Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 10 » Issue 1
Epithelial mesenchymal transition in smokers: large versus small airways and relation to airflow obstruction
Authors Mahmood M, Singh Sohal S, Shukla SD, Ward C, Hardikar A, Noor WD, Muller HK, Knight D, Walters EH
Received 16 January 2015
Accepted for publication 20 April 2015
Published 4 August 2015 Volume 2015:10(1) Pages 1515—1524
DOI https://doi.org/10.2147/COPD.S81032
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Malik Quasir Mahmood,1,* Sukhwinder Singh Sohal,1,2,* Shakti Dhar Shukla,1 Chris Ward,3 Ashutosh Hardikar,4 Wan Danial Noor,1 Hans Konrad Muller,1 Darryl A Knight,5 Eugene Haydn Walters1
1NHMRC Centre of Research Excellence for Chronic Respiratory Disease and Lung Ageing, School of Medicine, University of Tasmania, Hobart, TAS, Australia; 2School of Health Sciences, Faculty of Health, University of Tasmania, Launceston, TAS, Australia; 3Institute of Cellular Medicine, Newcastle University, Newcastle upon Tyne, Tyne and Wear, UK; 4Royal Hobart Hospital, Hobart, TAS, Australia; 5School of Biomedical Sciences and Pharmacy, University of Newcastle, Callaghan, NSW, Australia
*These authors contributed equally to this work
Background: Small airway fibrosis is the main contributor in airflow obstruction in chronic obstructive pulmonary disease. Epithelial mesenchymal transition (EMT) has been implicated in this process, and in large airways, is associated with angiogenesis, ie, Type-3, which is classically promalignant.
Objective: In this study we have investigated whether EMT biomarkers are expressed in small airways compared to large airways in subjects with chronic airflow limitation (CAL) and what type of EMT is present on the basis of vascularity.
Methods: We evaluated epithelial activation, reticular basement membrane fragmentation (core structural EMT marker) and EMT-related mesenchymal biomarkers in small and large airways from resected lung tissue from 18 lung cancer patients with CAL and 9 normal controls. Tissues were immunostained for epidermal growth factor receptor (EGFR; epithelial activation marker), vimentin (mesenchymal marker), and S100A4 (fibroblast epitope). Type-IV collagen was stained to demonstrate vessels.
Results: There was increased expression of EMT-related markers in CAL small airways compared to controls: EGFR (P<0.001), vimentin (P<0.001), S100A4 (P<0.001), and fragmentation (P<0.001), but this was less than that in large airways. Notably, there was no hypervascularity in small airway reticular basement membrane as in large airways. Epithelial activation and S100A4 expression were related to airflow obstruction.
Conclusion: EMT is active in small airways, but less so than in large airways in CAL, and may be relevant to the key pathologies of chronic obstructive pulmonary disease, small airway fibrosis, and airway cancers.
Keywords: EMT, EGFR, S100A4, vimentin, fragmentation, small airways
Background
Chronic obstructive pulmonary disease (COPD) is a huge global health problem.1 As its core is progressive narrowing of the airways caused by noxious particles and gases particularly from cigarette smoke.2 COPD airway remodeling changes are mostly related to reduced airflow due to small airway fibrosis, and ultimately, obliteration.3 Emphysema is a later variable secondary phenomenon.4
Recent observations have emphasized reticular basement membrane (Rbm) fragmentation, cellularity, and hypervascularity in large-airway biopsies from smokers and COPD patients.5–7 There is limited evidence that epithelial mesenchymal transition (EMT) is also active in small airways.8,9 Angiogenesis in the Rbm is specifically smoking related,10,11 while other features are most marked in COPD.5
In EMT, epithelial cells transition into a mesenchymal phenotype with subsequent migration into the underlying lamina propria and may be associated with accumulation of myofibroblasts immediately adjacent to the lamina propria. EMT has been implicated in tissue fibrosis12 in the so-called Type-2 form and when associated with hypervascularity (Type-3) with epithelial cancers development.13
COPD itself is a major risk factor for lung cancer.14,15 It is notable that epithelial cancers make up approximately 90% of all human malignancies, suggesting that epithelial cells are especially unstable and EMT-associated mechanisms may be the common factor. Local tissue angiogenesis is especially regarded as another key aspect of both the premalignant and malignant phases of epithelial cancer development.16
Our previous published work used large-airway endoscopic biopsies.7 The present study was designed to compare the degree of EMT activity in small versus matching large airways and also type of EMT in each compartment, using lung tissue resected from smokers undergoing lung resection for lung cancer. We also wished to explore the relationship between epithelial and EMT activity and the degree of airflow obstruction in this group selected to have a wide variation in chronic airflow limitation (CAL).
