Back to Journals » Local and Regional Anesthesia » Volume 13
Establishing a Technique for Pectoral II–Block Catheter Insertion with Ultrasound Guidance: A Randomized Controlled Trial
Authors Mansour MA , Fouad AZ, Amin SM , Dobal NM
Received 15 May 2020
Accepted for publication 20 July 2020
Published 11 August 2020 Volume 2020:13 Pages 85—93
DOI https://doi.org/10.2147/LRA.S262138
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Stefan Wirz
Mohamed A Mansour, Ahmed Z Fouad, Sarah M Amin, Nasser M Dobal
Department of Anesthesia, Intensive Care and Pain Management, Kasr Al Ainy Faculty of Medicine, Cairo University, Cairo, Egypt
Correspondence: Mohamed A Mansour
Intensive Care and Pain Management, Kasr Al Ainy Faculty of Medicine, Cairo University, Cairo, Egypt
Tel +20 11-1144-4058
Email [email protected]
Purpose: To assess the efficacy and safety of a modified technique for ultrasound-guided pectoral II block for postoperative pain control after mastectomy.
Methods: In this randomized controlled trial, patients were randomly allocated into two groups (40 patients each). Group I patients were subjected to ultrasound-guided pectoral II block with injection of 10 mL lidocaine 1% as a dissecting solution before attempting catheter insertion, while group II patients underwent the standard procedure without a dissecting solution. Measured outcomes included catheter visibility, pain, patient satisfaction, performance time, and complications.
Results: Compared with group II, group I had significantly lower median catheter-visibility scores, shorter block performance time, and fewer insertion attempts. Group I had a nonsignificantly higher rate of complications than group II.
Conclusion: The modified technique facilitated the procedure, shortened the catheter-insertion time, and showed higher patient satisfaction. However, it was associated with lower catheter visibility on ultrasonography. Further studies are required to confirm the present findings and assess the safety of the modified technique.
Keywords: analgesia, mastectomy, regional anesthesia, pectoral nerve block, postoperative pain
Introduction
Patients undergoing chest wall surgeries, such as mastectomy and anterior thoracotomy, may suffer from acute postoperative pain. Inadequately managed postoperative pain may result in impairment of pulmonary and immune-system functions. Moreover, the risk of serious complications, such as ileus, thromboembolism, and myocardial infarction, is higher in patients suffering from postoperative pain.1 In addition, a considerable proportion of patients — 25%–60% — may experience chronic pain.2 Consequently, postoperative pain may delay hospital discharge, which in turn increases health-care expenditure.3
The sole use of pain medications is not recommended, as a considerable proportion of patients may suffer adverse effects. Anesthesiologists used to achieve adequate pain control by performing paravertebral blocks or thoracic epidural analgesia.4 However, many complications were reported for both techniques. Complications that may result from thoracic paravertebral blocks are related to inadvertent puncture of blood vessels, epidural or intrathecal spaces, and pleura.5 Epidural analgesia causes hypotension and diverts blood away from the denervated flap to normal tissue, a complication that is known as steal phenomenon.6
In 2011, Blanco7 described a technique for pectoral nerve block in patients undergoing mastectomy and insertion of subpectoral implants by injecting local anesthetics between the pectoralis major and pectoralis minor muscles, which was later termed pectoral I block. The procedure was effective in most patients, but failed to control the pain adequately if the axilla were involved in the surgery. In 2012, Blanco et al8 proposed a solution to this problem by performing a second injection at the level of the third and fourth ribs into the plane between the pectoralis minor and serratus anterior muscles, which was termed pectoral II block. The two procedures can be performed under ultrasound guidance.7,8 Pectoral I block provides blockade to the medial and lateral pectoral nerves, while pectoral II block additionally targets the long thoracic nerve, thoracodorsal nerve, and anterior divisions of the thoracic intercostal nerves from T2 to T6.8–10 Pectoral blocks gained wide acceptance among anesthesiologists, as they are superficial, simple, effective, and relatively safe procedures that provide adequate postoperative analgesia (as evidenced by decreased pain scores and reduced need of postoperative pain medications).11–13 Several studies have reported the use of pectoral blocks in conjunction with superficial chest-wall surgeries.11–15
The space between the pectoralis minor and the serratus anterior muscles is a fascial plane that is occupied by connective tissue.10 We hypothesized that the use of a dissection fluid may help facilitate the expansion of the local anesthetic within this fascial plane to reach the targeted nerves. The present study assessed the efficacy and safety of a modified technique for ultrasound-guided pectoral II block for postoperative pain control following mastectomy. Catheter insertion was facilitated by injecting 10 mL lidocaine 1% as a dissecting solution. The study objectives included catheter visibility as a primary outcome, in addition to block performance time, number of catheter-insertion attempts, duration of the block, patient satisfaction, and incidence of complications as secondary outcomes.
