Back to Journals » Cancer Management and Research » Volume 10
Establishment and external validation of a prognostic model for predicting disease-free survival and risk stratification in breast cancer patients treated with neoadjuvant chemotherapy
Authors Lai JG , Wang HL, Peng JW, Chen PX, Pan ZH
Received 14 April 2018
Accepted for publication 19 May 2018
Published 1 August 2018 Volume 2018:10 Pages 2347—2356
DOI https://doi.org/10.2147/CMAR.S171129
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Antonella D'Anneo
Jianguo Lai,1,2 Hongli Wang,1,2 Jingwen Peng,3 Peixian Chen,4 Zihao Pan1,2
1Guangdong Provincial Key Laboratory of Malignant Tumor Epigenetics and Gene Regulation, Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University, Guangzhou, People’s Republic of China; 2Breast Tumor Center, Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University, Guangzhou, People’s Republic of China; 3Department of Rehabilitation, Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University, Guangzhou, People’s Republic of China; 4Department of Breast surgery, The First People’s Hospital of Foshan, Foshan, People’s Republic of China
Background: The eighth edition of the American Joint Committee on Cancer (AJCC) tumor-node-metastasis (TNM) staging system for survival prediction and risk stratification in breast cancer (BC) patients after neoadjuvant chemotherapy (NCT) is of limited efficacy. This study aimed to establish a novel prognostic nomogram for predicting disease-free survival (DFS) in BC patients after NCT.
Patients and methods: A total of 567 BC patients treated with NCT, from two independent centers, were included in this study. Cox proportional-hazards regression (CPHR) analysis was conducted to identify the independent prognostic factors for DFS, in order to develop a model. Subsequently, the discrimination and calibration ability of the prognostic model were assessed in terms of its concordance index (C-index), risk group stratification, and calibration curve. The performance of the nomogram was compared with that of the eighth edition of the AJCC TNM staging system via C-index.
Results: Based on the CPHR model, eight prognostic predictors were screened and entered into the nomogram. The prognostic model showed better performance (p<0.01) in terms of DFS prediction (C-index: 0.738; 95% CI: 0.698–0.779) than the eighth edition of the AJCC TNM staging system (C-index: 0.644; 95% CI: 0.604–0.684). Stratification into three risk groups highlighted significant differences between the survival curves in the training cohort and those in the validation cohort. The calibration curves for likelihood of 3- and 5-year DFS indicated optimal agreement between nomogram predictions and actual observations.
Conclusion: We constructed and externally validated a novel nomogram scoring system for individualized DFS estimation in BC patients treated with NCT. This user-friendly predictive tool may help oncologists to make optimal clinical decisions.
Keywords: breast cancer, neoadjuvant chemotherapy, disease-free survival, nomogram, prognosis
Introduction
Breast cancer (BC) remains the principal cause of cancer-related deaths in the People’s Republic of China1 and the second most common cause of cancer-related deaths worldwide.2 Neoadjuvant chemotherapy (NCT), which is increasingly offered to BC patients, may be used to downstage the primary tumor to enable breast-conserving surgery (BCS) and evaluate tumor chemosensitivity.3,4 Several studies have found that BC patients who attain pathologic complete response (pCR) after NCT exhibit improved survival.5,6 However, only 5%–38% of BC patients achieve pCR,7 meaning that the majority (who cannot attain pCR) have a higher risk of death and relapse.8 Therefore, the development of a practical tool for predicting survival in BC patients after NCT is necessary.
Currently, the eighth edition of the American Joint Committee on Cancer (AJCC) tumor-node-metastasis (TNM) staging system is widely used for cancer prognosis. In this system, patients with nonmetastatic BC are stratified by tumor size, invasion, and the extent of lymph node (LN) involvement. However, survival of BC patients after NCT varies greatly, even within the same stage. Some clinicopathological factors such as age, estrogen receptor (ER) status, histological grade, Ki67 status, and lymphovascular invasion (LVI) have been reported to be significantly associated with survival for BC patients treated with NCT.9–12 Therefore, the integration of an increased number of independent risk factors enables a practical tool to predict survival more accurately.
