Back to Journals » Therapeutics and Clinical Risk Management » Volume 19
Erythrocyte Sedimentation Rate for Assisted Diagnosis of Pediatric Osteomyelitis: A Meta-Analysis
Received 19 September 2023
Accepted for publication 3 December 2023
Published 7 December 2023 Volume 2023:19 Pages 1039—1049
DOI https://doi.org/10.2147/TCRM.S440996
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Garry Walsh
Han Qi,1 Zhitao Zhu,2 Dongsheng Zhu3
1Department of Emergency Surgery, The Second People’s Hospital of Lianyungang, Lianyungang, People’s Republic of China; 2Department of Radiology, The Second People’s Hospital of Lianyungang, Lianyungang, People’s Republic of China; 3Department of Pediatric Orthopedics, The First People’s Hospital of Lianyungang, Lianyungang, People’s Republic of China
Correspondence: Dongsheng Zhu, Department of Pediatric Orthopedics, The First People’s Hospital of Lianyungang, Lianyungang, 222000, People’s Republic of China, Email [email protected]
Objective: For the diagnosis of pediatric osteomyelitis, the sensitivity, specificity, and predictive value of erythrocyte sedimentation rate (ESR) were evaluated in this study.
Methods: A systematic computer-based search was performed for relevant articles focusing on the ESR diagnosis of pediatric osteomyelitis in PubMed, Embase, and the Cochrane Library with an inclusion criteria: 1) the diagnostic utility of ESR for diagnosing osteomyelitis patients under the age of 18;2) two-by-two contingency tables can be obtained. Case reports, review papers, and animal experiments were excluded.
Results: The diagnostic meta-analysis included 8 studies involving 348 children with osteomyelitis, all of whom were tested for ESR. Diagnostic meta-analysis revealed a sensitivity and specificity of 0.90, 95% confidence interval (CI) (0.86– 0.93), and 0.50 (95% CI,0.47– 0.54) for ESR in pediatric osteomyelitis diagnosis, respectively. The positive likelihood ratio (LR), negative LR, and diagnostic odds ratio were 1.38,(95% CI,1.08– 1.78), 0.46, (95% CI,0.26– 0.73), and 3.20, (95% CI,1.33– 7.69), respectively. The area under the curve (AUC) was determined to be 0.80 based on the summary receiver operating characteristic curve (SROC).
Conclusion: The literature on the use of ESR in pediatric osteomyelitis diagnosis was thoroughly reviewed in this study. It was also found that ESR may be useful as a biomarker for pediatric osteomyelitis diagnosis. Due to its low specificity, it should be used in combination with other markers such as C-reactive protein, neutrophil percentage, and white blood cell count.
Keywords: osteomyelitis, pediatric, ESR, diagnosis, meta-analysis
Introduction
Osteomyelitis is an inflammation of the bones that is brought on by an acute bacterial infection.1 Osteomyelitis is more common in boys, younger children, with incidence rates ranging from 2 to 13 per 10,000 young people.2 In clinical practice, the skin, oral cavity, or respiratory mucous membranes may all be the source of infection, as the bacteria responsible for the illness are sometimes difficult to identify, with Staphylococcus aureus being the most common cause of osteomyelitis, being implicated in more than 75% of cases.3 The main clinical signs of osteomyelitis are localized redness, swelling, suppuration, and pain, however the specificity is weak and the early characteristics of osteomyelitis are also unclear.4 Early diagnosis of acute osteomyelitis is often challenging, but timely treatment to minimize bone destruction is critical.5 Failure to diagnose and treat such infections can lead to chronic osteomyelitis, bone destruction or septicemia.6 After an infection has taken root, a rapid chain reaction of local and systemic inflammatory reactions, with the creation of various inflammatory mediators, ensues, and in severe cases, systemic inflammation may occur. Therefore, early diagnosis and timely treatment of osteomyelitis is of critical importance. Inflammatory markers in blood taken from the lab were reported to accurately identify infants with probable acute bone infections.7,8 The most common diagnostic auxiliary examination signs of osteomyelitis in pediatric patients at this time are the erythrocyte sedimentation rate (ESR), C-reactive protein, neutrophil percentage, and white blood cell count.5,8 ESR is a sensitive indicator of inflammation in the blood that can be used to predict the probability of bone infection.9 There have been several reports in the literature suggesting that ESR is useful in identifying acute osteomyelitis in children.10–12 In light of the expanding use of ESR in this sector, we conducted a thorough analysis of significant literature reports in order to provide a scientific basis for the clinical application of ESR in the diagnosis of pediatric osteomyelitis.