Materials and methods
Study design
The Tasmanian health and medical Human Research Ethics Committee approved this study (#EC00337). All subjects gave written, informed consent prior to participation.
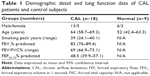
Eighteen patients with CAL were included. All had primary non small cell lung cancer and an approximately equal mix of squamous and adenocarcinoma, and were smokers. Nine patients had demonstrated stage 1 or 2 COPD17 on spirometry (forced expiratory ratio <70%), and nine patients had small airway disease only with scalloping of the expiratory limb of the flow–volume curve (forced expiratory flow25–75 <68% predicted). By selection, there was no history of other chronic respiratory disorders.
Sections from nine normal nonsmoker subjects (normal control [NC]) were included as a control group for small airways data and were provided from the bio bank at the University of British Columbia.
Study samples
Surgical resection material containing noncancer affected small and large airways were fixed in formalin within minutes of surgery. At processing, tissue blocks of large and small airways (<2.5 mm internal diameter)18 were separately embedded in paraffin for our analyses.
Immunostaining
Serial sections of resected tissue were immunostained for EMT mesenchymal biomarkers (S100A4, Vimentin), for the epithelial activation marker (epidermal growth factor receptor [EGFR]) and for vascularity using anti-Type-IV collagen for vessels in the Rbm. Sections of 3-μm thickness were mounted and stained with hematoxylin and eosin for morphological assessment and suitability for immunostaining. At room temperature, optimal sections were stained with the following antibodies: polyclonal anti-S100A4 (Dako, catalog no A5114, at 1:2,000 for 90 minutes), antivimentin monoclonal antibody (Dako, catalog no M7020, at 1:1,000 for 60 minutes), and Type-IV collagen monoclonal antibody (Dako, catalog no M0785, at 1:100 for 90 minutes) and monoclonal anti-EGFR (Dako, catalog no M3563, at 1:1,000 for 90 minutes). In each run, a section stained with immunoglobulin G1-negative control (X0931 clone DAKGO1; Dako Cytomation) was included to ensure absence of false-positive staining. Bound antibodies were elaborated by using horseradish peroxidase-conjugated DAKO Envision plus reagent (catalog no K4001, anti-mouse or K4003 anti-rabbit) and diaminobenzidine for a brown color resolution (catalog no K3468; Dako Cytomation). We have extensively used and published these methods.5,6,19,20
Tissue section analysis and quantitation
Computer-assisted image analysis was performed by using microscopy at 40× magnification (Leica DM 2500, Microsystems, Germany), a Spot insight 12 digital camera (Spot Imaging, MI, USA) and Image Pro V5.1 software (Media Cybernetics, Rockville, MD, USA). We randomly choose eight good fields for both large and small airways without a tumor interface, from each lung resection, for each of the biomarkers. We quantified the degree of Rbm fragmentation by measuring the length of linear clefts and expressed it as a percentage of total Rbm length. Slides were randomly mingled, coded, and assessed blind. All slides were quantified for epithelial activation (EGFR) and EMT biomarkers, ie, fragmentation, S100A4, vimentin, and vascularity (Type-IV collagen) in both large and small airways, in a single batch by an experienced observer (MQM). Quality assurance was provided by a professional academic pathologist (HKM).
EGFR was quantitated as a percentage of epithelial area stained while the EMT biomarkers (S100A4, vimentin) were quantitated as the number of basal cells in epithelium and cells in Rbm per millimeter of Rbm.
Statistical analysis
Large airways have previously been assessed against normal smokers and nonsmoking controls5 and that was not repeated. Current data for large and small airways in CAL were compared against each other, and then for small airways against NCs.
