Back to Journals » OncoTargets and Therapy » Volume 13
Essential Thrombocythaemia with Concomitant Waldenström Macroglobulinaemia: Case Report and Literature Review
Authors Lu N, Neoh CL, Ruan Z , Zhao L, Ying L, Zhang X, Chen S, Xu L
Received 14 January 2020
Accepted for publication 9 April 2020
Published 23 April 2020 Volume 2020:13 Pages 3431—3435
DOI https://doi.org/10.2147/OTT.S245950
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Leo Jen-Liang Su
Nina Lu,1 Chin Loon Neoh,2 Zhengying Ruan,3 Lei Zhao,4 Limei Ying,1 Xiaochang Zhang,1 Sai Chen,1 Linglong Xu1
1Department of Hematology, Taizhou Central Hospital (Taizhou University Hospital), Taizhou, Zhejiang 318000, People’s Republic of China; 2Department of Hematology, Royal Marsden Hospital, London SW3 6JJ, UK; 3Department of Pathology, Taizhou Central Hospital (Taizhou University Hospital), Taizhou, Zhejiang 318000, People’s Republic of China; 4Department of Laboratory Medicine, Taizhou Central Hospital (Taizhou University Hospital), Taizhou, Zhejiang 318000, People’s Republic of China
Correspondence: Linglong Xu Email [email protected]
Abstract: Essential thrombocythaemia (ET) and Waldenström macroglobulinaemia (WM) are two distinct disorders. Studies have reported several cases of myeloproliferative neoplasms (MPNs) with concomitant plasma cell dyscrasia. However, there were no reported cases of ET with concomitant WM to date. Here, we present a 55-year-old Chinese man with thrombocytosis and raised immunoglobulin level. Further investigations led to a diagnosis of ET and coexistent WM. Next-generation sequencing (NGS) of his bone marrow identified 3 mutated genes: JAK2 V617F, MYD88 L265P, and ATM F1036L. After being treated with pegylated interferon and low-dose aspirin, his platelet count normalized and immunoglobulin M (IgM) level reduced. To the best of our knowledge, this is the first reported case of dual pathology ET with WM.
Keywords: myeloproliferative neoplasm, Waldenström macroglobulinaemia, Janus kinase 2, myeloid differentiation factor 88
Introduction
Essential thrombocythaemia (ET) is one of the myeloproliferative neoplasms (MPNs). Waldenström macroglobulinaemia (WM) is a lymphoplasmacytic lymphoma with an IgM monoclonal gammopathy and bone marrow involvement. These are 2 distinct haematological disorders that arise from different haematopoietic clones. There were reported cases of MPNs with concomitant plasma cell disorders like monoclonal gammopathies of undetermined significance (MGUS) and multiple myeloma (MM). However, no cases of ET with coexistent WM have been reported to date.
Written informed consent was provided by the patient to publish the case details. The ethics committee of Taizhou Central Hospital approved the publication of the case details.
Case Report
A 55-year-old Chinese man was referred to our department with a history of asymptomatic thrombocytosis over 1 year’s duration. Apart from pleural tuberculosis 2 years ago treated with systemic anti-tuberculous therapy, his past medical history was unremarkable. His physical examination was unremarkable. A full blood count revealed a white blood cell count of 9.2×109/L with lymphocytes count of 3.0×109/L, haemoglobin 132g/L and platelet count of 629×109/L. Peripheral blood film showed thrombocytosis and there was no significant dysplasia. His biochemistry profile revealed a slightly raised total globulin level of 41.9g/L (normal range: 20–40g/L), uric acid: 507μmol/L (normal range: 208–428 μmol/L) and lactate dehydrogenase 242U/L (normal range: 109–245U/L). His serum immunoglobulin analysis revealed an elevated immunoglobulin M (IgM) of 21.5g/L (normal range: 0.46–3.04g/L), kappa light chain of 26.8g/L and lambda light chain lambda of 5.03g/L. Serum immunofixation confirmed the presence of monoclonal IgM. Ultrasound imaging study did not reveal lymphadenopathy, hepatomegaly or splenomegaly.