Nomograms are widely accepted as reliable tools in medicine, mainly because of their ability to provide an individual probability of an event by incorporating the important prognostic predictors.13 A nomogram, which is a user-friendly graphical prediction tool, could be used to obtain a numerical likelihood of an event, such as disease-free survival (DFS), for an individual patient.14 Nomograms have been shown to enable more accurate prediction for individual patients in diverse types of tumors, including extranodal NK/T-cell lymphoma, ovarian cancer, salivary gland cancer, BC, non-small-cell lung cancer, and nasopharyngeal carcinoma.15–21 However, prognostic nomograms for predicting survival and risk stratification in BC patients after NCT are scarce.
Therefore, the aim of this study was to establish a novel prognostic model incorporating the important clinicopathological variables for predicting DFS and risk stratification in BC patients after NCT.
Patients and methods
Patients and study design
A retrospective study was conducted, involving a training cohort of consecutive female BC patients who received NCT from August 2002 to December 2014 at the Sun Yat-sen Memorial Hospital (SYSMH) of Sun Yat-sen University. The eligibility criteria were as follows: 1) diagnosis with primary BC before NCT; 2) initial diagnosis without distant metastasis; 3) at least three cycles of NCT regimens before surgery; and 4) BCS or modified radical mastectomy.
On the basis of the same eligibility criteria, another independent cohort of consecutive female BC patients who had undergone NCT at the First People’s Hospital of Foshan (FPHF) between January 2009 and December 2011 were recruited as an external validation set. The following relevant medical information was retrospectively collected: demographic features; tumor-related characteristics (tumor size, extent of LN involvement, pathological type, histological grade, LVI, Ki67 status, ER status, progesterone receptor (PR) status, and HER2 status); treatment-related data (type of operation, NCT regimen, and pCR); and clinical outcome. The institutional review boards of SYSMH and FPHF approved this study. The requirement for informed consent was waived because of the retrospective nature of the study. We confirmed that the data from all the patients were anonymized in this study.
Pathological assessment
Pathologic TNM staging was performed using the AJCC TNM staging system, and pCR was defined as an absence of residual invasive carcinoma in both breast and LNs.10–12,22,23 ER, PR, and Ki67 statuses were evaluated by immunohistochemistry (IHC), which was conducted on formalin-fixed, paraffin-embedded tissue sections. The cutoff values for ER and PR expression (+) were set as 1%.24 HER2 status was estimated using IHC and/or fluorescence in situ hybridization (FISH). HER2 expression (+) was considered as 3 (+) by IHC or amplification identified by FISH.25,26 Ki67 status was classified as low expression (Ki67≤14%) or high expression (Ki67>14%).27 Hormone receptor (HR) expression (+) was determined as either ER+ or PR+, and both ER– and PR– were defined as HR–. BC subtypes were classified as follows: 1) HR+/HER2–; 2) HR+/HER2+; 3) HR–/HER2+; and 4) HR–/HER2–.28,29
NCT regimens, follow-up, and clinical outcome
In the NCT settings, anthracycline-based and/or taxane-based NCT regimens were used every 3 weeks: EC (epirubicin: 90 mg/m2, and cyclophosphamide: 600 mg/m2); TC (docetaxel: 75 mg/m2, and cyclophosphamide: 600 mg/m2); TEC (docetaxel: 75 mg/m2, epirubicin: 75 mg/m2, and cyclophosphamide: 500 mg/m2); CEF (cyclophosphamide: 500 mg/m2, epirubicin: 90 mg/m2, and 5-fluorouracil: 500 mg/m2); EC-T (epirubicin: 90 mg/m2, cyclophosphamide: 600 mg/m2, and sequential docetaxel 100 mg/m2); EC-TH (epirubicin: 90 mg/m2, cyclophosphamide: 600 mg/m2, and sequential docetaxel 100 mg/m2, trastuzumab: 8 mg/kg followed by 6 mg/kg); TCH (docetaxel: 75 mg/m2, carboplatin: area under the curve [AUC] =6 mg/mL/min, and trastuzumab: 8 mg/kg followed by 6 mg/kg). Follow-up was performed once every 3 months for the first 2 years after surgery, at intervals of every 6 months thereafter until 5 years, and at intervals of once every year subsequently after 5 years post surgery. Physical examinations were routinely conducted at follow-up visits, including blood tests, breast ultrasonography (US), abdominal US, gynecological US, and chest radiography, and a detailed medical history was taken. Mammography and magnetic resonance imaging were performed once every 1–2 years. Whole-body bone scan, biopsy, computed tomography scan, and positron emission tomography/computed tomography scans were conducted when appropriate. A relapse event was defined as the appearance of a newly detected local or regional relapse and distant metastasis. The primary study end point was DFS, which was determined from the date of surgery to relapse, second primary cancer, death, or the last follow-up.12,21
Establishment of the nomogram
First, univariate Cox proportional-hazards regression (CPHR) analysis was performed to identify the association between each prognostic factor and DFS. The potential risk factors were entered into the multivariate CPHR analysis (p<0.05). Then, the nomogram was constructed based on the multivariate CPHR model, as well as by consideration of both the clinical and statistical significances of the variables.