Because the data were normally distributed, results for each marker are presented as mean and 95% confidence interval (CI). Comparisons between different markers in large versus small airways, and small airways, normal versus CAL, used paired t-test. Associations between variables were assessed using Pearson’s rank test. Statistical analyses were performed using SPSS (statistics version 20.0; IBM Corporation, Armonk, NY, USA) for Windows 7.0 and a P-value of ≤0.05 was considered statistically significant.
Results
The overall demographics of two groups are presented in Table 1 and results in Tables 2 and 3.
EGFR expression in epithelium
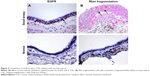
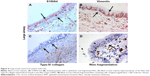
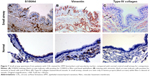
A greater proportion of the airway epithelium stained for EGFR in large airways of CAL patients (mean 11.4%, CI 9.9–12.8) (Figure 1) compared to CAL small airways (mean 6.8%, CI 5.5–8.1) (P<0.001) (Figure 2). There was also a marked difference between small airways in the CAL group and the normal small airways (mean 0.6%, CI 0.3–0.8) (P<0.001) (Figure 3).
Fragmentation in the Rbm
Fragmentation of the Rbm was more marked in large airways from CAL subjects (mean 27.9%, CI 24.3–31.6) (Figure 4) than in their small airways (mean 14.9%, CI 13–16.9) (P<0.001) (Figure 2). Small airways from CAL subjects showed significantly greater fragmentation than normal small airways (mean 2.4%, CI 2.1–2.7) (P<0.001) (Figure 3).
S100A4- and vimentin-positive cells in the basal epithelium
We observed S100A4 and vimentin staining in the basal layer of the epithelium in both large and small airways in CAL, but with considerably more marked expression in large airways (P<0.001 for each) (Figure 4) (S100A4, mean 42.9 cells/mm3, CI 38.5–47.4; vimentin, mean 69.7 cells/mm3, CI 60.1–79.3) than in small airways (S100A4, mean 25.9 cells/mm3, CI 22.8–28.9; vimentin, mean 41.5 cells/mm3, CI 35.8–47.4) (Figures 5 and 6). In turn there was significantly greater expression in CAL small airways than in NC small airways (S100A4, mean 6.4 cells/mm3, CI 4.7–7.9; vimentin, mean 8.2 cells/mm3, CI 6.9–9.4) (for both P<0.001) (Figure 7).
S100A4- and vimentin-positive cells in the Rbm
In comparison to CAL large airways (S100A4, mean 24.1 cell/mm3, CI 22.2–25.9; vimentin, mean 36.1 cells/mm3, CI 32.8–39.5) (Figure 4), there was a slight but significant low expression of these mesenchymal markers in small airways (S100A4, mean 13.9 cells/mm3, CI 12.5–15.3; vimentin, mean 27.8 cells/mm3, CI 25.4–30.2) (for both P<0.001) (Figures 5 and 6). However, once again, expression was greater in the small airways in CAL compared to NC small airways (S100A4, mean 5.3 cells/mm3, CI 4.1–6.5; vimentin, mean 8.1 cells/mm3, CI 6.7–9.5) (for both P<0.001) (Figure 7).
Blood vessels in the Rbm
Hypervascularity of the Rbm was found only in CAL large airways (mean 8.5 vessels per millimeter of Rbm, CI 7.6–9.4) (Figure 4) and not in the small airways either in CAL (mean 0.2 vessels per millimeter of Rbm, CI 0.05–0.3) (P<0.001) or the control group (mean 0.05 vessels per millimeter of Rbm, CI 0.02–0.13) (P= not significant) (Figure 7 and 8).
Regression data
In CAL subjects, there were significant relationships between increasing airflow obstruction and increasing epithelial activation (EGFR expression) and enhanced S100A4 expression in both epithelial basal cells and in Rbm cells, generally most marked for small airways pathology and for small airway obstruction (forced expiratory flow25–75% predicted) (Figure 9). There was quite a dichotomy between these relationships and the absence of such relationships for Rbm fragmentation and vimentin expression (Table 4).
