Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 14
Erythromycin Prevents Elastin Peptide-Induced Emphysema and Modulates CD4+T Cell Responses in Mice
Authors Tang S , Ma T, Zhang H, Zhang J, Zhong X, Tan C, Qiu Y, Zeng W, Feng X
Received 8 July 2019
Accepted for publication 13 November 2019
Published 29 November 2019 Volume 2019:14 Pages 2697—2709
DOI https://doi.org/10.2147/COPD.S222195
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Chunxue Bai
Shudan Tang,* Tingting Ma,* Hui Zhang,* Jianquan Zhang, Xiaoning Zhong, Caimei Tan, Ye Qiu, Wen Zeng, Xin Feng
Department of Respiratory Medicine, The First Affiliated Hospital of Guangxi Medical University, Nanning, Guangxi 530021, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jianquan Zhang
Department of Respiratory Medicine, The First Affiliated Hospital of Guangxi Medical University, 6 Shuangyong Road, Qingxiu, Nanning, Guangxi 530021, People’s Republic of China
Tel/Fax +86 771 535 1176
Email [email protected]
Purpose: Elastin peptides (EP) can induce lung inflammation and emphysema. Erythromycin has been shown to decrease acute exacerbation frequency and delay lung function decline in chronic obstructive pulmonary disease patients and ameliorate emphysema in murine models; however, the mechanism remains unclear. We aimed to observe the preventive and immunomodulatory effects of erythromycin in a mouse model of EP-induced emphysema.
Methods: In the in vivo study, Balb/c mice were treated with EP intranasally on day 0, and then administered erythromycin (100 mg/kg) or vehicle orally on day 1, which was continued every other day. Mice exposed to cigarette smoke were used as an emphysema positive control. The severity of emphysema and inflammation in the lungs of EP-exposed mice with or without erythromycin treatment were observed on day 40 after EP administration. In the in vitro study, naïve CD4+T cells were isolated from healthy mice spleens and stimulated by EP with or without erythromycin incubation. Flow cytometry was used to measure the proportions of Th1, Th17, and Treg cells. ELISA was used to detect cytokine levels of IFN-γ, IL-17, IL-6, and TGF-β. Transcript levels of Ifnγ, IL17a, and Foxp3 were evaluated by qRT-PCR.
Results: After exposure to EP, Th1 and Th17 cell percentages and the levels of inflammatory cytokines increased in vivo and in vitro, while Treg cells decreased in vivo. Erythromycin reduced IFN-γ, IL-17, IL-6 inflammatory cytokines, MLI, and the inflammation score in the lungs of EP-exposed mice. In vitro, erythromycin also limited Th17 and Th1 cell differentiation and downregulated transcript levels of Ifnγ and IL17a in the EP-stimulated CD4+T cells.
Conclusion: The Th1 and Th17 cell responses were increased in EP-induced emphysema. Prophylactic use of erythromycin effectively ameliorated emphysema and modulated CD4+T cells responses in EP-induced lung inflammation in mice.
Keywords: Th1, Th17, Treg cells, inflammation, lungs
Introduction
Chronic obstructive pulmonary disease (COPD) is characterized by persistent, chronic inflammation and airflow obstruction.1 It is a worldwide public health problem with high prevalence; in China alone there are 99.9 million COPD patients.2 Many pathogenesis and treatment methods of COPD have been proposed.3–6 However, the optimal prevention and treatment regimen for COPD still needs to be explored.
Elastin fibers, which are abundant in the extracellular matrix of lung tissue, are crucial for maintaining reversible contraction and dilation of the lungs during breathing.7,8 However, evidence indicates that elastin peptides (EP), the product of elastolysis, could become a pro-inflammatory trigger in the initiation of COPD/emphysema.9 The persistence of EP stimulation in lung tissue is likely attributable to the persistence of inflammation. Previous studies have shown that smoke-induced emphysema is accompanied by elastin degradation.3,9,10 This is caused by elastase released from inflammatory cells, principally neutrophils and macrophages, in the context of cigarette smoke exposure or under other inflammatory stimulation.11,12 EP can induce macrophage accumulation,10,13 regulate cell proliferation,3,14 and modulate the release of proinflammatory cytokines.15,16 Moreover, it has been demonstrated that EP-induced emphysema was accompanied not only by increased numbers of macrophages and neutrophils, but also was associated with greater differentiation of T-cytotoxic 1 (Tc1), T helper 1 (Th1), and T helper 17 (Th17) cells.3,9,17 EP accumulating in the lungs appear to act as an antigen, activating the adaptive immune response and resulting in abnormal polarization of T cells.3,17 The activation of adaptive immunity is likely to explain why the progress of COPD does not stop after smoking cessation, and EP may contribute to this self-enhanced inflammatory immune response.3
Erythromycin belongs to the family of 14-membered ring macrolide antibiotics and has been reported to alleviate cigarette smoke-induced emphysema and inflammation in rats.18 Since the late 1980s, long-term and low-dose macrolides have been considered to have anti-inflammatory and immunomodulatory activity in addition to being antimicrobial.19 In recent years, the anti-inflammatory and immunomodulatory effects of macrolides have been established in chronic respiratory disease.6,18,20–22 Our previous work, and work by other researchers, demonstrated that long-term treatment with prophylactic macrolides has a significant clinical benefit by decreasing pro-inflammatory cytokines and the frequency of COPD exacerbations, as well as improving quality of life for COPD patients.20,22–25 The GOLD 2017 standard stressed and recommended the use of macrolides in group D patients with repeated and uncontrollable exacerbations.1 We have also done some work to confirm the anti-inflammatory and immunomodulatory effects of erythromycin.23,26 In vitro, erythromycin has been shown to recover histone deacetylase protein expression and inhibit nuclear factor-κB activity in cigarette smoke extract-stimulated human macrophages.27 In vivo, it can reduce pulmonary inflammation and emphysema, while increasing regulatory T (Treg) cells in rats exposed to cigarette smoke.26 Recently, we also found that erythromycin could suppress EP-induced increase in Th17 cells in vitro.28 It appears that erythromycin could also modulate EP-induced inflammation and CD4+T cells dysregulation, not only in the setting of cigarette exposure. However, the effects of erythromycin on adaptive immunity and the differentiation of T cells in EP-exposed mice have not been confirmed in vivo.