Validation and calibration of the nomogram
External validation of the model was conducted by 1,000 bootstrap resamples in the FPHF cohort. The discrimination ability of the model for predicting DFS was estimated using the C-index. In addition, the C-index of the model was compared with that of the eighth edition of the AJCC TNM staging system. Calibration of the model for 3- and 5-year DFS was carried out by comparing the nomogram-predicted survival with the actual survival.
Risk stratification based on the nomogram score system
On the basis of the prognostic model, risk scores were assigned for each variable. Considering the total risk scores in the SYSMH cohort, the X-tile statistical software was used to choose the optimal cutoff values of risk scores.30 Subsequently, the patients were classified as low-risk, intermediate-risk, and high-risk groups on the basis of the optimal cutoff values. The cutoff values were also used in the FPHF cohort to provide reliable risk stratification.
Statistical analyses
Descriptive analysis was performed for demographic and clinicopathological features. The optimal cutoff values of the risk scores were confirmed using X-tile.30 Survival curves of different factors values were evaluated via Kaplan–Meier method and compared using the log-rank test. Univariate and multivariate CPHR analyses were performed to screen the independent prognostic variables associated with DFS. Statistical significance was defined as p<0.05. Stata/MP, version 13.0 (StataCorp LP, College Station, TX, USA) and R version 3.4.1 were used for statistical analyses.
Results
Patient characteristics
The study participants comprised a total of 567 consecutive BC patients who underwent NCT, including the primary cohort from SYSMH (n=425) and the validation cohort from FPHF (n=142). The baseline characteristics of study patients are listed in Table 1. There were 177 events over a median follow-up time of 68 months (range: 1.9–183.3 months) in the SYSMH cohort and 54 events over a median follow-up time of 66.9 months (range: 2.2–92 months) in the FPHF cohort. In the entire cohort, the 3- and 5-year DFS rates were 74.0% and 67.6%, respectively, by the Kaplan–Meier method. The pCR rates were 11.5% and 10.6% in the training and validation groups, respectively.
Independent prognostic predictors in the SYSMH cohort
The results of the univariate CPHR analysis are shown in Table 2. Age (p=0.012), T stage (p<0.001), histological grade (p<0.001), N stage (p<0.001), ER status (p=0.022), Ki67 status (p<0.001), LVI (p<0.001), and pCR (p=0.018) were found to be significantly correlated with DFS. All the significant indicators in the univariate CPHR analysis were then entered into the multivariate CPHR analysis (p<0.05). The results of the multivariate CPHR analysis, listed in Table 2, indicate that age, T stage, histological grade, N stage, ER status, Ki67 status, and LVI were independent prognostic factors (p<0.05).
Prognostic model for DFS
Considering the clinical significance of pCR, we included pCR in the final prognostic model, without impairing the discrimination ability of the nomogram. Thus, a novel nomogram that combined the significant independent prognostic predictors with pCR was developed (Figure 1). According to this model, T stage made the largest contribution to DFS, followed by LVI, ER status, histological grade, and N stage. By calculating the total score, oncologists could easily obtain the nomogram-predicted probability of DFS for individual patients.
Assessment of the model
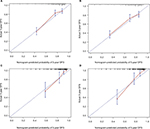
The C-index values for the nomogram were 0.738 (95% CI: 0.698–0.779) and 0.768 (95% CI: 0.706–0.831) in the SYSMH and FPHF cohorts, respectively. In the training cohort, the C-index of the prognostic model (0.738; 95% CI: 0.698–0.779) was significantly greater than that of the AJCC TNM staging system (0.644; 95% CI: 0.604–0.684; p<0.01). The C-index values of the nomogram were 0.735, 0.726, 0.784, and 0.711 in the four subtypes, which demonstrated that our nomogram was reliable and could predict prognosis regardless of the subtypes of BC. The calibration curves also showed an excellent agreement between the nomogram-predicted and actual probabilities of 3- and 5-year DFS, in both the SYSMH and FPHF cohorts (Figure 2).