Methods
Study Design and Settings
This randomized controlled clinical trial was carried out on patients scheduled for simple mastectomy from November 2018 to August 2019 at Kasr Al Ainy Hospital. Informed consent was obtained from each patient. This study was conducted in accordance with the Declaration of Helsinki. The trial protocol obtained approval from the Research Ethics Committee, Kasr Al Ainy Faculty of Medicine, Cairo University, Cairo, Egypt (N-26-2018). The trial was registered at ClinicalTrials.gov (NCT03030677). We intend to share the study protocol, as well as individual deidentified participant data. Data will be accessible through direct contact with the corresponding author, beginning at 12 months and ending 36 months following article publication.
Eligibility Criteria
Female patients 21–60 years old with American Society of Anesthesiologists (ASA) physical status I– III scheduled for simple mastectomy surgery were included in the present study. Exclusion criteria included administration of anticoagulants, presence of chest-wall infection, and patient refusal.
Randomization and Blinding
Patients were randomly allocated by a closed-envelope method into two groups (40 patients each). Group I was scheduled for ultrasound-guided pectoral II block with catheter insertion after injection of 10 mL lidocaine 1% as a dissecting solution. Group II patients were scheduled for ultrasound-guided pectoral II block with direct catheter insertion. Generation of random-allocation sequence was carried out using computer software (www.randomizer.org) to obtain two sets of random numbers. Prior to performing the block, an experienced anesthesiologist chose a slip of paper from a dark envelope that contained two slips, with each slip marked with one of the two patient groups. Whichever group was written on the paper determined the block technique that was used in that patient. Blinding of patients or the surgical team was not feasible in the present study.
Interventions
After arrival of the patients at the operating room, a five-lead electrocardiogram, pulse oximeter, and noninvasive blood-pressure monitor were applied. A peripheral venous cannula was inserted contralaterally to the surgical site and saline solution started at 2 mL/kg/hour. Premedication with 0.1 mg/kg midazolam was performed. Surgery was done under general anesthesia (GA) using intravenous fentanyl 2 µg/kg, propofol 2 mg/kg, laryngeal mask, and inhaled isoflurane anesthetic. No other analgesic was given intraoperatively.
