Back to Journals » International Journal of General Medicine » Volume 15
Establishment and Evaluation of an Internal Quality Control Method for ESR Relays
Authors Fu S , Zhang L, Hu QL, Li ZJ
Received 16 December 2021
Accepted for publication 1 March 2022
Published 22 March 2022 Volume 2022:15 Pages 3247—3254
DOI https://doi.org/10.2147/IJGM.S354260
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Shui Fu,1 Liang Zhang,1 Qi-Lei Hu,1,2 Zuo-Jie Li3
1Department of Clinical Laboratory, Linping Campus, The Second Affiliated Hospital of Zhejiang University School of Medicine, Hangzhou, Zhejiang, 311000, People’s Republic of China; 2Quality Management Section, Linping Campus, The Second Affiliated Hospital of Zhejiang University School of Medicine, Hangzhou, Zhejiang, 311100, People’s Republic of China; 3Department of Clinical Laboratory, The People’s Hospital of Cangnan Zhejiang, Cangnan County, Zhejiang, 325800, People’s Republic of China
Correspondence: Zuo-Jie Li, Department of Clinical Laboratory, The People’s Hospital of Cangnan Zhejiang, No. 2288 Yucang Road, Cangnan County, Zhejiang, 325800, People’s Republic of China, Tel +86 577-64810162, Email [email protected]
Objective: This study establish and evaluate an internal quality control system for erythrocyte sedimentation rate (ESR) by a “relay” mode based on samples from relevant patients.
Methods: The method for establishing a new internal quality control system for ESR by a “relay” mode based on patient’s samples was executed from February 2021 to July 2021. In this paper, a total of 219 outpatients were recruited for ESR determination, and their blood samples were stored at 4 °C or room temperature for 24 h. Subsequently, the samples were re-measured for ESR, and the re-measured values were compared with the initial values. The patient samples (15± 1mm/h and 50± 3mm/h) were selected after the TEST1 ESR analyzer was calibrated, and were stored overnight at 4 °C and measured again the following day. The percentage deviation was determined and entered into the quality control management module for internal quality control. Next, we analyzed the median distribution trend of the patients’ ESR values measured by our laboratory every day over five months, as well as the external quality assessment (EQA) results for ESR obtained from the National Center for Clinical Laboratories (NCCL).
Results: The ESR of the room temperature samples after 24 h of storage had significantly decreased (P=0.001), while there was no noticeable difference for those stored at 4 °C (P=0.197). Results of the internal quality control in March were satisfactory, and there was no significant deviation in the median ESR relay results within five months. Besides, the EQA results for the ESR data obtained from NCCL were excellent.
Conclusion: As a precise and practical new method, the ESR relay internal quality control method can be used to scientifically determine the stability and accuracy of the TEST1 ESR analyzer.
Keywords: erythrocyte sedimentation rate, ESR, fully automatic ESR analyzer, quality control, fresh blood sample, Westgard rules
As one of the most commonly used nonspecific indicators in clinical diagnosis and prognosis evaluation, erythrocyte sedimentation rate (ESR) has the twin benefits of low cost and high efficiency.1,2 The TEST1 ESR Analyzer (Alifax, Italy) is now widely used for rapid ESR testing as an alternative to the traditional quantitative capillary spectrophotometry. In 2017, this method was listed by the International Council for Standardization in Haematology (ICSH) as a practical alternative to the Westergren method for ESR testing.3,4 Good practice for internal quality control is an important component in the overall quality system of a Laboratory and is also the foundation for guaranteeing the accuracy of test results.5,6 Applying operating calibrator and quality control for the TEST1 ESR Analyzer are both costly and long ordering period and matrix effect,7 because the equipment is imported, although the charge for taking an ESR test is very low. Therefore, it is difficult to comprehensively apply quality control procedures regularly. In addition, Operators may pay more attention to data changes of the commercial quality control system itself while ignoring variations in the samples, which is the real purpose of quality control. This makes it very difficult to improve the quality control of sample analysis. Since ordinary fresh blood samples are the most natural and ideal quality controls, a scientific and practical internal quality control method based on fresh blood samples may generate great social value and economic benefit. In recent years, there have been several studies on self-developed ESR quality control methods, but it is still difficult to promote them in laboratories because whole blood samples are difficult to store.8,9 Based on the medical decision level (MDL) and the bias of external quality assessment criterion (EQA) for ESR according to the National Center for Clinical Laboratories (NCCL), our laboratory selected two levels of fresh blood samples to establish a new method for the internal quality control of the ESR relay by utilizing software from the quality control management module of the laboratory information management system (LIMS). Subsequently, we carried out relevant evaluations using big data distribution for patients who were given ESR tests by our laboratory, as well as EQA results for ESR obtained from NCCL.