A predominant pattern in the distribution of Th1 and Th17 cells in EP-induced emphysema has been reported.3,17 Treg, another subset of CD4+T cells, which play a role in maintaining immune tolerance,29 is also involved in the development of COPD.30 In this study, we aimed to observe whether prophylactic use of erythromycin could prevent emphysema and modulate the responses of Th1, Th17, and Treg cells in EP-induced inflammation.
Materials and Methods
Animals
Six to eight-week-old male BALB/c mice (Guangxi Medical University Laboratory Animal Center, China) were housed individually in standard laboratory cages with a 12 h light-dark cycle. They had ad libitum access to chow and tap water. The experiments were approved by the Animal Research Care Committee of Guangxi Medical University. Animal ethics review followed the Guiding Opinions on the Treatment of Laboratory Animals issued by the Ministry of Science and Technology of the People’s Republic of China and the Laboratory Animal-Guideline for Ethical Review of Animal Welfare issued by the National Standard GB/T35892-2018 of the People’s Republic of China.
Models
Referring to the protocol used by Sellami et al9 with slight adjustments, mice were intranasally administered 50 µl suspensions of EP (VGVAPG, Genepep, Prades-le-Lez, Paris, France; 20 µg EP/50 µl ddH2O) or an identical volume of vehicle after being anesthetized with 10% chloral hydrate (0.3 mL/100 g) to construct the emphysema model (Figure 1). Sixteen EP-treated mice were randomly distributed into an EM group (n=8) and a vehicle group (n=8) and orally administered either erythromycin (Solarbio, 100 mg/kg) or an identical volume of vehicle. On the 40th day, all mice were anesthetized and sacrificed by cervical dislocation. Blood, lungs, spleens, and bronchoalveolar lavage fluid (BALF) were collected. The right lungs of mice were lavaged three times to obtain BALF through a tracheal cannula with 0.75 mL PBS each time. Then, the lavage fluid was centrifuged at 1500 rpm for 5 min at 4 °C to remove cell debris, and the supernatant was stored at −80 °C for enzyme-linked immunosorbent assays (ELISAs). After lavage, the lungs were used to isolate single-cell suspension. To compare the differences between EP-induced emphysema and a traditional emphysema model, we also set up a CS exposure group (n=8) as we previously described.31 All experiments were replicated three times.
Histology
The left lungs of mice were fixed in 10% formalin for 18–24 h and embedded in paraffin. After sectioning, they were stained with hematoxylin and eosin (H&E) to observe and quantify enlargement of alveolar airspace and inflammatory infiltration of emphysema. The mean linear intercept (MLI) was used to assess the enlargement of alveolar airspace and an inflammation score was used to document the severity of pulmonary inflammation by two independent individuals in a blinded manner.32 As previously described, the degree of peribronchial and perivascular inflammation was assessed on a subjective score of 0, 1, 2, and 3.33
Peripheral Blood Mononuclear Cells (PBMCs) of Mice
Post-anesthetic, blood was collected from the retro-orbital vein into EDTA-treated tubes. PBMCs were isolated from peripheral blood by Ficoll-Hypaque (Solarbio) gradient centrifugation.
Mouse Single-Cell Preparation and Isolation
Spleens were obtained from BALB/c mice and gently ground into single cells using a piston of a 5 mL syringe. The suspension was filtered through a 70-μm cell strainer, to prepare a single-cell suspension. The spleen cell suspensions were collected by centrifugation at 1500 rpm for 5 min at 4 °C. Erythrocytes were removed with RBC lysis buffer (R1010, Solarbio) for 2–3 min at room temperature in the dark, and washed twice with cold phosphate buffer saline (PBS) and centrifugation at 1500 rpm for 5 min.
