings of this study are available from the corresponding authors upon request. The corresponding authors: Xiang-jun QIU ([email protected])
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors have declared that no competing interests exist.
References
1. Karyopharm Therapeutics Inc. XPOVIO™ (selinexor) tablets, for oral use: US prescribing information; 2019. Available from: www.fda.gov.
2. Gounder MM, Zer A, Tap WD, et al. Phase IB study of selinexor, a first-in-class inhibitor of nuclear export, in patients with advanced refractory bone or soft tissue sarcoma. J Clin Oncol. 2016;34(26):3166–3174. doi:10.1200/JCO.2016.67.6346.
3. Abdul Razak AR, Mau-Soerensen M, Gabrail NY, et al. First-in-class, first-in-human Phase I study of selinexor, a selective inhibitor of nuclear export, in patients with advanced solid tumors. J Clin Oncol. 2016;34(34):4142–4150. doi:10.1200/JCO.2015.65.3949.
4. Gandhi UH, Senapedis W, Baloglu E, et al. Clinical implications of targeting XPO1-mediated nuclear export in multiple myeloma. Clin Lymphoma Myeloma Leuk. 2018;18(5):335–345. doi:10.1016/j.clml.2018.03.003.
5. Turner JG, Sullivan DM. CRM1-mediated nuclear export of proteins and drug resistance in cancer. Curr Med Chem. 2008;15:2648–2655. doi:10.2174/092986708786242859
6. Garg M, Kanojia D, Mayakonda A, et al. Selinexor (KPT-330) has antitumor activity against anaplastic thyroid carcinoma in vitro and in vivo and enhances sensitivity to doxorubicin. Sci Rep. 2017;7(1):9749. doi:10.1038/s41598-017-10325-x.
7. Ranganathan P, Kashyap T, Yu X, Meng X, Garzon R. XPO1 inhibition using selinexor synergizes with chemotherapy in acute myeloid leukemia (AML) by targeting DNA repair and restoring topoisomerase IIα to the nucleus. Clin Cancer Res. 2016;22(24):6142. doi:10.1158/1078-0432.CCR-15-2885.
8. Liu XJ, Chong YL, Tu YM, et al. CRM1/XPO1 is associated with clinical outcome in glioma and represents a therapeutic target by perturbing multiple core pathways. J Hematol Oncol. 2016;9:108. doi:10.1186/s13045-016-0338-2.
9. Saulino DM, Younes PS, Biley JM, Younes M. Expression of exportin 1 (XPO1/CRM1) in pancreatic adenocarcinoma. J Clin Oncol. 2017;35(4suppl):327. doi:10.1200/JCO.2017.35.4_suppl.327.
10. Corno C, Stucchi S, De Cesare M, et al. FoxO-1 contributes to the efficacy of the combination of the XPO1 inhibitor selinexor and cisplatin in ovarian carcinoma preclinical models. Biochem Pharmacol. 2018;147:93–103. doi:10.1016/j.bcp.2017.11.009.
11. Nair JS, Musi E, Schwartz GK. Selinexor (KPT-330) induces tumor suppression through nuclear sequestration of IkappaB and down-regulation of survivin. Clin Cancer Res. 2017;23(15):4301–4311. doi:10.1158/1078-0432.CCR-16-2632.
12. Karyopharm Therapeutics. Karyopharm announces FDA approval of XPOVIO™ (selinexor) for the treatment of patients with relapsed or refractory multiple myeloma [media release]. 2019.
13. Karyopharm Receives Positive CHMP Opinion for NEXPOVIO® (selinexor) for the Treatment of Patients with Refractory Multiple Myeloma. Available from: https://investors.karyopharm.com/2021-01-29-Karyopharm-Receives-Positive-CHMP-Opinion-for-NEXPOVIO-R-selinexor-for-the-Treatment-of-Patients-with-Refractory-Multiple-Myeloma.
14. Lin Q, Xie S, Qiu X, Chen J, Xu RA. Drug–drug interaction study of imatinib and voriconazole in vitro and in vivo. Infect Drug Resist. 2019;12:1021–1027. doi:10.2147/IDR.S199526
15. Li SL, Zhu YL, Zhu CY, et al. Simultaneous determination of parecoxib and its metabolite valdecoxib concentrations in beagle plasma by UPLC-MS/MS and application for pharmacokinetics study. Drug Des Devel Ther. 2020;14:1117–1125. doi:10.2147/DDDT.S226349
16. Zhou W, Li SL, Zhao T, et al. Effects of dexmedetomidine on the pharmacokinetics of dezocine, midazolam and its metabolite 1-hydroxymidazolam in beagles by UPLC-MS/MS. Drug Des Devel Ther. 2020;14:2595–2605. doi:10.2147/DDDT.S254055
17. US Food and Drug Administration. Guidance for industry: bioanalytical method validation. Rockville, MD, USA: US Department of Health and Human Services, US FDA, Center for Drug Evaluation and Research; 2018. Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/bioanalyticalmethod-validation-guidance-industry.
18. Li SL, Zhang Y, Cheng QS, et al. UPLC-MS/MS measurement of the effect of isavuconazole, itraconazole and fluconazole on the pharmacokinetics of selinexor in rats. Infect Drug Resist. 2020;13:3153–3161. doi:10.2147/IDR.S269831
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