Prior to surgical incision and after the induction of GA, the ultrasound-guided pectoral block was performed as described by Blanco et al8 with an ultrasound machine (Siemens, Acuson X300) equipped with a linear transducer (8–15 MH). The ultrasound probe was initially placed under the lateral third of the clavicle to identify the location of the subclavian muscle, axillary artery, and axillary vein. Then, the probe was moved distally toward the axilla until the pectoralis minor muscle was identified. From this point, the ribs were counted while the probe was moved caudally and laterally until the lateral border of pectoralis minor muscle was identified between the third and fourth ribs, where the edge of the serratus anterior muscle was visualized (Figure 1). Levobupivacaine 10 mL 0.25% was injected between the pectoralis major and minor muscles. Then, according to the intervention group, either 10 mL lidocaine 1% was injected as a dissecting solution before attempting catheter insertion (group I) or catheter insertion was directly attempted without a dissecting solution (group II). A catheter kit (Silverstim 18 G, 50 mm needle, including a catheter 20 G, 300 mm with an echogenic tip; Vygon) was used for continuous infusion. The catheter was inserted in the needle and advanced 3 cm beyond the needle tip. The needle was then withdrawn and the catheter tunneled and fixed with sterile medication (Figures 2 and 3). The procedure was completed after confirming the position of the echogenic catheter tip in the correct intramuscular plane using the ultrasound probe. Under the pectoralis minor muscle and above the serratus anterior muscle, 20 mL (5 mL lidocaine 1% and 15 mL bupivacaine 0.25%) were injected in group I, while 30 mL (15 mL lidocaine 1% and 15 mL bupivacaine 0.25%) were injected in group II. The procedure was performed by an anesthesiologist skilled in performing ultrasound-guided pectoral blocks. Surgery was only commenced 15 minutes after finalizing the pectoral block.
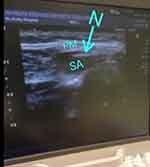 |
Figure 1 Needle between pectoralis major and serratus muscles. |
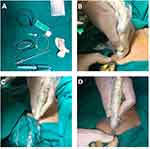 |
Figure 2 (A) Set prepared; (B) needle insertion; (C) injection of dissecting volume; (D) catheter threading. |
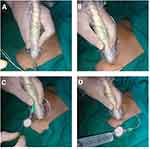 |
Figure 3 (A) Catheter threading; (B) confirming catheter position; (C) catheter ready for injection; (D) injecting the rest. |
At the end of the surgery and after recovery, a continuous-infusion pump was connected to the catheter. An infusion regimen was started using local anesthetic (plain bupivacaine 0.125% + fentanyl 2 µg/mL) at a rate of 6–10 mL/hr for 24 hours. The catheter was removed after 24 hours. The quality of sensory block was assessed by bilateral application of ice over the breast area 30 minutes postprocedure once the patient was alert and oriented in the recovery room. Assessment was done using the 3-point scale for sensory assessment (0 = complete loss, 1 = partial loss, 2 = normal sensation). Pain intensity was measured using the VAS (1–10), starting 30 minutes after transfer to the recovery room and repeated every hour. If VAS score was >3, the patient was given 1 g paracetamol infusion over 20 minutes, and if pain persisted after 20 minutes, 0.05 mg/kg intravenous morphine was given. If pain persisted for another 20 minutes, another dose of morphine 0.025 mg/kg was given. The overall level of patient satisfaction with the procedure and postoperative analgesia were assessed using a 0- to 3-point scale (0 = poor, 1 = good, 2 = very good, 3 = excellent).
Outcomes
The primary outcome was the catheter position confirmed by ultrasound (catheter-visibility score), which was recorded on a scale from 1 to 3 (1 = poor, 2 = good, 3 = excellent), where visualization of the whole echogenic catheter tip or at least the tip of the catheter in place was considered excellent, visualization of “none” of the echogenic catheter tip as poor, and partial visualization of the echogenic catheter tip as good. Secondary outcomes were block performance time (time from applying the probe to the skin till finishing the injection of the local anesthetic), number of catheter-insertion attempts, duration of the block, patient satisfaction, and incidence of complications.
Sample Size
To the best of the authors’ knowledge, no previous studies have evaluated catheter or needle visibility in pectoral II block. Sample size was calculated using MedCalc version 15.8. The calculation was based on VAS scores, as reported in a previous paper,16 assuming an effect size (mean difference in VAS score) of 1, SD in both groups of 1.5, statistical power of 80%, α-error of 5%, CI of 95%, and significance at p<0.05. The minimum sample size for each of the two study groups was 37 patients. We enrolled 40 cases per group to accommodate for dropouts.