Since lung tissue contains more connective tissue fibers, mechanical disruption and collagenase digestion are required prior to grinding for collection of lung single cell suspensions. First, 10 mL PBS was injected into the right ventricle to rinse cells in lung vasculature. The hearts were then removed, and the lungs were cut into 0.1 cm pieces and incubated in 2 mL RPMI 1640 medium containing 1 mg/mL collagenase type IV (Sigma-Aldrich) for 40 min at 37 °C in the shaker. The remaining intact tissues were treated following the spleen protocol until single-cell lung suspensions were obtained.
Cell Culture
Spleen single-cell suspensions were obtained and CD4+T cells were isolated by immuno-magnetic positive selection using mouse CD4 (L3T4) microbeads according to the manufacturer’s instructions (Miltenyi Biotec). Cell purity and viability were over 93% and 90%, as determined by flow cytometry analysis and Trypan Blue Dye Exclusion Method, respectively. Referring to the method described by Meghraoui-Kheddar et al17 with slight adjustments, isolated CD4+ splenic T cells were cultured in 24-well culture plates (2×106 cells/mL) and incubated with T-activator anti-CD3e/CD28 (Invitrogen). Cells were preincubated with or without the presence of EM (100 µg/mL) for 2 h, and then stimulated with VGVAPG peptide (20 µg/mL). After 48 h of incubation, cells were collected for flow cytometry and quantitative real-time PCR (qRT-PCR). All experiments were replicated five times.
Flow Cytometry
For intracellular cytokine analysis, PBMCs, spleen, and lung single-cell suspensions isolated from mice were stimulated with 25 ng/mL phorbol myristate acetate (PMA; Sigma-Aldrich, USA) and 1 µg/mL ionomycin (Cell Signaling Technology, USA) in the presence of 2 µl/mL GolgiStop™ (BD Biosciences) for 4 h at 37 °C in 5% CO2. The cells were then washed and stained for the surface markers PerCP-anti-mouse CD4 (Clone RM4-5) and PE-anti-mouse CD25 (Clone PC61.5). For intracellular staining, cells were fixed and permeabilized with BD Pharmingen fixation/permeabilization solution or eBioscienceTM Foxp3/Transcription Factor Staining Buffer Set Kit, according to the manufacturer’s protocol. Following fixation/permeabilization, APC-anti-mouse IFN-γ mAb (Clone XMG1.2), PE-anti-mouse IL-17 mAb (Clone TC11-18H10), and APC-anti-mouse Foxp3 mAb (Clone FJK-16s) were stained for 30 min at 4 °C. CD4, IFN-γ, and IL-17 mAbs were purchased from BD Pharmingen and CD25, and Foxp3 mAbs were purchased from eBioscience. For both Th1 and Th17 cells, we used unstimulated cells, which did not show intracellular cytokine expression, as a biological comparison control, according to the method described by Maecker and Trotter.34 For Treg cells, fluorescence-minus-one controls (FMO) were used as a gating control to determine positivity or negativity for CD4, CD25, and Foxp3.34 Flow cytometry was performed using a BD FACS Canto II (BD Biosciences) and data were analyzed using Flow Jo 7.6 (Treestar).
Quantitative Real-Time PCR
Total RNA was obtained from lung tissue stored at −80 °C, and from cultured cells, with TRIzol reagent according to the manufacturer’s protocol (Takara, China). The quality and quantity of total RNA were detected by a spectrophotometer (NARODROP200, Thermo Scientific). Complementary DNA (cDNA) was transcribed from total RNA with PrimeScript RT reagent Kit with gDNA Eraser following the manufacturer’s protocol (Takara). Real-time PCR was conducted using SYBR® Premix Ex Taq™ II (Perfect Real Time; TaKaRa). A typical 20 μL PCR mixture included 10 μL of SYBR® Premix Ex Taq™ II, 0.8 μL of each PCR primer, 2 μL of template cDNA, 0.4 μL ROX Reference Dye, and 6 μL ddH2O. Cycling conditions were 95 °C for 30 s, followed by 40 cycles at 95 °C for 5 s, and 60 °C for 30 s. The reactions were performed on an ABI 7500 Real-Time PCR System.