Materials and Methods
Search Strategy
For the meta-analysis, we searched PubMed, Embase, and the Cochrane Library for publications with the following subject terms: “osteomyelitis” or “bone infection” and “erythrocyte sedimentation rate” or “ESR” and “adolescent” or “children” or “pediatrics” (last search: April 01, 2023). Search time: April 15, 2023. Only English literatures were included. We also looked over the list of references for earlier systematic reviews. In conducting and reporting this systematic review, we followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 statement.
Inclusion and Exclusion Criteria
The following topics were included in this study: (a) diagnostic utility of ESR for the diagnosis of osteomyelitis; (b) the clinical gold standard for the determination of osteomyelitis was bacterial culture of bone aspirates; (c) feasibility of the two-by-two contingency table; and (d) children under the age of 18. Case reports, review papers, and animal experiments were among the types of research not included in the analysis.
Quality Assessment
The technique was validated using the Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2); the methodological quality of the included studies was assessed.
Literature Screening and Data Extraction
The inclusion and exclusion criteria were independently evaluated by two reviewers. The third reviewer settled the disagreement. The following information was taken from the literature: study, year, country, age, sample size, ESR threshold, values of true positives (TP), false negatives (FN), false positives (FP), and true negatives (TN) per study.
Criteria of Heterogeneity Test
First, there were variations in diagnostic tests due to threshold and non-threshold effects. The receiver operating characteristic (ROC) plane graph would have a “shoulder-arm” point distribution when the threshold effect was present. If threshold effects were present, it was best to calculate the area and Q-index using the Summary ROC (SROC) curve method. Individual evaluation measures, such as sensitivity and specificity, can be immediately incorporated when there were no threshold effects.
Statistical Methods
Software such as Review Manager 5.3, Meta-disc 1.4, STATA 12.0, and R 3.4 were used for statistical analysis. Threshold effects were found using Spearman correlation analysis. Sensitivity, specificity, positive likelihood ratio, negative likelihood ratio, and diagnostic odds ratio were calculated. I2 values were used to gauge the heterogeneity between trials. I2 values more than 50% indicated the presence of considerable heterogeneity. Potential publication bias was ruled out by using a Deeks’ regression test for funnel plot asymmetry.
Results
Basic Characteristic
The aforementioned search approach resulted in 260 papers, of which 8 were eventually included in the qualitative analysis. (Figure 1): Butbul-Aviel et al,12 Riise et al,11 Paakkonen et al,10 Reitzenstein et al,8 Nduaguba et al,13 Maciej K et al,14 Zulian et al,15 Chen et al.16 Of this, 2 were from USA, 1 each from Finland, Israel, Norway, China, Switzerland, and Italy. A total of 1016 patients were enrolled from 2005 until 2021. (Table 1 and Table 2).
 |
Table 1 Basic Characteristic of the Included Studies |
 |
Table 2 Basic Data of the Included Studies |
 |
Figure 1 Diagram of workflow in the systematic review and meta-analysis. |
Quality Evaluation
The QUADAS-2 criteria, which encompass patient selection, index test, reference standard, and patient flow, were used to assess the quality of the included studies. Figure 2 presented the outcome of the quality assessment criteria.
 |
Figure 2 Methodological quality summaries.(A)Risk of bias summary; (B)Risk of bias graph. |
Threshold Effect Detection
The initial stage in this study was to determine whether a threshold effect exists, as several important factors could give rise to a threshold effect. When the ROC plane graph was examined, it was discovered that there is no “shoulder-arm” point distribution, and the Spearman correlation coefficient was 0.38, P=0.34, indicating that no threshold effect has occurred. (Figure 3).