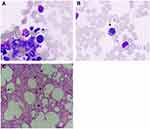
A bone marrow (BM) aspiration and trephine biopsy were performed. The BM aspirate morphology showed an excess of platelets and lymphoplasmacytoid lymphocytes (Figure 1A and B). The trephine reported cellularity of 55%, with increased numbers of lymphocytes and mature megakaryocytes with hyperlobated nuclei (Figure 1C). Flow cytometric immunophenotyping of his BM aspirate confirmed monoclonal B lymphocytes, which were positive for CD19, CD20 and cell membrane kappa light chain, but negative for CD5, CD10 and CD23 (Figure 2). Next-generation sequencing (NGS) with a 126-mutation panel was adopted to further investigate his gene/molecular mutation status which detected JAK2 V617F, MYD88 L265P, and ATM F1036L. BCR-ABL1 mRNA was not detected by quantitive real time-PCR.
Taken together, a diagnosis of ET with concomitant WM was made. He was treated with pegylated interferon and low-dose aspirin (100mg per day). After two months of treatment, his platelet count dropped to 324×109/L and IgM level to 11.80g/L.
Discussion
ET is characterized by clonal hematopoiesis thrombocytosis and susceptibility to thrombosis. About 50% of ET patients demonstrate JAK2 V617F mutation. Although JAK2 V617F is more commonly seen in MPN, it can also be found in other myeloid malignancies, such as acute myeloid leukemia and myelodysplastic syndrome. This patient met the WHO 2016 diagnostic criteria of ET with thrombocytosis, typical bone marrow finding, exclusion of BCR-ABL1 and presence of JAK2 V617F.1
WM is an indolent B cell neoplasm with an MYD88 L265P mutation.2 Although this mutation is not strictly required for the diagnosis of WM, it is found to be highly characteristic and present in about 90–95% of cases. When taken into consideration the BM aspirate morphology, immunophenotype, presence of IgM gammopathy and MYD88 mutation, a diagnosis of WM can be made in this patient. It remains important to note that MYD88 L265P can exist in a minority of other low-grade B cell neoplasms such as chronic lymphocytic leukaemia (CLL), mantle cell lymphoma (MCL), splenic margin zone lymphoma and follicular lymphoma.3–6
It is unclear if MPN directly correlates with lymphoid neoplasms. On reviewing some literature, the reported cases of MPN coexist with MGUS or MM.7 Eskazan AE et al reported a patient with ET developed MM after being treated with hydroxycarbamide for 3 years.8 Kuroda J et al also reported a case with concomitant ET and MM in which both the haematological neoplasms were shown to originate from separate malignant clones at hierarchically different differentiation levels.9 There are other case reports and reviews reporting patients with coexisting MPN and plasma cell disease.10 Overall, most patients in these cases were over 55 years old. It seems that concomitant plasma cell diseases do not accelerate the evolution of MPN to blastic phase. And there is no definitive evidence showing that these 2 entities arise from a common-ancestor hematopoietic stem cell.7,9,10 The management of these patients should be individual based. We treated the patient with pegylated interferon and aspirin. And he had his thrombocythemia and IgM level ameliorated after 2 months’ treatment. However, further study is warranted to establish optimal management.
The mechanism of developing MPN with concomitant plasma cell dyscrasia remains to be elucidated. Recent studies indicate that oxidative stress plays a major role in carcinogenesis as well as in genomic instability. Chronic inflammation, oxidative stress might trigger the clonal evolution in MPN.11–13 An increased level of reactive oxygen species (ROS) and inflammation markers were observed in ET patients.14,15 ROS levels and total antioxidant capacity seemed to be reduced after current risk-adapted therapy.16 On the other hand, there are growing data to support external microbial and antigen stimulation can trigger the development of lymphoproliferative diseases, including WM.17,18 Perrot A found that a peroxidase PRDX6 was most under-expressed in WM, which indicates oxidative stress might contribute to the pathogenesis of WM.19
ATM, which controls genome stability and cell survival, is part of cell metabolism and growth and oxidative stress.20 Oxidation can activate ATM directly.21 ATM-deficient mice show elevated ROS, indicating ATM can reduce oxidative stress.22 In this case, the patient gained an additional ATM F1036L mutation, which might correlate to the excess of oxidative stress. Taken together, oxidative stress might play a role in the development of co-occurrence of ET and WM in the patient. And further study is warranted.