Primer sequences were as follows: β–Actin (forward 5ʹ-CATCCGTAAAGACCTCTATGCCAAC-3ʹ, reverse 5ʹ-ATGGAGCCACCGATCCACA-3ʹ); IL17a (forward 5ʹ-GGTCCATATTTGACTTTTCCCACT-3ʹ, reverse 5ʹ-GATGTTCCACTCTCCTCTTCTCTTG-3ʹ); Ifnγ (forward 5ʹ-AGGAACTGGCAAAAGGATGGT-3ʹ, reverse 5ʹ-ACGCTTATGTTGTTGCTGATGG-3ʹ); Foxp3 (forward 5ʹ-AGTGCCTGTGTCCTCAATGGTC-3ʹ, reverse 5ʹ-AGGGCCAGCATAGGTGCAAG-3ʹ). Relative gene expression was calculated using the 2−∆∆Ct method.
Measurements of Cytokines
The levels of IL-17, IFN-γ, IL-6, and TGF-β in the serum and BALF were detected using commercially available ELISA kits (Cusabio Biotech Co., Ltd., China), according to the manufacturer’s protocols.
Statistical Analysis
Data are shown as the median with interquartile range. Since our data were not all normally distributed, comparisons between two groups were performed with a Mann–Whitney U-test. Comparisons among multiple groups were done using the Kruskal-Wallis test with the Bonferroni adjustment. All statistical tests were analyzed using the software SPSS 22.0 (SPSS, Chicago, IL, USA). P values <0.05 were considered statistically significant.
Results
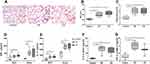
EP-Induced Emphysema, Pulmonary Inflammation, and Inflammatory Cytokines Mimic CS-Induced Emphysema in Mice
Lung tissues obtained from control, EP, and CS mice were stained with H&E and the airspace and inflammatory infiltrates were quantitatively analyzed (Figure 2). In control mice, we observed minimal inflammatory infiltrates and destruction of alveolar walls. Lung sections from the EP mice showed alveolar enlargement and many inflammatory infiltrates. Inflammatory cells mainly accumulated around the terminal bronchiole and blood vessels. Similar pathologies, including alveolar enlargement, rupture, confluence of alveolar walls, and inflammatory infiltrates, were seen in the lungs of CS mice (Figure 2A). Although CS mice showed higher MLI values (representing alveolar size) than EP mice, this difference was not significant (P >0.05). Logically, with alveolar enlargement, the MLI values and inflammation score of the EP and CS groups were higher than control mice (P<0.05, Figure 2B and C). We also detected the concentration of IFN-γ, IL-17, IL-6, and TGF-β in either the serum or/and BALF. There were no significant differences in the serum or BALF concentrations of IFN-γ or IL-17 between EP and CS mice (P >0.05), while these were lower in control mice (P <0.05, Figure 2D and E). Similarly, the BALF concentrations of IL-6 and TGF-β were not significantly different between EP and CS mice (P >0.05) but were lower in control mice (P <0.05, Figure 2F and G).
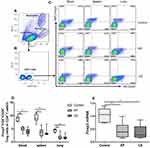
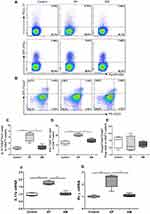
Increased Percentages of IL-17+CD4+T (Th17) and IFN-γ+ CD4+T (Th1) Cells in Peripheral Blood, Spleens, and Lungs of EP Mice
The representative flow cytometric dot plots of Th1 and Th17 cells in the peripheral blood, spleen, and lung tissue are shown in Figure 3A and B. With stimulation by PMA and ionomycin, IL-17+CD4+T and IFN-γ+CD4+T cells (Figure 3B) were detected by flow cytometry while unstimulated cells showed no cytokine production (data not shown). Percentages of Th17 and Th1 cells were significantly higher in the peripheral blood, spleens, and lungs of EP and CS mice than control mice (Figure 3C and D). No significant differences in the percentages of Th1 or Th17 cells were observed between EP mice and CS mice, except for Th17 cells in the spleen (Figure 3C and D). Furthermore, both the relative levels of Ifnγ and IL17a mRNA transcripts were significantly higher (P <0.05, Figure 3E and F) in the lungs of EP and CS mice than in control mice, while there was no detectable difference between EP and CS mice (P>0.05, Figure 3E and F).
Decreased Expression of Foxp3+CD4+CD25+T (Treg) Cells in Peripheral Blood, Spleens, and Lungs of EP Mice
Foxp3+CD4+CD25+ Tregs are a subset of lymphocytes that are essential for reducing effector T cells proliferation in order to establish peripheral tolerance.35 Representative flow cytometric dot plots of Foxp3+CD4+CD25+T cells in the peripheral blood, spleens, and lungs of control, EP, and CS mice are shown in Figure 4A–C. Lymphocytes, neutrophils, and monocytes were identified based on their characteristic properties shown in the FSC and SSC (Figure 4A). The percentages of Treg cells were significantly lower in peripheral blood, spleens, and lungs of EP and CS mice than in control mice (P <0.05, Figure 4D). Additionally, the relative levels of Foxp3 mRNA transcripts were significantly lower in lungs of EP and CS mice than in control mice, while there was no significant difference between EP and CS mice (P >0.05, Figure 4E).