Performance of risk stratification based on the nomogram score system
The point assignments and prognostic scores of each variable according to the nomogram score system are listed in Table 3. The optimal cutoff points were confirmed using X-tile. Thus, we achieved effective risk stratification into three groups (low risk: ≤22.1; intermediate risk: 22.1–33.8; high risk: >33.8). Additionally, Kaplan–Meier curves for DFS were constructed based on risk group stratification in both the training set and the validation set (Figure 3). In the entire cohort, the 5-year DFS rates were 85.1%, 57.5%, and 11.1% in the low-risk, intermediate-risk, and high-risk groups, respectively.
  | Table 3 Point assignment and prognostic score for each variable Abbreviations: LVI, lymphovascular invasion; ER, estrogen receptor; pCR, pathologic complete response. |
Discussion
In the present study, we established and externally validated a novel prognostic nomogram for individualized DFS estimation and risk stratification in BC patients after NCT. On the basis of univariate and multivariate CPHR analyses, age, T stage, histological grade, N stage, ER status, Ki67 status, and LVI were screened as the independent prognostic predictors, consistent with previous reports on the prognostic factors for BC after NCT.9–12 More recently, a study with a large sample size (n=1,033) found that post-NCT LVI was a strong independent risk factor for poor survival and strongly recommended including LVI in NCT score systems.9 We included LVI to improve the predictive accuracy of the nomogram, whereas previous models have ignored the predictive value of LVI for estimation survival.10–12 A previous Korean nomogram was reported to predict the probability of 2-year relapse-free survival.11 However, the Korean model did not taken into account histological grade and LVI as covariates, and the follow-up time was too short (<3 years). On the other hand, it should be noted that pCR was not an independent prognostic factor in our multivariate CPHR analysis. On the basis of both clinical and statistical significances, pCR was included without compromising the performance of the nomogram.14
Recently, the Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) 2017 meta-analysis indicated that patients who underwent NCT might have a higher probability of local recurrence than patients who did not undergo NCT.31 This finding suggested that a practical tool for predicting DFS in the NCT setting was imperative. To determine the generalizability of the model, it was necessary to assess the performance of the established nomogram. With regard to the discrimination ability, the C-index of our nomogram (0.738; 95% CI: 0.698–0.779) was superior to that of the AJCC TNM staging system (0.644; 95% CI: 0.604–0.684; p<0.01). This is not surprising, as the nomogram integrated more number of strong predictors to predict survival. Meanwhile, the calibration curves indicated an optimal agreement between the nomogram-predicted and actual probabilities of DFS, which confirmed the repeatability of our model.14 Thus, both oncologists and patients could obtain accurate, individualized estimates of DFS after NCT by using our user-friendly nomogram, with high discrimination and calibration ability.
To date, there has been no clear consensus regarding optimal post-NCT therapeutic schemes, especially adjuvant chemotherapy. Moreover, uncertainty remains over how to identify post-NCT patients at high risk of relapse. In the risk group stratification in this study, significant differences were found among the survival curves of the three risk groups in the primary and validation cohorts (p<0.001). Unsurprisingly, patients in the low-risk group had better DFS than those in the higher risk groups. More importantly, screening subgroups of high-risk patients for poor DFS might have a positive effect on treatment strategies. For example, in low-risk patients, it may be preferable to tailor adjuvant chemotherapy regimens to reduce side effects, such as by using a low cumulative dose and fewer cycles of the chemotherapy drug, especially in older patients. Conversely, intermediate- and high-risk patients might require more cycles of adequately dosed adjuvant chemotherapy to achieve optimal therapeutic outcomes, especially in younger patients. Intensive follow-up may also be imperative for intermediate- and high-risk patients. In addition, the prognostic tool could enable more rational risk stratification of patients when designing clinical trials.