Subjects and Methods
Subjects
A total of 219 outpatients who received relevant testing in our Hospital in February 2021 were included in this study. Among them, there were 88 males with an average age of 46 years (range: 2 ~ 89 years) and 131 females with an average age of 49 years (range: 1 ~ 87 years).
Instruments and Reagents
The TEST1 ESR Analyzer (Alifax, Italy) was used for ESR testing and was operated according to the relevant calibrator. Besides, the laboratory information management system (LIMS) from Lianzhong Medical (Hangzhou Lianzhong Medical Science Co., Ltd.) was utilized for the internal quality control and management of ESR.
The study flow
For the establishment and verification process of the ESR relay internal quality control method, see Figure 1.
 |
Figure 1 Establishment and verification process of ESR relay internal quality control. Abbreviation: NHC, National Health Commission of the People’s Republic of China. |
Methods
Analysis of the Stability of ESR Samples Stored at Different Temperatures
Two tubes of 2 mL EDTA-K2 venous blood were collected from each of the 219 subjects and tested on the TEST1 ESR Analyzer within 1 hour of collection. For each patient, the average value of the two ESR tests was used as the initial ESR value. Then, one tube was stored at room temperature for 24 h, while the other tube was stored at 4 °C for 24 h. On the following day, ESR tests were performed using the same equipment for the samples in both tubes. Subsequently, a statistical analysis was performed on the two sets of data.
Requirements and Testing of Quality Control for the ESR Relay Internal Quality Control Method
Every day, two fresh blood samples containing EDTA-K2 anticoagulant were taken from the different patients. These two samples, which excluded patients with cold agglutinin disease, acted as quality controls, had ESR values of 15±1mm/h and 50±3mm/h, based on the medical decision level (MDL) and EQA criterion for the ESR according to the NCCL. The samples were tested immediately after calibration of the TEST1 ESR Analyzer, according to the requirements for quality control mentioned above. Two quality control samples were stored overnight in a refrigerator at 4 °C. On the next day, the samples were removed from refrigeration and warmed to room temperature, before being tested. Thereafter, the above practice was repeated every day using a controlled approach. The samples with test results within the tolerance were discarded, while those with test results beyond the control levels were tested again, with 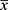 denoting the test result.
denoting the test result.
Setting and Calculation of Variables for ESR Relay Internal Quality Control Method
Here, we established values for the “measured value”, “target value”, “standard deviation” and “coefficient of variation”.
The deviation percentage of the two levels was calculated every day except for the first day, and the calculated results were taken as the “measured values”, which were input into the LIMS system as quality control data. The initial “target value” were the comprehensive percentage deviation based on the stability of the ESR samples during the relay interval (24 h). It was a value located within the normal range and the abnormally high value. Moreover, a third of the allowable biases for the value within the normal range and the abnormally high value were based on the quality evaluation of NHC as the “standard deviation”. Besides, the subsequent “target value” and “standard deviation” accumulated by the LIMS system were the “target value” and “standard deviation” of the following month. This practice was repeated for three to five months. The coefficient of variation adopted a value equal to double the standard deviation.
Rules and Application of Relay Internal Quality Control Method
The Westgard multiple rule approach (12s, 13s, 22s, R4s, 41s, and 10x), which is built into the LIMS system, was used for relevant determination. Based on the established quality control rules, if a run was out of control, the type of error could be determined and the source of the error could also be identified. Thus, relevant adjustments could be accurately made according to the standard operating procedures for internal quality control.