Discussion
This study sought to analyze the expression of classic EMT structural and mesenchymal biomarkers, epithelial activation, and also vascular changes, in matching large and small airways from smoking patients with airflow obstruction (CAL). We have previously demonstrated that in large-airway biopsies from smokers compared to NC tissue, epithelial activation, EMT biomarkers, and related classic structural changes are highly expressed and that these changes are greatest in those with COPD.5 Here we show that small airways from a group of CAL patients also demonstrated active EMT significantly above normal, but uniformly less so than in large airway. However, in small airways, EMT changes are not associated with hypervascularity, ie, could be considered as the profibrotic Type-2 EMT rather than the more malignancy-associated Type-3 EMT.
In both large and small airways in this CAL group, staining for S100A4 and vimentin was focused in the basal cell layer of the epithelium. This may highlight the likelihood of these cells being especially involved in undergoing transition to a mesenchymal phenotype. Similar positive staining of cells in the clefts of the disrupted Rbm further strengthens this concept of transition and migration of these basal cells. We have previously shown that the Rbm cells are not immune or inflammatory cells and that they do strongly express matrix metalloprotein-9, ie, with proteolytic capacity to digest their way through the lamina propria, thereby likely causing Rbm fragmentation.5,6
The differentiation into Type-2 EMT in small airways (<2 mm internal diameter) is consistent with fibrosis and obliteration here. Classically more cancer develops in large airways,18,21,22 especially for the squamous cell variety where Type-3 EMT predominates. Myofibroblast accumulation accompanies the damage to small airways in COPD.23 The origin of these myofibroblasts is not fully understood, but our data suggest that they might be derived from EMT. Endothelial mesenchymal transition, pericyte transformation, or stroma cells are other possible sources.24,25 Further work is needed to delineate these possibilities.
We have now shown that EMT is active in small airways and that at least S100A4 expression is associated with airflow obstruction, suggesting that this fibroblast marker may indeed be relevant to airway fibrosis. We are analyzing in detail the fibroblast subtypes and related changes in extracellular matrix. Interestingly, EMT is also thought to be involved in the development of idiopathic pulmonary fibrosis,26 and because this is also frequently smoking related, it suggests a certain pathological unity with the process in the small airways.
In agreement with our findings, Milara et al recently also observed upregulation of EMT biomarkers in small airways in COPD.9 Such EMT changes could also be induced in culture by cigarette smoke. Our study takes this further by quantifying Rbm structural changes, comparing large and small airways and characterizing Type-2 versus Type-3 EMT. Milara et al also implicated increasing cyclic adenosine monophosphate in EMT activity in the airways,27 while others implicated an urokinase plasminogen activator receptor-dependent signaling pathway28 or a muscarinic mechanism.29
COPD is a major independent risk factor for the development of non small cell lung cancer22 with squamous cell tumors developing in central airways and adenocarcinoma more in the peripheral airway tree. Multiple mechanisms are associated with the development of malignancy including genetic changes secondary to environmental insult (tobacco smoke) but it is also known that EMT, especially in association with hypervascularity, ie, Type-3, also has a strong procancerous influence.11,30 Thus, the local airway microenvironment is very important in carcinogenesis and metastasis.31 It is thought that vessels are immunoprotective through the expression of endocan, which inhibits the local antitumor activity of natural killer lymphocytes.32 It is also suggested that EMT gives rise to cancer-stimulating stromal cells (cancer-associated fibroblasts), which produce tumor-enhancing growth factors.33
The main strength of this study is that we directly investigated human tissue from CAL-affected individuals. Further, we have a fairly robust number of participants with good spread of airflow obstruction in large and small airways. Some limitations are, however, inevitable, especially the availability of NC small airway samples and international collaboration has been needed to provide this. We have focused on the empiric but classically described34 “downstream” phenotypic manifestation of EMT, ie, structural changes and protein expression. We will follow-up with detailed comparative analysis of more upstream pathways and expression of EMT-related transcription factors in large- versus small airway tissue and will perform molecular studies on both whole tissues and cell cultures.
Finally, due to the number of patients available to analyze, we have treated those with frank COPD (forced expiratory ratio <70%) and those only with abnormal small airway function (scalloping of the expiratory limb of the volume loop and low forced expiratory flow25–75%) as a single group. The advantage that this gave us is that we had a wide range of physiological values for regression analyses, which have produced extremely interesting results. Our current group of cancer patients contained only four subjects without evidence of any airflow obstruction. Their details have not been included in this paper, although we will follow-up as numbers increase. It is interesting how relatively uncommon completely physiologically normal smoking cancer patients seem to be, but it is also noteworthy that they do seem to have EMT changes in both large and small airways, though less marked. Indeed, had their data been added to our regressions, these would have been even more significant, ie, less obstruction and less EMT together.