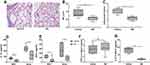
Erythromycin Prevented Emphysema and Decreased Inflammatory Cytokines in Lungs of EP-Induced Mice
Following EP nasal inhalation and treatment with erythromycin or ddH2O every other day for 40 days, lung tissue sections of mice were stained with H&E (Figure 5A). Compared with vehicle control mice, EM mice showed reduced alveolar space, as represented by the MLI value (P <0.01, Figure 5B) and less inflammatory infiltration, as represented by the inflammation score (P <0.05, Figure 5C). The proinflammatory factors IL-17 and IFN-γ were both significantly decreased in the serum and BALF of EM mice (P <0.05, Figure 5D and E). In addition, the concentration of TGF-β in BALF was higher in EM mice than in vehicle control mice, but there were no significant differences (P >0.05, Figure 5F). The inflammatory cytokine IL-6 was significantly lower in BALF of EM mice than in EP mice (P <0.01, Figure 5G).
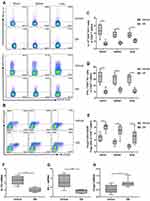
Erythromycin Modulated Th17, Th1, and Treg Cells in Peripheral Blood, Spleens, and Lungs of EP-Induced Mice
To observe the role of erythromycin, we used flow cytometry to detect the percentages of Th17, Th1, and Treg cells in the peripheral blood, spleens, and lungs of EM treated mice (Figure 6A and B). We observed that Th17 and Th1 cells were lower in the peripheral blood, spleens, and lungs of the EM group mice than in vehicle control mice (P <0.01, Figure 6C and D). Treg cell numbers were higher in the peripheral blood, spleens, and lungs of EM mice than in vehicle control mice (P <0.01, Figure 6E). The relative levels of IL17a and Ifnγ mRNA transcription were lower in the lungs of EM mice compared with vehicle control mice (P <0.01, Figure 6F and G). However, the relative level of Foxp3 mRNA transcript was higher in lungs of EM mice (P <0.05, Figure 6H). These data demonstrated that administration of erythromycin significantly ameliorated EP-induced inflammatory cell infiltration in the local organs and whole bodies of mice.
Increased Percentages of IL-17+CD4+T (Th17) and IFN-γ+ CD4+T (Th1) Cells with EP Stimulation Were Decreased with Erythromycin Treatment in vitro
In vitro, the naïve CD4+T cells were stimulated with EP in the presence of anti-CD3e/CD28. The percentages of Th17 and Th1 cells were higher in the CD4+T cells treated with EP compared with untreated controls, but there was no difference in the percentage of Treg cells between EP- treated cells and untreated controls (Figure 7A–E). Th1 and Th17 cells both significantly decreased after erythromycin treatment (P <0.05, Figure 7C and D), which was consistent with our findings in vivo. In addition, our results on relative levels of IL17a and Ifnγ mRNA transcripts were in line with our flow cytometry results (Figure 7F and G).
Discussion
Cigarette smoke is a major risk factor for COPD. However, smoking cessation cannot completely reverse pulmonary inflammation or emphysema.36 Thus, it is likely that cigarette smoke exposure is not the only trigger in the pathogenesis of COPD. EP, which exists in the lungs of individuals exposed to cigarette smoke, has been proven to play a substantial role in the progression of emphysema.3,9,10 To confirm that elastin fragments could cause emphysema, pulmonary inflammation, and dysregulation, we gave EP alone to mice at a single dose by nasal inhalation, and observed the effects 40 days post EP administration. As expected, we found similar histological changes in emphysema induced by EP and CS.9 Moreover, EP-induced emphysema was associated with a remarkable increase of Th1 and Th17 cells and a decrease of Treg cells in the lung tissue, which is consistent with the immunophenotype of COPD/emphysema derived from other causes.4,37 We also found systemic inflammation with increasing inflammatory cytokines and dysregulation of immune cells in blood and spleen of EP-induced mice. Additionally, we compared pulmonary damage with MLI at day 21, which corresponded to the modeling time in the article by Sellami et al.9 However, we found no significant difference between control and EP mice (data not shown). These differences may be due to different experimental environments and operational experimenters.
It seems that EP is a peptide with immunogenic characteristics. EP has been shown to act as an antigen to activate the adaptive immune response and promote the generation of elastin-specific Th1 and Th17 cells in vivo and in vitro.17,38 In our study, EP stimulation did not significantly promote the activation and differentiation of naïve CD4+T cells in the absence of the activator anti-CD3e/CD28 in vitro (data not shown). In contrast, the proportions of Th1 and Th17 cells relative to the total CD4+T cell population were increased with the activator anti-CD3e/CD28 when stimulated with EP. This finding is in line with previous studies.17,38 Meghraoui-Kheddar and his colleagues thought that the interaction between EP and spliced-galactosidase (S-gal), which is an elastin receptor expressed on T cells, neutrophils, and macrophages, plays a crucial role in the emphysema-associated inflammatory process.17 In addition, Shan et al found that EP could act as cognate antigens and promote Th1 and Th17 generation.38 Thus, we speculate that EP may have the ability to stimulate T cells directly and influence their differentiation.