Evaluation of Relevant Relay Internal Quality Control Method
The trend of the median ESR for the patients based on daily testing using the TEST1 ESR Analyzer was assessed, to evaluate the stability and verify the relay internal quality control method. Furthermore, while running the relay internal quality control, our laboratory participated in the EQA for ESR program with the NCCL. The EQA results further verified the accuracy of the relay internal quality control method.
Statistical Analysis
For relevant data processing, we used SPSS 26.0 software. Also, the Kolmogorov–Smirnov normality test was used for the data distribution analysis, with data of abnormal distribution expressed as M(Q1,Q3). The result of the rank sum test was used for comparison between groups that did not follow a normal distribution, and values of P<0. 05 signified that the differences had statistical significance.
Results
Change in ESR of Samples After Storage for 24 h at Different Temperatures
The ESR of samples decreased by 20% (P=0.001) after 24 h of storage at room temperature but decreased by merely 8.45% (P=0.197) after being stored at 4 °C for 24 h. However, the difference did not qualify as being statistically significant (P=0.197). The results are presented in Table 1 and Figure 2.
 |
Table 1 Change in ESR of Samples Stored for 24 h at Different Temperatures |
 |
Figure 2 Comparison with initial ESR for samples stored for 24 h at different temperatures. |
Establishment of ESR Relay Internal Quality Control Method
After we kept the samples at 4 °C for 24 h, we firstly decreased the relevant value within the normal range by about 8.65% and the abnormally high value by about 7.23%. They were set as the initial target values of two quality control levels, with 1/3 of allowable biases for the value within the normal range and abnormally high value, based on the quality evaluation of NHC (6.85% and 6.63%) as the “standard deviation”. Finally, the ESR relay internal quality control chart (see Figure 3 for an example from March 2021) was completed according to the guidelines described in the document entitled “Internal quality control for quantitative measurement in clinical laboratories”.10
 |
Figure 3 ESR relay internal quality control chart from March 2021. |
Verification of Internal Quality Control Method for ESR Relay
Consistency of ESR Results Obtained Using the TEST1 ESR Analyzer
From March to July 2021, there was no significant divergence in the median ESR for the patients. The maximum value was 22mm/h while the minimum value was 10mm/h, The median ESR of patients everyday was stabilized at 16±3mm/h for a long period, with no more than 2 standard deviations out of control, suggesting that the performance of the TEST1 ESR Analyzer was stable throughout this period. Figure 4 provides details of the median ESR values during these months.
 |
Figure 4 Median ESR for patients from March to July 2021. |
Comparability of ESR Results Based on the TEST1 ESR Analyzer
The median ESR values from March to July 2021 were compared between the months, and the percentage differences between the months are listed in Table 2. The largest median difference (Value A), 2×bias limit (Value B), and the ratio of Value A to Value B are also presented here. An A/B ratio of 0.52 ≤2 means that there is high comparability between months, since it is below the threshold level.11
 |
Table 2 Comparison of Median ESR Between Months from March to July 2021 |
EQA Results for ESR in the First Set of Data in 2021 Obtained from the NCCL
The maximum bias of the three samples was −4.26%, which was far lower than the allowable bias of about ±20%, as Table 3 shows.
 |
Table 3 EQA Results for ESR Obtained from NCCL (mm/h) |
Discussion
In this study, two fresh blood samples were collected from each outpatient. After initial testing, one sample was stored at 4 °C while the other was stored at room temperature for 24 h. Then they were measured for ESR again. The re-measured ESR values were compared with the average initial ESR values, thus avoiding accidental errors caused by a single flawed test. It was discovered that the ESR results of outpatients were abnormal distribution, which was inconsistent with the results of Jiang Fei12 and Yu Xiaoyang,13 and might be due to the different sample sources. Besides, results from this study show that the ESR value fell for both sets of samples after 24 h of storage, but the decrease rate for the 4 °C samples was significantly smaller than for the samples kept at room temperature. This was different from the results of Yu Xiaoyang,13 but generally consistent with the results from the studies of Jiang Fei12 and Mario Plebani,8 who used different test methods from Yu13. Therefore, in this study, the fresh blood samples used for quality control were stored at 4 °C for TEST1 ESR analysis the next day.