Statistical Analysis
Statistical analysis was carried out using SPSS version 22. Categorical data are expressed as numbers and percentages and compared using Fisher’s exact or Fisher–Freeman–Halton exact tests. Continuous data were first tested for normality with the Shapiro–Wilk test. Normally distributed data are expressed as means ± SD and were compared with independent-sample t-tests. Data that did not follow normal distribution are presented as medians and IQRs (expressed as 25th–75th percentiles). Comparison of abnormally distributed continuous data was done using the Mann–Whitney U test. p<0.05 was considered statistically significant.
Results
This study included 80 patients aged 20–40 years with ASA physical status I– III scheduled for mastectomy. Out of 103 patients, 80 fulfilled the eligibility criteria, while 23 were excluded: 12 refused to participate, seven were administering anticoagulants, and four had chest-wall infection. Eligible patients were randomly allocated into groups I and II (40 patients each). Both groups underwent pectoral II block by ultrasound-guided catheter insertion. Lidocaine 10 mL 1% was injected as a dissecting solution before attempting catheter insertion in group I, while catheter insertion was attempted directly without a dissecting solution in group II. All patients underwent the interventions as allocated and were followed up after the intervention without violation of the protocol at any time point. There was no loss to follow-up, and all 80 cases were included in all statistical analyses (Figure 4).
 |
Figure 4 CONSORT flowchart of enrolled participants. |
Table 1 shows homogeneous distribution of age and ASA of the patients studied, with no significant (p>0.05) differences between groups I and II.
 |
Table 1 Age and ASA Distribution of the Patients Studied (n=80) |
Table 2 shows that the median catheter-position score was significantly (p<0.001) higher in group II than group I (3 vs 2, respectively). However, median block performance time was significantly lower in group I (187.5 seconds) than group II (229 seconds). Otherwise, there were no significant differences between groups I and II regarding quality of sensory block, VAS scores, or patient satisfaction.
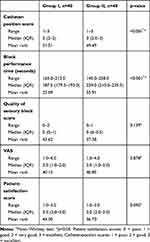 |
Table 2 Comparison of Block Performance Time, Catheter Position, Quality of Sensory Block, VAS, and Patient-Satisfaction Scores Between the Groups |
Regarding the number of attempts for catheter insertion, this was significantly (p<0.001) lower in group I than group II. Frequency of successful catheter insertion from the first attempt was significantly higher in group I than group II (97.5% vs 65.0%, respectively). Further, the need for second and third attempts was significantly higher in group II. The incidence of complications was nonsignificantly (p=0.615) higher in group I than group II (7.5% vs 2.5%), where three cases (two with nausea and vomiting and one with itching) were reported in group I, but only one case (suffering nausea and vomiting) was reported in group II (Table 3).
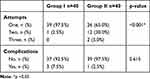 |
Table 3 Number of Catheter-Insertion Attempts and Incidence of Complications in the Two Groups |
Discussion
The pectoral block is a technically simple procedure that is used to alleviate pain in patients undergoing superficial chest-wall surgeries, such as mastectomy and insertion of prosthetic implants. The present study attempted to maximize the benefit of pectoral II block by catheter insertion under ultrasound guidance to enable continuous infusion of bupivacaine for controlling postoperative pain in patients undergoing simple mastectomy procedures. We evaluated the effect of injecting 10 mL lidocaine 1% as a dissecting solution to facilitate catheter insertion. Patients were randomly allocated into two groups (40 patients each). Group I patients were subjected to ultrasound-guided pectoral II block with injection of 10 mL lidocaine 1% as a dissecting solution before attempting catheter insertion, while group II patients underwent the standard procedure without a dissecting solution. The studied groups were matching in age and ASA, as evidenced by lack of significant differences between them.