Although COPD is characterized by chronic inflammation in the airway, it is challenging to find an effective anti-inflammatory agent for COPD treatment. Even glucocorticoids cannot effectively inhibit chronic airway inflammation or delay the progression of COPD/emphysema in clinical situation. Macrolides possess anti-inflammatory and immunomodulatory properties and are used for the treatment of many diseases involving pulmonary inflammation, including COPD.1 The use of continuous and intermittent prophylactic macrolides leads to a clinically significant benefit in reducing exacerbations in COPD patients.25 Yutaka et al used clarithromycin, a type of macrolide, to observe the preventative effects in a cigarette smoke-induced emphysema mouse model. Their data showed that administering clarithromycin at the beginning of cigarette smoke exposure lowers neutrophils and macrophages in the BALF and significantly reduces the increase in lung compliance.39 Among the variety of macrolides, erythromycin is effective, well tolerated, and inexpensive. Our previous study found that erythromycin can reduce airway pro-inflammatory cytokines IL-17 and IL-23 in COPD patients.23 Treatment with erythromycin has been found to decrease neutrophil and lymphocyte counts, lower levels of IL-8 and TNF-α in the BALF, and downregulate keratinocyte-derived chemokine and TNF-α transcription in CS-exposed mice.21,26 Emerging evidence shows that macrolides could play an immunomodulatory role in addition to the known antibiotic role, including the regulation of innate and adaptive immune cells.40,41 We previously showed that erythromycin modulated inflammatory immune cells in the airway and restored the decrease of Treg through immunomodulation in rats exposed to cigarette smoke.26 Thus, we prophylactically used erythromycin to observe whether it has a preventive effect on EP-induced emphysema. As expected, we found decreased percentages of Th1 and Th17 cells and decreased levels of pro-inflammatory factors including IFN-γ, IL-17, and IL-6 in the BALF and serum. These changes were accompanied by amelioration of emphysema in EP mice following erythromycin treatment. In vitro, erythromycin could also reduce EP-induced Th1 and Th17 differentiation. Thus, we speculated that erythromycin could prevent lung inflammation and emphysema progression in EP-triggered emphysema, which may contribute to interrupting the self-amplification adaptive immune loop.
Further, we observed elevated Treg cell numbers in the peripheral blood, spleens, and lungs of EM mice in comparison to vehicle control mice. TGF-β along with IL-6 can promote differentiation of naïve CD4+T cells to Th17 cells.42 In our study, erythromycin treatment was associated with decreased IL-6 and Th17, but TGF-β remained elevated. We postulate that erythromycin may downregulate IL-6 levels, which may cause a decrease in Th17 cells. The observed high level of TGF-β may be involved in maintaining immune tolerance in this condition, which is consistent with the maintained Treg cell population. However, our study showed that there were no differences in the proportion of Treg cells neither stimulated with EP nor treated with erythromycin in vitro. In a previous study, the unaltered Treg cell response was also observed in vivo.17 These contradictory results might be attributable to the complexity of the immune system in vivo and the differences in time course and dose of EP inhalation.
To our knowledge, we are the first to observe the effect of erythromycin on preventing EP-triggered emphysema, and the regulatory effect of erythromycin on Th1, Th17, and Treg cells in vivo. However, our study has some limitations that need further consideration. Firstly, our data showed that erythromycin restored the immune dysregulation observed in EP-induced emphysema. However, it remains unclear whether it acts directly on the receptor S-gal of the elastin peptide, or by reducing synergistic inflammatory factors. In addition, there are many other subsets of CD4+T cells, but our study was not designed to identify the effects of erythromycin on subsets beyond Th1, Th17, and Treg cells.
Conclusion
Our findings support the evidence that EP plays an important role in the development of COPD/emphysema. Mice that inhaled EP presented prominent characteristic of emphysema. These were accompanied by lung and systematic immune dysregulation including elevated Th1 and Th17 cells, and decreased Treg cells, similarly to CS-exposed mice. Prophylactic use of erythromycin could effectively attenuate the inflammation and CD4+T cell responses triggered by EP and prevent the progression of emphysema. This supports the potential value of erythromycin as a therapeutic agent in COPD with dysregulation of CD4+T cells.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81460009) and (81760010). The content of this report does not necessarily reflect the position or policy of the Chinese Government.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am J Respir Crit Care Med. 2017;195(5):557–582. doi:10.1164/rccm.201701-0218PP
2. Wang C, Xu J, Yang L, et al. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China Pulmonary Health [CPH] study): a national cross-sectional study. Lancet. 2018;391(10131):1706–1717. doi:10.1016/S0140-6736(18)30841-9
3. Lee SH, Goswami S, Grudo A, et al. Antielastin autoimmunity in tobacco smoking-induced emphysema. Nat Med. 2007;13(5):567–569. doi:10.1038/nm1583
4. Barnes PJ. Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2016;138(1):16–27. doi:10.1016/j.jaci.2016.05.011
5. Obeidat M, Faiz A, Li X. et al. The pharmacogenomics of inhaled corticosteroids and lung function decline in COPD. Eur Respir J;2019. 1900521. doi:10.1183/13993003.00521-2019
6. Segal LN, Clemente JC, Wu BG, et al. Randomised, double-blind, placebo-controlled trial with azithromycin selects for anti-inflammatory microbial metabolites in the emphysematous lung. Thorax. 2017;72(1):13–22. doi:10.1136/thoraxjnl-2016-208599