The deviation between batches for the ESR of samples in a specified concentration range was taken as the measured value for this study. Also, the indeterminate absolute deviation for ESR was converted into an associated stable relative deviation. Thus, the inaccuracies associated with the variability of samples in ESR were effectively overcome. Furthermore, the quality control management module in the LIMS system was used to calculate the standard deviation and automatically determine internal quality control results, thus avoiding complicated calculations and manual interpretations of formulas. Based on this, the internal quality control for ESR was implemented strictly following the “Internal quality control for quantitative measurement in clinical laboratories document”. In March 2021, the internal quality control of the ESR relay was good, and the daily errors were all within the permissible range. This study adopted the calculated relative deviation as the measured value, and the variation coefficient of the LIMS system was based on the process overlap obtained by a calculation using the standard deviation/target value of the quantitative results. Therefore, the variation coefficient in the LIMS system quality control chart is not a real variations of relay internal quality control. This is one deficiency of this study that needs to be addressed. For the variation coefficient of ESR relay internal quality control, we adopted a value equal to double the standard deviation. This study is consistent with the study of Wei Zhiwu14 in principle but slightly different in method. In the study of Wei Zhiwu, relevant parameters were first manually calculated based on formulas, then a quality control chart was constructed. This study took full advantage of the quality control management module in the LIMS system to calculate relevant parameters automatically and determine whether they were outside the given thresholds based on the quality control rules that had been set. This can not only reduce errors but also improve the efficiency and quality of the relevant work. Giavarina et al15 utilized the absolute difference of fresh blood samples over two consecutive days for the internal quality control of ESR, and many false alarms or outlying results were discovered within one month. In this study, the internal quality control of ESR was carried out after calculating the relative deviation of fresh blood samples across two consecutive days. By conducting tests in this manner the in adequacies of the study of Giavarina et al.15 were effectively avoided. Overall, results showed that the internal quality control of the ESR analysis was satisfactory.
The moving median method based on the laboratory data of patients can be used to supplement evidence for routine internal quality control.7,11 In this study, five months of ESR results revealed no significant threshold excursion of the median ESR, which is consistent with the fact that no outlying results were found in the internal quality control system established by our laboratory. These results are consistent with the study of Plebani,8 thus verifying the reliability of the ESR relay internal quality control method. Furthermore, our laboratory participated in an EQA program for ESR with the National Center for Clinical Laboratories (NCCL), and the EQA results were excellent. All three of the samples were close to the target value (the maximum deviation was –4.26%), without homolateral excursion. This further verifies the accuracy of the TEST1 ESR Analyzer in ESR testing, and also proves that the ESR relay internal quality control method was precise. Considering the high cost of commercial ESR quality control products, in this study, we did not complete internal quality control of a commercial ESR system synchronously. Thus, there was no comparison between the proposed and commercial ESR internal quality control methods, which is the main limitation of this study. Our comparison has been included in the plan for subsequent research so that the ESR relay internal quality control method may be evaluated more comprehensively and systematically.
In summary, the ESR relay internal quality control method and the conventional method based on commercial quality control are essentially the same, but different materials are utilized for the quality control process. By utilizing the “measured value” obtained by the data exchange and adjusting the relay method, an effect similar to the Levey-Jennings rule based on quality controls can be achieved. In other words, the two methods can reach the same goal by different means. Additionally, for the ESR relay internal quality control method, the cost is low and there is no matrix effect or interchangeability. The ESR relay internal quality control method is revolutionary of the conventional method, which may be very conducive to its wider application. The proposed internal quality control method for ESR may be used as an example of internal quality control for other tests using fresh blood samples.
Conclusion
The ESR relay internal quality control method not only lowers costs but also effectively eliminates the matrix effect. Therefore, it should be implemented in laboratories due to its reliability and practicability. The relevant steps of the process are as follows: First, identify conditions of the samples under a steady state within 24 h. Next, use these samples as the quality control products, depending on the medically determined level and the biologically reasonable level of the coefficient of variation. Then, utilize relevant information technology to construct a convenient and scientific internal quality control system. Finally, verify the system by a variety of methods, and if relevant results are satisfactory, it can be employed in practical situations.