The insertion of needles and catheters during the procedure of nerve block can cause injury to tissue in the vicinity of the nerve. In addition, the efficacy of the procedure — in terms of analgesia — depends on precise positioning of the catheter tip. Therefore, the performance of regional anesthesia for nerve block under ultrasound guidance is recommended to provide good visualization during catheter insertion, rendering the procedure safer.17 However, the tip of the needle used for nerve block is typically thinner than that of an interventional needle, and hence it is more difficult to visualize the exact location of the former’s tip.18 Moreover, catheters used for regional anesthesia are hard to distinguish on ultrasonography.19–21
The primary outcome of the present study was the visibility of the catheter position confirmed by ultrasound, measured by catheter-visibility score. Group I had a significantly lower median catheter-visibility score than group II (2 vs 3, respectively). Visibility of in-plane needles depends on the difference between the needle surface and the background.22 In our patients, a potential explanation for the lower catheter-visibility score in group I is that injection of lidocaine as a dissecting solution might have caused blunting of the contrast between the catheter and the background, making distinction of the catheter more difficult.
Other factors that may affect catheter visibility include the angle of positioning and the catheter structure. Takatani et al23 found that some catheters were better visualized when placed at 0° or 30° angles. They also found that catheters that enhance the dark at their centers have better visibility. Therefore, it is recommended to place catheters at shallow angles to improve visualization by ultrasonography. Furthermore, it has been reported that obesity can impact the visibility of the catheter.24 Unfortunately, the BMI of patients was not recorded in the current study, though the randomization process used in allocating the patients should have negated variations in basic patient characteristics between the two groups.
The current study investigated secondary outcomes of block performance time, number of catheter-insertion attempts, duration of the block, patient satisfaction, and incidence of complications. The number of attempts for catheter insertion was significantly lower in group I than group II in this study. Consequently, the frequency of successful catheter insertion from the first attempt was significantly higher in group I than group II (97.5% vs 65.0% respectively), while the frequency of second and third attempts was significantly higher in group II. This indicates that the modified technique employed in group I facilitated the insertion of the catheter from the first time without requiring further attempts in most subjects of this group.
The median block performance time in the current study was significantly shorter in group I than group II (187.5 vs 229.0 seconds), which may be attributed to the higher success rate of catheter insertion at first attempt in group I. This may also indicate that lower catheter-visibility scores in group I did not impact successful insertion of the catheter markedly. As regards the quality of sensory block, VAS scores, and patient satisfaction, we found no significant differences between the groups. VAS scores in the two groups were comparable to those reported in earlier studies.11,16,25 A slightly higher-quality sensory block was recorded in group I patients than group II, which may have contributed to the higher patient-satisfaction scores in group I. Further research is required to clarify the mechanism of better sensory block with the modified technique and whether it is related to better spread of lidocaine to axilla and other fascial planes, producing more effective block of the targeted nerves.
The potential complications of pectoral nerve blocks include local anesthetic toxicity, pneumothorax, hematoma, nausea, vomiting, and urinary retention.26 Local anesthetic toxicity may arise due to inadvertent injection into the thoracoacromial artery. Pneumothorax may occur due to puncturing of the pleura.8,27,28 The incidence of complications in the current study was non significantly higher in group I than group II (7.5% vs 2.5%). Reported complications included nausea, vomiting and itching, most probably related to the effect of the anesthetic drugs, rather than being caused by the technique of catheter insertion.
In addition to the aforementioned complications of pectoral II block, other disadvantages have been identified. The technique requires ultrasound-trained personnel. Moreover, it is a common practice to inject a single dose of local anesthetic without leaving a catheter in the site for continuous injection,9 though Blanco et al8 described catheter insertion in the original technique. In the current study, a catheter was left in place to enable continuous infusion of local anesthetics, thereby providing longer duration and better control of postoperative analgesia.
The present study was subject to some limitations. The BMI of the studied patients was not recorded. Variations in BMI between the two groups and analysis of subgroups divided according to BMI may have explained the lower catheter-visibility scores in group I or at least helped in excluding the effect of obesity. Moreover, the amount of opioids administered postoperatively in each group was not recorded.