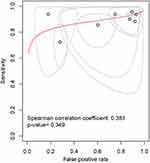 |
Figure 3 ROC plane diagram for threshold effect detection. |
The Value of ESR in Diagnosing Osteomyelitis in Children
Data for 8 studies were collected and these articles were included in the final meta-analysis. Based on these results, it was found that the pooled sensitivity of CRP for the diagnosis of osteomyelitis in children was 0.90 (95% Confidence interval (CI), 0.86–0.93) (Figure 4A), the pooled specificity was 0.50 (95% CI, 0.47–0.54) (Figure 4B), and pooled positive LR, negative LR, diagnostic OR, were 1.38 (95% CI, 1.08–1.78)(Figure 5A), 0.46 (95% CI, 0.29–0.73)(Figure 5B), 3.20, 95% CI (1.33−7.69)(Figure 5C), respectively. After meta-analysis, ESR’s positive predictive capacity in the diagnosis of pediatric osteomyelitis increased by 10% (from 0.2 to 0.22), while it’s negative predictive ability increased by 50% (from 0.2 to 0.1). This was demonstrated using Fagan’s nomogram (Figure 6A). Due to pooled positive LR 10 and pooled negative LR >0.1, the predictive practicality, however, was not very good. (Figure 6B). The area under the sROC curve was 0.80 (Figure 7).
 |
Figure 4 (A) Sensitivity of the forest to the ESR diagnosis of osteomyelitis in children; (B) Specificity of the forest to the ESR diagnosis of osteomyelitis in children. |
 |
Figure 7 Summary receiver operating characteristics curve for ESR diagnosis in children with osteomyelitis. |
Publication Bias
According to Deeks’ regression test, there was no discernible publication bias. (P=0.33) (Figure 8).
 |
Figure 8 Deeks’ funnel plot for ESR diagnosis of osteomyelitis in children. |
Discussion
Osteomyelitis is an inflammatory bone disease, which translates from the Greek as “inflammation of the bone marrow”.17 Osteomyelitis is brought on by a bacterial infection. The most common route for infection to migrate into bone tissue is through the bloodstream, although other routes of infection include trauma, local diffusion and surgery.1 Staphylococcus aureus is the most frequent pathogen and accounts for the majority of cases of hematologic osteomyelitis cases in children.3 Currently, bacterial culture of bone aspirates is the gold standard for osteomyelitis diagnosis.12 However, children sometimes refuse to participate in bone aspirates, and bacterial cultures typically take several days, making it difficult to diagnose osteomyelitis in children at an early stage.
The ESR calculates the rate in millimeters (mm)/hour, at which erythrocytes fall or settle in the plasma of a randomly collected anticoagulated blood specimen over a given period of time (often 60 minutes).18 Dr. Edmund Faustyn Biernacki made the first observations of this phenomena in 1897, finding that blood settled at different rates in different people and that red blood cells settled more quickly in the presence of more fibrinogen.19 The ESR can also be significantly affected by changes in multiple plasma proteins and their interactions with each other, such as cytokine-induced elevations in acute phase proteins in response to infection, inflammation or trauma.20 ESR is currently frequently used in the diagnosis of acute bone inflammatory diseases such as septic arthritis, osteomyelitis and acute rheumatic fever.12. Since ESR is a sensitive biomarker of an essential inflammatory component of infections, it is now commonly used as a marker of infection. Despite our best efforts, we were unable to find any meta-analyses using ESR to identify pediatric osteomyelitis. So, we conducted a meta-analysis to examine the utility of ESR as a diagnostic indicator for pediatric osteomyelitis, and eventually included 8 studies. 6 retrospective studies and 2 prospective trials with a total of 1106 children were included in our analysis, which pooled data from 8 studies with excellent overall quality. Of those, 348 children developed osteomyelitis. Our meta-analysis revealed a joint sensitivity and specificity of 90% and 50% for pediatric osteomyelitis, respectively. These findings suggested that while pediatric osteomyelitis has a significant rate of missed diagnoses; there are few cases of misdiagnosis.