7. Rauscher S, Pomes R. The liquid structure of elastin. Elife. 2017;6:e26526.
8. Gaggar A, Weathington N. Bioactive extracellular matrix fragments in lung health and disease. J Clin Invest. 2016;126(9):3176–3184. doi:10.1172/JCI83147
9. Sellami M, Meghraoui-Kheddar A, Terryn C, et al. Induction and regulation of murine emphysema by elastin peptides. Am J Physiol Lung Cell Mol Physiol. 2016;310(1):L8–L23. doi:10.1152/ajplung.00068.2015
10. Houghton AM, Quintero PA, Perkins DL, et al. Elastin fragments drive disease progression in a murine model of emphysema. J Clin Invest. 2006;116(3):753–759.
11. Ofulue AF, Ko M. Effects of depletion of neutrophils or macrophages on development of cigarette smoke-induced emphysema. Am J Physiol. 1999;277(1 Pt 1):L97–L105. doi:10.1152/ajplung.1999.277.1.L97
12. Dhami R, Gilks B, Xie C, et al. Acute cigarette smoke-induced connective tissue breakdown is mediated by neutrophils and prevented by alpha1-antitrypsin. Am J Respir Cell Mol Biol. 2000;22(2):244–252. doi:10.1165/ajrcmb.22.2.3809
13. Senior RM, Griffin GL, Mecham RP. Chemotactic responses of fibroblasts to tropoelastin and elastin-derived peptides. J Clin Invest. 1982;70(3):614–618. doi:10.1172/JCI110654
14. Ghuysen-Itard AF, Robert L, Jacob: MP. Effet des peptides d'élastine sur la prolifération cellulaire. [Effect of elastin peptides on cell proliferation]. C R Acad Sci III. 1992;315(12):473–478.
15. Baranek T, Debret R, Antonicelli F, et al. Elastin receptor (spliced galactosidase) occupancy by elastin peptides counteracts proinflammatory cytokine expression in lipopolysaccharide-stimulated human monocytes through NF-kappaB down-regulation. J Immunol. 2007;179(9):6184–6192. doi:10.4049/jimmunol.179.9.6184
16. Debret R, Le Naour RR, Sallenave JM, et al. Elastin fragments induce IL-1beta upregulation via NF-kappaB pathway in melanoma cells. J Invest Dermatol. 2006;126(8):1860–1868. doi:10.1038/sj.jid.5700337
17. Meghraoui-Kheddar A, Pierre A, Sellami M, et al. Elastin receptor (S-gal) occupancy by elastin peptides modulates T-cell response during murine emphysema. Am J Physiol Lung Cell Mol Physiol. 2017;313(3):L534–l547. doi:10.1152/ajplung.00465.2016
18. Zhou Y, Tan X, Kuang W, et al. Erythromycin ameliorates cigarette-smoke-induced emphysema and inflammation in rats. Transl Res. 2012;159(6):464–472. doi:10.1016/j.trsl.2011.09.007
19. Kudoh S, Azuma A, Yamamoto M, et al. Improvement of survival in patients with diffuse panbronchiolitis treated with low-dose erythromycin. Am J Respir Crit Care Med. 1998;157(6 Pt 1):1829–1832. doi:10.1164/ajrccm.157.6.9710075
20. Albert RK, Connett J, Bailey WC, et al. Azithromycin for prevention of exacerbations of COPD. N Engl J Med. 2011;365(8):689–698. doi:10.1056/NEJMoa1104623
21. Mikura S, Wada H, Higaki M, et al. Erythromycin prevents the pulmonary inflammation induced by exposure to cigarette smoke. Transl Res. 2011;158(1):30–37. doi:10.1016/j.trsl.2011.03.001
22. He ZY, Ou LM, Zhang JQ, et al. Effect of 6 months of erythromycin treatment on inflammatory cells in induced sputum and exacerbations in chronic obstructive pulmonary disease. Respiration. 2010;80(6):445–452. doi:10.1159/000321374
23. Tan C, Huang H, Zhang J, et al. Effects of low-dose and long-term treatment with erythromycin on interleukin-17 and interleukin-23 in peripheral blood and induced sputum in patients with stable chronic obstructive pulmonary disease. Mediators Inflamm. 2016;2016:4173962. doi:10.1155/2016/4173962
24. Naderi N, Assayag D, Mostafavi-Pour-Manshadi SM, et al. Long-term azithromycin therapy to reduce acute exacerbations in patients with severe chronic obstructive pulmonary disease. Respir Med. 2018;138:129–136. doi:10.1016/j.rmed.2018.03.035
25. Herath SC, Normansell R, Maisey S, et al. Prophylactic antibiotic therapy for chronic obstructive pulmonary disease (COPD). Cochrane Database Syst Rev. 2018;10:Cd009764.