Conclusion
The present study showed that injection of lidocaine as a dissecting solution in pectoral nerve II block facilitated the performance of the procedure, as evidenced by the shortened catheter-insertion time, and showed higher patient satisfaction. However, it was associated with lower catheter visibility on ultrasonography, and no significant differences were detected regarding the quality of sensory block or VAS scores. Therefore, based on the benefits and disadvantages of the new technique, more evaluation by future studies is mandated. Further studies with more participants are required to confirm the current findings and the safety of the modified technique.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Joshi GP, Ogunnaike BO. Consequences of inadequate postoperative pain relief and chronic persistent postoperative pain. Anesthesiol Clin North Am. 2005;23(1):21–36. doi:10.1016/j.atc.2004.11.013
2. Andersen KG, Kehlet H. Persistent pain after breast cancer treatment: a critical review of risk factors and strategies for prevention. J Pain. 2011;12(7):725–746. doi:10.1016/j.jpain.2010.12.005
3. Apfelbaum JL, Chen C, Mehta SS, Gan TJ. Postoperative pain experience: results from a national survey suggest postoperative pain continues to be undermanaged. Anesth Analg. 2003;97(2):534. doi:10.1213/01.ANE.0000068822.10113.9E
4. Terheggen MA, Wille F, Borel Rinkes IH, Ionescu TI, Knape JT. Paravertebral blockade for minor breast surgery. Anesth Analg. 2002;94(2):355–359.
5. Naja Z, Lönnqvist PA. Somatic paravertebral nerve blockade. Incidence of failed block and complications. Anaesthesia. 2001;56(12):1184–1188. doi:10.1046/j.1365-2044.2001.02084-2.x
6. Bakshi SG, Pokhale S, Sharma S. Role of regional catheters for postoperative analgesia following reconstructive surgeries for breast cancer. Indian J Cancer. 2016;53(2):243. doi:10.4103/0019-509X.197712
7. Blanco R. The ‘pecs block’: a novel technique for providing analgesia after breast surgery. Anaesthesia. 2011;66(9):847–848. doi:10.1111/j.1365-2044.2011.06838.x
8. Blanco R, Fajardo M, Parras Maldonado T. Ultrasound description of Pecs II (modified Pecs I): a novel approach to breast surgery. Rev Esp Anestesiol Reanim. 2012;59(9):470–475. doi:10.1016/j.redar.2012.07.003
9. Hinchcliff KM, Hylton JR, Orbay H, Wong MS. Intraoperative placement of pectoral nerve block catheters: description of a novel technique and review of the literature. Ann Plast Surg. 2017;78(5 Suppl 4):S189–S193. doi:10.1097/SAP.0000000000000954
10. Porzionato A, Macchi V, Stecco C, Loukas M, Tubbs RS, De Caro R. Surgical anatomy of the pectoral nerves and the pectoral musculature. Clin Anat. 2012;25(5):559–575. doi:10.1002/ca.21301
11. Bashandy GMN, Abbas DN. Pectoral nerves I and II blocks in multimodal analgesia for breast cancer surgery: a randomized clinical trial. Reg Anesth Pain Med. 2015;40(1):68–74. doi:10.1097/AAP.0000000000000163
12. Morioka H, Kamiya Y, Yoshida T, Baba H. Pectoral nerve block combined with general anesthesia for breast cancer surgery: a retrospective comparison. JA Clin Rep. 2015;1(1):15. doi:10.1186/s40981-015-0018-1
13. Versyck B, van Geffen G-J, Van Houwe P. Prospective double blind randomized placebo-controlled clinical trial of the pectoral nerves (Pecs) block type II. J Clin Anesth. 2017;40:46–50. doi:10.1016/j.jclinane.2017.03.054