The previous study reported MPN patients can develop secondary malignancy, including squamous cell carcinoma, basal cell carcinoma, bladder cancer, rectal carcinoma, and hematological malignancy. A propensity score weighted competing risk analysis revealed a statistically significant increased risk of second malignancy (HR 10.81 [95% CI 2.54–45.92], p=0.001) with ruxolitinib treatment.23 However, such data should be interpreted with caution, because of the very low prevalence of secondary malignancy. Of note, the use of JAK1/2 inhibitor seems to be associated with a higher frequency of aggressive B-cell lymphomas. Porpaczy E et al conducted a retrospective study and found that B-cell lymphomas evolved in 4 (5.8%) out of 69 patients receiving JAK1/2 inhibitor compared to 2 (0.36%) of 557 patients with conventional treatment (hence, a 16-fold increased risk).24
Conclusion
To the best of our knowledge, MYD88 L265P is seen in JAK2 V617F positive ET. The case we present is the first case of ET with coexistent WM. It is not clear whether these two conditions originate from the same or separate malignant clones. As such, the management of such cases should be individualized as there is insufficient evidence to guide treatment.
Disclosure
The authors have no conflicts to declare.
References
1. Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391–2405. doi:10.1182/blood-2016-03-643544
2. Treon SP, Xu L, Yang G, et al. MYD88 L265P somatic mutation in Waldenstrom’s macroglobulinemia. N Engl J Med. 2012;367(9):826–833. doi:10.1056/NEJMoa1200710
3. Swerdlow SH, Kuzu I, Dogan A, et al. The many faces of small B cell lymphomas with plasmacytic differentiation and the contribution of MYD88 testing. Virchows Arch. 2016;468(3):259–275. doi:10.1007/s00428-015-1858-9
4. Bogusz AM, Bagg A. Genetic aberrations in small B-cell lymphomas and leukemias: molecular pathology, clinical relevance and therapeutic targets. Leuk Lymphoma. 2016;57(9):1991–2013. doi:10.3109/10428194.2016.1173212
5. Jiménez C, Sebastián E, Chillón MC, et al. MYD88 L265P is a marker highly characteristic of, but not restricted to, Waldenstrom’s macroglobulinemia. Leukemia. 2013;27(8):1722–1728. doi:10.1038/leu.2013.62
6. Xu L, Ding X, Ying L, Zhang X, Lu N. Follicular lymphoma presenting with monoclonal IgM and MYD88 mutation: a case report and review of the literature. Onco Targets Ther. 2019;12:7833–7842. doi:10.2147/OTT.S211436
7. Malhotra J, Kremyanskaya M, Schorr E, Hoffman R, Mascarenhas J. Coexistence of myeloproliferative neoplasm and plasma-cell dyscrasia. Clin Lymphoma Myeloma Leuk. 2014;14(1):31–36. doi:10.1016/j.clml.2013.09.015
8. Eskazan AE, Ongoren S, Ar MC, et al. Essential thrombocythemia and multiple myeloma: two rare diseases in one patient. Clin Lymphoma Myeloma Leuk. 2011;11(5):442–445. doi:10.1016/j.clml.2011.04.001
9. Kuroda J, Matsumoto Y, Tanaka R, et al. JAK2V617F-positive essential thrombocythemia and multiple myeloma with IGH/CCND1 gene translocation coexist, but originate from separate clones. Acta Haematol. 2008;120(3):177–181. doi:10.1159/000187645
10. Masarova L, Newberry KJ, Pierce SA, et al. Association of lymphoid malignancies and Philadelphia-chromosome negative myeloproliferative neoplasms: clinical characteristics, therapy and outcome. Leuk Res. 2015;39(8):822–827. doi:10.1016/j.leukres.2015.05.002
11. Hasselbalch HC. A role of NF-E2 in chronic inflammation and clonal evolution in essential thrombocythemia, polycythemia vera and myelofibrosis? Leuk Res. 2014;38(2):263–266. doi:10.1016/j.leukres.2013.07.002
12. Hasselbalch HC, Thomassen M, Riley CH, et al. Whole blood transcriptional profiling reveals deregulation of oxidative and antioxidative defence genes in myelofibrosis and related neoplasms. Potential implications of downregulation of Nrf2 for genomic instability and disease progression. PLoS One. 2014;9(11):e112786. doi:10.1371/journal.pone.0112786
13. Moisă C, Găman M-A, Pascu EG, et al. The role of oxidative stress in essential thrombocythemia. Arch Balkan Med Union. 2018;53(1):70–75.