Several potential limitations of the current study should be acknowledged. First, there was a lack of data concerning the use of adjuvant chemotherapy, antihormonal therapy, and anti-HER2 therapy, which are the important predictors for survival. Thus, future studies should incorporate these prognostic factors to enhance the nomogram. Second, the sample size of this retrospective study was limited. There was difference between the primary and external validation cohorts, which might be due to the relatively limited sample size. Therefore, the prognostic nomogram presented here should be prospectively tested in future research using a larger sample size with adequate information on the applied NCT regimens, dose intensity, and postneoadjuvant therapy strategies. Third, molecular biomarkers were not available for the vast majority of patients in this study. Hence, reliable molecular biomarkers should be included in future research to improve the predictive accuracy of the nomogram. Fourth, the included patients were from two centers in the People’s Republic of China, and the pCR rate was relatively low in this study. The most likely reason was that many patients with HER2-positive BC could not afford the high expenses of targeted therapy (trastuzumab), especially in the low-income families,32 since trastuzumab was not covered by health insurance until July 19, 2017, in the People’s Republic of China. Moreover, patients with HER2-positive BC were unable to receive treatment with trastuzumab until 2009 in the People’s Republic of China.32 These were the potential main causes for the low pCR rate in this study, which may have an influence on the nomogram. The prognostic model should therefore be validated in non-Asian patients before it is widely applied.
Conclusion
We have developed and externally validated a novel prognostic model for predicting DFS in BC patients treated with NCT. This user-friendly tool could enable oncologists to more accurately predict survival for individual patients after NCT and identify high-risk patients in need of a specific therapeutic scheme.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (ID: 81472731), Guangdong Natural Science Funds for Distinguished Young Scholar (ID: S20120011199), Foundation for the Author of National Excellent Doctoral Dissertation of the People’s Republic of China (FANEDD, 81000-3149001), the Key Laboratory of Malignant Tumor Gene Regulation and Target Therapy of Guangdong Higher Education Institutes, Sun Yat-sen University (ID: KLB09001), and the Key Laboratory of Malignant Tumor Molecular Mechanism and Translational Medicine of Guangzhou Bureau of Science and Information Technology (ID: [2013]163).
Author contributions
ZHP and JGL made substantial contributions to conception, design, analysis, and interpretation of data, and wrote and revised the manuscript. HLW collected and analyzed the data, as well as took part in the drafting of the manuscript. JWP and PXC helped to collect and analyze the data. All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work. All authors have read and approved the final version of the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
Chen W, Zheng R, Zeng H, Zhang S. The updated incidences and mortalities of major cancers in China, 2011. Chin J Cancer. 2015;34(11):502–507. | ||
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. | ||
Golshan M, Cirrincione CT, Sikov WM, et al; Alliance for Clinical Trials in Oncology. Impact of neoadjuvant chemotherapy in stage II-III triple negative breast cancer on eligibility for breast-conserving surgery and breast conservation rates: surgical results from CALGB 40603 (alliance). Ann Surg. 2015;262(3):434–439; discussion 438–439. | ||
Bear HD, Anderson S, Brown A, et al; National Surgical Adjuvant Breast and Bowel Project Protocol B-27. The effect on tumor response of adding sequential preoperative docetaxel to preoperative doxorubicin and cyclophosphamide: preliminary results from National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol. 2003;21(22):4165–4174. | ||
Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384(9938):164–172. | ||
Abrial SC, Penault-Llorca F, Delva R, et al. High prognostic significance of residual disease after neoadjuvant chemotherapy: a retrospective study in 710 patients with operable breast cancer. Breast Cancer Res Treat. 2005;94(3):255–263. | ||
von Minckwitz G, Untch M, Nuesch E, et al. Impact of treatment characteristics on response of different breast cancer phenotypes: pooled analysis of the German neo-adjuvant chemotherapy trials. Breast Cancer Res Treat. 2011;125(1):145–156. | ||
Mougalian SS, Soulos PR, Killelea BK, et al. Use of neoadjuvant chemotherapy for patients with stage I to III breast cancer in the United States. Cancer. 2015;121(15):2544–2552. | ||