14. Leiman D, Barlow M, Carpin K, Piña EM, Casso D. Medial and lateral pectoral nerve block with liposomal bupivacaine for the management of postsurgical pain after submuscular breast augmentation. Plast Reconstr Surg Glob Open. 2015;2(12):e282. doi:10.1097/GOX.0000000000000253
15. Haydon NB, van der Rijt R, Downs C, Buckland G. A novel technique of intraoperative lateral pectoral nerve block during subpectoral breast implant placement. Plast Reconstr Surg Glob Open. 2016;4(3):e646. doi:10.1097/GOX.0000000000000625
16. Karaca O, Pınar HU, Arpacı E, Dogan R, Cok OY, Ahiskalioglu A. The efficacy of ultrasound-guided type-I and type-II pectoral nerve blocks for postoperative analgesia after breast augmentation: a prospective, randomised study. Anaesth Crit Care Pain Med. 2019;38(1):47–52. doi:10.1016/j.accpm.2018.03.009
17. Chapman GA, Johnson D, Bodenham AR. Visualisation of needle position using ultrasonography. Anaesthesia. 2006;61(2):148–158. doi:10.1111/j.1365-2044.2005.04475.x
18. Perrella RR, Kimme-Smith C, Tessler FN, Ragavendra N, Grant EG. A new electronically enhanced biopsy system: value in improving needle-tip visibility during sonographically guided interventional procedures. Am J Roentgenol. 1992;158(1):195–198. doi:10.2214/ajr.158.1.1727345
19. Swenson JD, Davis JJ, DeCou JA. A novel approach for assessing catheter position after ultrasound-guided placement of continuous interscalene block. Anesth Analg. 2008;106(3):1015. doi:10.1213/ane.0b013e318161528a
20. Koscielniak-Nielsen ZJ, Rasmussen H, Hesselbjerg L. Long-axis ultrasound imaging of the nerves and advancement of perineural catheters under direct vision: a preliminary report of four cases. Reg Anesth Pain Med. 2008;33(5):477–482. doi:10.1097/00115550-200809000-00013
21. Chelly JE, Casati A. Perineural infusion of local anesthetics: “more to the review”. Anesthesiology. 2007;106(1):191–192. doi:10.1097/00000542-200701000-00031
22. Schafhalter-Zoppoth I, McCulloch CE, Gray AT. Ultrasound visibility of needles used for regional nerve block: an in vitro study. Reg Anesth Pain Med. 2004;29(5):480–488. doi:10.1097/00115550-200409000-00014
23. Takatani J, Takeshima N, Okuda K, Uchino T, Noguchi T. Ultrasound visibility of regional anesthesia catheters: an in vitro study. Korean J Anesthesiol. 2012;63(1):59–64. doi:10.4097/kjae.2012.63.1.59
24. Brookes J, Sondekoppam R, Armstrong K, et al. Comparative evaluation of the visibility and block characteristics of a stimulating needle and catheter vs an echogenic needle and catheter for sciatic nerve block with a low-frequency ultrasound probe. Br J Anaesth. 2015;115(6):912–919. doi:10.1093/bja/aev351
25. Wang K, Zhang X, Zhang T, et al. The efficacy of ultrasound-guided type II pectoral nerve blocks in perioperative pain management for immediate reconstruction after modified radical mastectomy: a prospective, randomized study. Clin J Pain. 2018;34(3):231–236. doi:10.1097/AJP.0000000000000529
26. Kim D-H, Kim S, Kim CS, et al. Efficacy of pectoral nerve block type II for breast-conserving surgery and sentinel lymph node biopsy: a prospective randomized controlled study. Pain Res Manag. 2018;2018:4315931. doi:10.1155/2018/4315931
27. Diéguez P, Casas P, López S, Fajardo M. Ultrasound guided nerve block for breast surgery. Rev Esp Anestesiol Reanim. 2016;63(3):159–167. doi:10.1016/j.redar.2015.11.003
28. Bolin ED, Harvey NR, Wilson SH. Regional anesthesia for breast surgery: techniques and benefits. Curr Anesthesiol Rep. 2015;5(2):217–224. doi:10.1007/s40140-015-0102-0
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