Both positive and negative LR are clinically significant indicators of efficacy in diagnostic trials and are relatively independent. They are frequently employed to calculate post-test probabilities in order to improve the therapeutic relevance of the findings. The meta-analysis revealed a pooled positive LR of 1.38 and a pooled negative LR of 0.46. In addition, the Fagan's nomogram revealed that a pooled negative LR of 0.46, and reduced the post-test probability from 20% to 10%. However, prior studies have demonstrated that the likelihood of diagnosing or eliminating a certain ailment increases considerably when pooled positive LR >10 and pooled negative LR < 0.1.21 This suggested that ESR still has some limitations when used to diagnose pediatric osteomyelitis.
When AUC is less than 0.5, it shows no diagnostic value; it signals a low diagnostic value when it is between 0.5 and 0.7; a value between 0.7 and 0.9 indicates a high diagnostic value; moreover, when it exceeds 0.9, it represents the best diagnostic accuracy.22 The results showed a good level of diagnostic precision with an AUC for this research of 0.80.
This study also had a number of other shortcomings, including: 1) This meta-analysis included both prospective and perspective studies, which may have had some bearing on the results; 2) The diagnostic value of serological biomarkers for osteomyelitis is influenced by multiple factors, such as infection stage, site of infection, previous treatment, and many confounding factors were not well controlled; 3) Some information were not provided about the clinical characteristics of the patients, such as infection site, stage of infection, pathogen type; 4) There may have been a linguistic bias as only English literature was looked at in this study.
Conclusion
To diagnose osteomyelitis in pediatric patients, this study set out to thoroughly evaluate the literature on the use of ESR as a biomarker. The specificity of ESR for the diagnosis of pediatric osteomyelitis is low, though. To diagnose pediatric osteomyelitis, it should therefore be used in conjunction with other tests rather than as a single biomarker. Due to the limitation of this study, much more high-quality studies will be required in the future to evaluate the applicability value of their combined diagnosis.
Abbreviations
ESR, erythrocyte sedimentation rate; QUADAS-2, Quality Assessment of Diagnostic Accuracy Studies-2; TP, true positive; FP, false positive; TN, true negative; FN, false negative; ROC, receiver operating characteristic; SROC, summary receiver operating characteristic; LR, likelihood ratio; DOR, diagnostic odds ratio; CI, Confidence interval.
Data Sharing Statement
They may get from correspondence author.
Ethical Approval
This study was approved by the ethics committee of The Second People’s Hospital of Lianyungang.
Funding
There is no funding to report.
Disclosure
The authors declare that they have no competing interest in this work.
References
1. Lew DP, Waldvogel FA. Osteomyelitis. Lancet. 2004;364(9431):369–379. doi:10.1016/S0140-6736(04)16727-5
2. Funk SS, Copley LA. Acute hematogenous osteomyelitis in children: pathogenesis, diagnosis, and treatment. Orthop Clin North Am. 2017;48(2):199–208. doi:10.1016/j.ocl.2016.12.007
3. Silago V, Mushi MF, Remi BA, et al. Methicillin resistant Staphylococcus aureus causing osteomyelitis in a tertiary hospital, Mwanza, Tanzania. J Orthop Surg Res. 2020;15(1):95. doi:10.1186/s13018-020-01618-5
4. Lee YJ, Sadigh S, Mankad K, Kapse N, Rajeswaran G. The imaging of osteomyelitis. Quant Imaging Med Surg. 2016;6(2):184–198. doi:10.21037/qims.2016.04.01
5. Deng S, Wang Y, Liu S, et al. Extracellular vesicles: a potential biomarker for quick identification of infectious osteomyelitis. Front Cell Infect Microbiol. 2020;10:323.
6. Dunsmuir RA, Barnes SJ, McGarrity G. ”Goalkeeper’s Hip”: acute haematogenous osteomyelitis secondary to apophyseal fractures. Br J Sports Med. 2006;40(9):808–809. doi:10.1136/bjsm.2005.025031
7. Lyons TW, Kharbanda AB, Thompson AD, et al. A clinical prediction rule for bacterial musculoskeletal infections in children with monoarthritis in lyme endemic regions. Ann Emergency Med. 2022;80(3):225–234.