Conclusion and summary
Our data provide evidence for active EMT in small as well as the previously demonstrated large airways of smokers with CAL. On the basis of Rbm hypervascularity, the EMT in large airways is characteristic of so-called Type-3 EMT, while Type-2 EMT is predominant in small airways. This is consistent with the major pathologies in the two compartments: in large airways, the process might especially form a microenvironment for the development of lung cancer, while Type-2 EMT is thought to be especially profibrotic and might be more related to small airway obstruction and obliteration. The relationship seen between the fibroblastic marker S100A4 and airflow obstruction would certainly fit with this.
Acknowledgments
This study was funded by the National Health and Medical Research Council grant 490023 and the Royal Hobart Hospital Research Foundation. The sponsors had no role in the study design; in the collection, analysis, and interpretation of the data; or in the decision to submit the article for publication.
Author contributions
Performed the literature search, histological and statistical analysis, data interpretation, clinical assessment, and writing and revision of the manuscript: MQM. Assisted in histological and statistical analysis, and writing of paper: SSS. Assisted in histological analysis and writing of manuscript: SDS. Project advisor and assisted in drafting of article: CW. Performed surgical resection of lung tissue and contributed in manuscript writing: AH. Assisted in recruiting patients and contributed in data acquisition and the writing of the manuscript: WDN. Advised on histology strategies, quality control and writing of manuscript: HKM. Provided normal lung tissue and contributed in writing and revising the paper: DK. Designed the study and contributed to clinical assessments, overview of all analyses, data interpretation, writing and revision of the paper: EHW.
Disclosure
The authors report no conflicts of interest in this work.
References
Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3(11):e442. | ||
Fullerton DG, Bruce N, Gordon SB. Indoor air pollution from biomass fuel smoke is a major health concern in the developing world. Trans R Soc Trop Med Hyg. 2008;102(9):843–851. | ||
Hogg JC, McDonough JE, Suzuki M. Small airway obstruction in COPD: new insights based on micro-CT imaging and MRI imaging. Chest. 2013;143(5):1436–1443. | ||
Dunnill MS, Ryder R. A quantitative study of the relationship between chronic bronchitis, emphysema and smoking. Chest. 1971;59(suppl):35S+. | ||
Sohal SS, Reid D, Soltani A, et al. Reticular basement membrane fragmentation and potential epithelial mesenchymal transition is exaggerated in the airways of smokers with chronic obstructive pulmonary disease. Respirology. 2010;15(6):930–938. | ||
Sohal SS, Reid D, Soltani A, et al. Evaluation of epithelial mesenchymal transition in patients with chronic obstructive pulmonary. Respir Res. 2011;12:130. | ||
Soltani A, Muller HK, Sohal SS, et al. Distinctive characteristics of bronchial reticular basement membrane and vessel remodelling in chronic obstructive pulmonary disease (COPD) and in asthma: they are not the same disease. Histopathology. 2012;60(6):964–970. | ||
Sohal SS, Walters EH. Epithelial mesenchymal transition (EMT) in small airways of COPD patients. Thorax. 2013;68(8):783–784. | ||
Milara J, Peiro T, Serrano A, Cortijo J. Epithelial to mesenchymal transition is increased in patients with COPD and induced by cigarette smoke. Thorax. 2013;68(5):410–420. | ||
Walters EH, Reid D, Soltani A, Ward C. Angiogenesis: a potentially critical part of remodelling in chronic airway diseases? Pharmacol Ther. 2008;118(1):128–137. | ||
Hiroshima K, Iyoda A, Shibuya K, et al. Evidence of neoangiogenesis and an increase in the number of proliferating cells within the bronchial epithelium of smokers. Cancer. 2002;95(7):1539–1545. | ||