14. Gaman AM, Moisa C, Diaconu CC, Gaman MA. Crosstalk between oxidative stress, chronic inflammation and disease progression in essential thrombocythemia. Rev Chim. 2019;70(10):3486–3489. doi:10.37358/RC.19.10.7581
15. Moisa C, Gaman MA, Diaconu CC, Gaman AM, Levels OS. JAK2V617F mutational status and thrombotic complications in patients with essential thrombocythemia. Rev Chim. 2019;70(8):2822–2825. doi:10.37358/RC.19.8.7435
16. Moisă C, Găman M-A, Diaconu CC, Assani AD, Gaman AM. The evaluation of oxidative stress in patients with essential thrombocythemia treated with risk-adapted therapy. Arch Balkan Med Union. 2018;53(4):529–534. doi:10.31688/ABMU.2018.53.4.07
17. Burger JA, Ghia P, Rosenwald A, Caligaris-Cappio F. The microenvironment in mature B-cell malignancies: a target for new treatment strategies. Blood. 2009;114(16):3367–3375. doi:10.1182/blood-2009-06-225326
18. Stone MJ, Pascual V. Pathophysiology of Waldenström’s macroglobulinemia. Haematologica. 2010;95(3):359–364. doi:10.3324/haematol.2009.017251
19. Perrot A, Pionneau C, N BC A, et al. Waldenström’s macroglobulinemia harbors a unique proteome where Ku70 is severely underexpressed as compared with other B-lymphoproliferative disorders. Blood Cancer J. 2012;2(9):e88. doi:10.1038/bcj.2012.35
20. Cremona CA, Behrens A. ATM signaling and cancer. Oncogene. 2013;33(26):3351–3360. doi:10.1038/onc.2013.275
21. Guo Z, Kozlov S, Lavin MF, Person MD, Paull TT. ATM activation by oxidative stress. Science. 2010;330(6003):517–521. doi:10.1126/science.1192912
22. Semlitsch M, Shackelford RE, Zirkl S, Sattler W, Malle E. ATM protects against oxidative stress induced by oxidized low-density lipoprotein. DNA Repair (Amst). 2011;10(8):848–860. doi:10.1016/j.dnarep.2011.05.004
23. Tremblay D, King A, Li L, et al. Risk factors for infections and secondary malignancies in patients with a myeloproliferative neoplasm treated with ruxolitinib: a dual-center, propensity score-matched analysis. Leuk Lymphoma. 2020;61(3):660–667. doi:10.1080/10428194.2019.1688323
24. Porpaczy E, Tripolt S, Hoelbl-Kovacic A, et al. Aggressive B-cell lymphomas in patients with myelofibrosis receiving JAK1/2 inhibitor therapy. Blood. 2018;132(7):694–706. doi:10.1182/blood-2017-10-810739
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.