8. Reitzenstein JE, Yamamoto LG, Mavoori H. Similar erythrocyte sedimentation rate and C-reactive protein sensitivities at the onset of septic arthritis, osteomyelitis, acute rheumatic fever. Pediatr Rep. 2010;2(1):e10. doi:10.4081/pr.2010.e10
9. Li G, Weng J, Xu C, Wang D, Xiong A, Zeng H. Factors associated with the length of stay in total knee arthroplasty patients with the enhanced recovery after surgery model. J Orthopaed Surg Res. 2019;14(1):343. doi:10.1186/s13018-019-1389-1
10. Pääkkönen M, Kallio MJ, Kallio PE, Peltola H. Sensitivity of erythrocyte sedimentation rate and C-reactive protein in childhood bone and joint infections. Clin Orthop Relat Res. 2010;468(3):861–866. doi:10.1007/s11999-009-0936-1
11. Riise ØR, Kirkhus E, Handeland KS, et al. Childhood osteomyelitis-incidence and differentiation from other acute onset musculoskeletal features in a population-based study. BMC Pediatr. 2008;8(1):45. doi:10.1186/1471-2431-8-45
12. Butbul-Aviel Y, Koren A, Halevy R, Sakran W. Procalcitonin as a diagnostic aid in osteomyelitis and septic arthritis. Pediatr Emerg Care. 2005;21(12):828–832. doi:10.1097/01.pec.0000190226.12610.24
13. Nduaguba AM, Flynn JM, Sankar WN. Septic Arthritis of the Elbow in Children: clinical Presentation and Microbiological Profile. J Pediatr Orthop. 2016;36(1):75–79. doi:10.1097/BPO.0000000000000390
14. Albiński MK, Lutz N, Ceroni D, N’Dele D, Zambelli PY, Bregou A. Paediatric musculoskeletal infections with Panton-Valentine leucocidin. Swiss Med. Wkly. 2018;148:w14669.
15. Zulian F, Marigo E, Ardenti-Morini F, et al. Osteoperiostitis in children: proposal for a diagnostic algorithm. Eur J Pediatr. 2021;180(10):3229–3235. doi:10.1007/s00431-021-04058-3
16. Chen JA, Lin HC, Wei HM, et al. Clinical characteristics and outcomes of culture-negative versus culture-positive osteomyelitis in children treated at a tertiary hospital in central Taiwan. J Microbiol Immunol Infect. 2021;54(6):1061–1069. doi:10.1016/j.jmii.2020.08.005
17. Kavanagh N, Ryan EJ, Widaa A, et al. Staphylococcal osteomyelitis: disease progression, treatment challenges, and future directions. Clin Microbiol Rev. 2018;31(2). doi:10.1128/CMR.00084-17
18. Batlivala SP. Focus on diagnosis: the erythrocyte sedimentation rate and the C-reactive protein test. Pediatr Rev. 2009;30(2):72–74. doi:10.1542/pir.30.2.72
19. Grzybowski A, Sak JJ. Who discovered the erythrocyte sedimentation rate? J Rheumatol. 2011;38(7):1521–1522; author reply 1523. doi:10.3899/jrheum.101312
20. Bray C, Bell LN, Liang H, et al. Erythrocyte Sedimentation Rate and C-reactive Protein Measurements and Their Relevance in Clinical Medicine. WMJ. 2016;115(6):317–321.
21. Kraft CS, Parrott JS, Cornish NE, et al. A laboratory medicine best practices systematic review and meta-analysis of Nucleic Acid Amplification Tests (NAATs) and algorithms including NAATs for the diagnosis of clostridioides (Clostridium) difficile in adults. Clin Microbiol Rev. 2019;32(3).
22. Redding DW, Pigot AL, Dyer EE, Şekercioğlu ÇH, Kark S, Blackburn TM. Location-level processes drive the establishment of alien bird populations worldwide. Nature. 2019;571(7763):103–106. doi:10.1038/s41586-019-1292-2
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