Zeisberg EM, Potenta SE, Sugimoto H, Zeisberg M, Kalluri R. Fibroblasts in kidney fibrosis emerge via endothelial-to-mesenchymal transition. J Am Soc Nephrol. 2008;19(12):2282–2287. | ||
Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119(6):1420–1428. | ||
Parimon T, Chien JW, Bryson CL, McDonell MB, Udris EM, Au DH. Inhaled corticosteroids and risk of lung cancer among patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;175(7):712–719. | ||
Yang IA, Relan V, Wright CM, et al. Common pathogenic mechanisms and pathways in the development of COPD and lung cancer. Expert Opin Ther Targets. 2011;15(4):439–456. | ||
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. | ||
GOLD. Global Initiative for Chronic Obstructive Pulmonary Lung Disease. 2013. Available from: http://www.goldcopd.org | ||
Hogg JC, Chu F, Utokaparch S, et al. The nature of small airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(26):2645–2653. | ||
Sohal SS, Soltani A, Reid D, et al. A randomized controlled trial of inhaled corticosteroids (ICS) on markers of epithelial-mesenchymal transition (EMT) in large airway samples in COPD: an exploratory proof of concept study. Int J Chron Obstruct Pulmon Dis. 2014;9:533–542. | ||
Shukla SD, Sohal SS, Mahmood MQ, Reid D, Muller HK, Walters EH. Airway epithelial platelet-activating factor receptor expression is markedly upregulated in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2014;9:853–861. | ||
Yanai M, Sekizawa K, Ohrui T, Sasaki H, Takishima T. Site of airway obstruction in pulmonary disease: direct measurement of intrabronchial pressure. J Appl Physiol (1985). 1992;72(3):1016–1023. | ||
Prudkin L, Liu DD, Ozburn NC, et al. Epithelial-to-mesenchymal transition in the development and progression of adenocarcinoma and squamous cell carcinoma of the lung. Mod Pathol. 2009;22(5):668–678. | ||
Burgel PR, Bourdin A, Chanez P, et al. Update on the roles of distal airways in COPD. Eur Respir Rev. 2011;20(119):7–22. | ||
Scotton CJ, Chambers RC. Molecular targets in pulmonary fibrosis*: the myofibroblast in focus. Chest. 2007;132(4):1311–1321. | ||
Milara J, Serrano A, Peiró T, et al. Aclidinium inhibits cigarette smoke-induced lung fibroblast-to-myofibroblast transition. Eur Respir J. 2013;41(6):1264–1274. | ||
Coward W, Deacon K, Pang L. Activation of epidermal growth factor receptor (EGFR) is required for Tgfβ1-induced epithelial-mesenchymal transition (EMT) in idiopathic pulmonary fibrosis (IPF). Am J Respir Crit Care Med. 2013;187:A4818. | ||
Milara J, Peiró T, Serrano A, et al. Roflumilast N-oxide inhibits bronchial epithelial to mesenchymal transition induced by cigarette smoke in smokers with COPD. Pulm Pharmacol Ther. 2014;28(2):138–148. | ||
Wang Q, Wang Y, Zhang Y, Zhang Y, Xiao W. The role of uPAR in epithelial-mesenchymal transition in small airway epithelium of patients with chronic obstructive pulmonary disease. Respir Res. 2013;14:67. | ||
Yang K, Song Y, Tang YB, et al. mAChRs activation induces epithelial-mesenchymal transition on lung epithelial cells. BMC Pulm Med. 2014;14:53. | ||
Keith RL, Miller YE, Gemmill RM, et al. Angiogenic squamous dysplasia in bronchi of individuals at high risk for lung cancer. Clin Cancer Res. 2000;6(5):1616–1625. | ||
Fontanini G, Vignati S, Bigini D, et al. Neoangiogenesis: a putative marker of malignancy in non-small-cell lung cancer (NSCLC) development. Int J Cancer. 1996;67(5):615–619. | ||
Sarrazin S, Maurage C, Delmas D, Lassalle P, Delehedde M. Endocan as a biomarker of endothelial dysfunction in cancer. J Cancer Sci Ther. 2010;2(6):47–52. | ||
Iwatsuki M, Mimori K, Yokobori T, et al. Epithelial-mesenchymal transition in cancer development and its clinical significance. Cancer Sci. 2010;101(2):293–299. | ||
Pain M, Bermudez O, Lacoste P, et al. Tissue remodelling in chronic bronchial diseases: from the epithelial to mesenchymal phenotype. Eur Respir Rev. 2014;23(131):118–130. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.