Hamy A-S, Lam G-T, Laas E, et al. Lymphovascular invasion after neoadjuvant chemotherapy is strongly associated with poor prognosis in breast carcinoma. Breast Cancer Res Treat. 2018;169(2):295–304. | ||
Rouzier R, Pusztai L, Delaloge S, et al. Nomograms to predict pathologic complete response and metastasis-free survival after preoperative chemotherapy for breast cancer. J Clin Oncol. 2005;23(33):8331–8339. | ||
Keam B, Im SA, Park S, et al. Nomogram predicting clinical outcomes in breast cancer patients treated with neoadjuvant chemotherapy. J Cancer Res Clin Oncol. 2011;137(9):1301–1308. | ||
Colleoni M, Bagnardi V, Rotmensz N, et al. A risk score to predict disease-free survival in patients not achieving a pathological complete remission after preoperative chemotherapy for breast cancer. Ann Oncol. 2009;20(7):1178–1184. | ||
Balachandran VP, Gonen M, Smith JJ, DeMatteo RP. Nomograms in oncology: more than meets the eye. Lancet Oncol. 2015;16(4):e173–e180. | ||
Iasonos A, Schrag D, Raj GV, Panageas KS. How to build and interpret a nomogram for cancer prognosis. J Clin Oncol. 2008;26(8):1364–1370. | ||
Lu CH, Liu CT, Chang PH, et al. Develop and validation a nomogram to predict the recurrent probability in patients with major salivary gland cancer. J Cancer. 2017;8(12):2247–2255. | ||
Yang Y, Zhang YJ, Zhu Y, et al. Prognostic nomogram for overall survival in previously untreated patients with extranodal NK/T-cell lymphoma, nasal-type: a multicenter study. Leukemia. 2015;29(7):1571–1577. | ||
Lee CK, Simes RJ, Brown C, et al. A prognostic nomogram to predict overall survival in patients with platinum-sensitive recurrent ovarian cancer. Ann Oncol. 2013;24(4):937–943. | ||
Hirabayashi S, Kosugi S, Isobe Y, et al. Development and external validation of a nomogram for overall survival after curative resection in serosa-negative, locally advanced gastric cancer. Ann Oncol. 2014;25(6):1179–1184. | ||
Liang W, Zhang L, Jiang G, et al. Development and validation of a nomogram for predicting survival in patients with resected non-small-cell lung cancer. J Clin Oncol. 2015;33(8):861–869. | ||
Wang Y, Li J, Xia Y, et al. Prognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomy. J Clin Oncol. 2013;31(9):1188–1195. | ||
Tang LQ, Li CF, Li J, et al. Establishment and validation of prognostic nomograms for endemic nasopharyngeal carcinoma. J Natl Cancer Inst. 2016;108(1):djv291. | ||
Mazouni C, Peintinger F, Wan-Kau S, et al. Residual ductal carcinoma in situ in patients with complete eradication of invasive breast cancer after neoadjuvant chemotherapy does not adversely affect patient outcome. J Clin Oncol. 2007;25(19):2650–2655. | ||
Tadros AB, Yang WT, Krishnamurthy S, et al. Identification of patients with documented pathologic complete response in the breast after neoadjuvant chemotherapy for omission of axillary surgery. JAMA Surg. 2017;152(7):665–670. | ||
Hammond ME, Hayes DF, Dowsett M, et al; American Society of Clinical Oncology; College of American Pathologists. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010;28(16):2784–2795. | ||
Perez EA, Dueck AC, McCullough AE, et al. Predictability of adjuvant trastuzumab benefit in N9831 patients using the ASCO/CAP HER2-positivity criteria. J Natl Cancer Inst. 2012;104(2):159–162. | ||
Wolff AC, Hammond ME, Schwartz JN, et al; American Society of Clinical Oncology/College of American Pathologists. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25(1):118–145. | ||
Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thurlimann B, Senn HJ. Strategies for subtypes – dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the primary therapy of early breast cancer 2011. Ann Oncol. 2011;22(8):1736–1747. | ||
Swisher SK, Vila J, Tucker SL, et al. Locoregional control according to breast cancer subtype and response to neoadjuvant chemotherapy in breast cancer patients undergoing breast-conserving therapy. Ann Surg Oncol. 2016;23(3):749–756. | ||
Houssami N, Macaskill P, von Minckwitz G, Marinovich ML, Mamounas E. Meta-analysis of the association of breast cancer subtype and pathologic complete response to neoadjuvant chemotherapy. Eur J Cancer. 2012;48(18):3342–3354. | ||
Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004;10(21):7252–7259. | ||
Asselain B, Barlow W, Bartlett J, et al. Long-term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: meta-analysis of individual patient data from ten randomised trials. Lancet Oncol. 2017;19(1):27–39. | ||
Zhou P, Jiang YZ, Hu X, et al. Clinicopathological characteristics of patients with HER2-positive breast cancer and the efficacy of trastuzumab in the People’s Republic of China. Onco Targets Ther. 2016;9:2287–2295. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.