Ethics Approval and Consent to Participate
This study was conducted with approval from the Ethics Committee of Linping Campus, The Second Affiliated Hospital of Zhejiang University School of Medicine. This study was conducted in accordance with the declaration of Helsinki. Written informed consent was obtained from all participants. And the patient guardians under 18 years of age provided informed consent.
Acknowledgments
We would like to acknowledge the hard and dedicated work of all the staff that implemented the intervention and evaluation components of the study.
Funding
There is no funding to report.
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Lapić I, Padoan A, Bozzato D, et al. Erythrocyte sedimentation rate and c-reactive protein in acute inflammation. Am J Clin Pathol. 2020;153(1):14–29.
2. Ali A, Abbasi AS, Amjad T, et al. Erythrocyte sedimentation rate and C-reactive protein as marker of acute versus chronic medical conditions. J Ayub Med Coll Abbottabad. 2019;31(1):39–45.
3. Ilardo C, Richerd C, Rostain V. Impact of preanalytical storage on the measurement of erythrocyte sedimentation rate using an infrared microphotometersystem (TEST1). Scand J Clin Lab Invest. 2020;80(6):523–524. doi:10.1080/00365513.2020.1786887
4. Kratz A, Plebani M, Peng M, et al.; International Council for Standardization in Haematology (ICSH). ICSH recommendations for modified and alternate methods measuring the erythrocyte sedimentation rate. Int J Lab Hematol. 2017;39(5):448–457. doi:10.1111/ijlh.12693.
5. Wu ZQ, Xu HG. Comparison of two commercial quality control sera for adrenocorticotropin (ACTH) used in Elecsys® immunoassay system. J Clin Lab Anal. 2019;33(1):e22618. doi:10.1002/jcla.22618
6. Zhang S, Wang W, Zhao H, et al. Status of internal quality control for thyroid hormones immunoassays from 2011 to 2016 in China. J Clin Lab Anal. 2018;32(1):e22154. doi:10.1002/jcla.22154
7. Shukang HE, Wei WANG, Yuxuan DU. Research and progress of clinical laboratory based on patient data for indoor quality control. Int J Lab Med. 2020;41(11):1390–1395. doi:10.3969/j.issn.1673-4130.2020.11.026
8. MarioPlebani M, Piva E. Erythrocyte sedimentation rate: use of fresh blood for quality control. Am J Clin Pathol. 2002;117(4):621–626.
9. Cuiling ZHENG, Li WANG, Yan CHENG. Fresh blood comparison program in daily internal quality control of hematology analyzers. Lab Med. 2021;36(6):662–666. doi:10.3969/j.issn.1673-8640.2021.06.018
10. National Health Commission of the People’s Republic of China. WS/T 641-2018 “Internal quality control for quantitative measurement in clinical laboratory “. Ministry of Health, the People’s Republic of China; 2018.
11. Xiaoyan ZHANG, Wei WANG, Haijian ZHAO, Zhiguo. WANG. Use the median of patient data to assess and monitor the comparability and stability of clinical test indicators. Chin J Clin Lab Sci. 2016;34(7):599–601. doi:10.13602/j.cnki.jcls.2016.07.17
12. Fei JIANG, Shenlin. RUAN. Evaluation of the quality monitoring effect of automatic erythrocyte sedimentation rate analyzer. Lab Med. 2020;35(6):624–625. doi:10.3969/j.issn.1673-8640.2020.06.022
13. Xiaoyang Y, Chengming X. The influence of time and temperature placing the samples on the ESR results. Guangzhou Med J. 2013;44(2):50–51. doi:10.3969/j.issn.1000-8535.2013.02.023
14. Zhi-wu WEI, Jun JU. Initial applications of quality controlling Method for sample following-detection. J Mod Lab Med. 2011;26(2):15–18. doi:10.3969/j.issn.1671-7414.2011.02.005
15. Giavarina D, Capuzzo S, Cauduro F, et al. Internal quality control for erythrocyte sedimentation rate measured by TEST-1 analyzer. Clin Lab. 2002;48(9–10):459–462.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