26. Bai J, Qiu SL, Zhong XN, et al. Erythromycin enhances CD4+Foxp3+ regulatory T-cell responses in a rat model of smoke-induced lung inflammation. Mediators Inflamm. 2012;2012:410232. doi:10.1155/2012/410232
27. Li M, Zhong X, He Z, et al. Effect of erythromycin on cigarette-induced histone deacetylase protein expression and nuclear factor-kappaB activity in human macrophages in vitro. Int Immunopharmacol. 2012;12(4):643–650. doi:10.1016/j.intimp.2011.12.022
28. Liu J, Zhong X, He Z, et al. [Erythromycin inhibits elastin peptides-induced differentiation of CD4(+)T cells into Th17 cells]. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2015;31(3):289–292.
29. Panduro M, Benoist C, Mathis D. Tissue tregs. Annu Rev Immunol. 2016;34:609–633. doi:10.1146/annurev-immunol-032712-095948
30. Chiappori A, Folli C, Balbi F, et al. CD4(+)CD25(high)CD127(-) regulatory T-cells in COPD: smoke and drugs effect. World Allergy Organ J. 2016;9:5. doi:10.1186/s40413-016-0095-2
31. Kuang LJ, Deng TT, Wang Q, et al. Dendritic cells induce Tc1 cell differentiation via the CD40/CD40L pathway in mice after exposure to cigarette smoke. Am J Physiol Lung Cell Mol Physiol. 2016;311(3):L581–L589. doi:10.1152/ajplung.00002.2016
32. Thurlbeck WM. Internal surface area and other measurements in emphysema. Thorax. 1967;22(6):483–496. doi:10.1136/thx.22.6.483
33. Wu YP, Cao C, Wu YF, et al. Activating transcription factor 3 represses cigarette smoke-induced IL6 and IL8 expression via suppressing NF-kappaB activation. Toxicol Lett. 2017;270:17–24. doi:10.1016/j.toxlet.2017.02.002
34. Maecker HT, Trotter J. Flow cytometry controls, instrument setup, and the determination of positivity. Cytometry A. 2006;69(9):1037–1042. doi:10.1002/cyto.a.20333
35. Whitehouse G, Gray E, Mastoridis S, et al. IL-2 therapy restores regulatory T-cell dysfunction induced by calcineurin inhibitors. Proc Natl Acad Sci U S A. 2017;114(27):7083–7088. doi:10.1073/pnas.1620835114
36. Duan MC, Tang HJ, Zhong XN, et al. Persistence of Th17/Tc17 cell expression upon smoking cessation in mice with cigarette smoke-induced emphysema. Clin Dev Immunol. 2013;2013:350727. doi:10.1155/2013/350727
37. Yang L, Ma QL, Yao W, et al. Relationship between the anti-inflammatory properties of salmeterol/fluticasone and the expression of CD4(+)CD25(+)Foxp3(+) regulatory T cells in COPD. Respir Res. 2011;12:142. doi:10.1186/1465-9921-12-142
38. Shan M, Cheng HF, Song LZ, et al. Lung myeloid dendritic cells coordinately induce TH1 and TH17 responses in human emphysema. Sci Transl Med. 2009;1(4):4ra10. doi:10.1126/scitranlsmed.3000154
39. Nakanishi Y, Kobayashi D, Asano Y, et al. Clarithromycin prevents smoke-induced emphysema in mice. Am J Respir Crit Care Med. 2009;179(4):271–278. doi:10.1164/rccm.200806-905OC
40. Marjanovic N, Bosnar M, Michielin F, et al. Macrolide antibiotics broadly and distinctively inhibit cytokine and chemokine production by COPD sputum cells in vitro. Pharmacol Res. 2011;63(5):389–397. doi:10.1016/j.phrs.2011.02.001
41. Hodge S, Hodge G, Holmes M, et al. Increased CD8 T-cell granzyme B in COPD is suppressed by treatment with low-dose azithromycin. Respirology. 2015;20(1):95–100. doi:10.1111/resp.12415
42. Veldhoen M, Hocking RJ, Atkins CJ, et al. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24(2):179–189. doi:10.1016/j.immuni.2006.01.001
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.