Back to Journals » Cancer Management and Research » Volume 10
Epidermal growth factor receptor gene mutations in non-small-cell lung cancer cells are associated with increased radiosensitivity in vitro
Authors Xie B, Sun LY , Cheng YJ, Zhou J, Zheng JH, Zhang WM
Received 18 February 2018
Accepted for publication 14 June 2018
Published 13 September 2018 Volume 2018:10 Pages 3551—3560
DOI https://doi.org/10.2147/CMAR.S165831
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Antonella D'Anneo
Bo Xie, Liyue Sun, Yanjun Cheng, Juan Zhou, Jihua Zheng, Weimin Zhang
Department of Oncology, Guangzhou General Hospital of Guangzhou Military Command, Guangzhou, Guangdong, People’s Republic of China
Introduction: There is still lack of specific biomarkers in predicting the radiosensitivity of non-small cell lung cancer (NSCLC) patients in clinic. Previous studies have shown that the EGFR gene status may correlate with radiosensitivity of NSCLC. However, the underlying mechanisms remain unknown. The aim of this study was to further investigate the correlation between EGFR mutation status and the NSCLC cell radiosensitivity and to explore the possible cellular mechanism.
Methods: Eight NSCLC cell lines with different EGFR gene status were irradiated by linear accelerator, and the radiosensitivity between the cell lines was compared by cell colony formation assay and cell proliferation assay. Cell cycle and apoptosis were analysed by flow cytometry. Radiosensitivity-related protein expression was detected by Western blotting.
Results: In the present study, we found that NSCLC cell lines with the epidermal growth factor receptor (EGFR) gene mutations were more sensitive to X-ray irradiation than those with wild-type EGFR (P<0.05). No difference in radiosensitivity was observed between NSCLC cells with EGFR exon19 deletion (Del 19) mutation and exon 21 point mutation at position 858 (L858R) with or without T790M mutation (P<0.05), as well as between NSCLC cells with EGFR mutation and those with acquired EGFR-tyrosine kinase inhibitors (TKIs) resistance. Mechanistically, EGFR mutations promoted NSCLC cell apoptosis in response to X-ray irradiation through the upregulation of proapoptotic protein Bax and downregulation of anti-apoptotic proteins such as Bcl-2 and DNA-dependent protein kinase catalytic subunit. In addition, phosphorylated histone (γ-H2AX) foci assay showed that EGFR mutations sustained irradiation-induced DNA damage.
Conclusion: Taken together, our study demonstrates that EGFR mutations are closely associated with the increased sensitivity of NSCLC cell lines to X-ray irradiation and that EGFR mutation status is a potentially useful indicator to evaluate the effectiveness of radiotherapy in the treatment of NSCLC.
Keywords: non-small-cell lung cancer, epidermal growth factor receptor, mutation, radiosensitivity
Erratum for this paper has been published
Introduction
Non-small-cell lung cancer (NSCLC) is the most common type of lung cancer, representing more than 80% of the total number of lung cancer cases.1 Many patients with NSCLC are inoperable due to locally advanced or metastatic disease upon diagnosis.1 Thus, radiotherapy alone or chemo-radiotherapy are very important in the treatment of NSCLC.2,3 However, wide heterogeneity is observed in the response to radiotherapy in patients with NSCLC. Specifically, some patients have a strong response to radiotherapy with an effective local control whereas others have local relapses even with increased radiation doses,4 which highlights the necessity for identifying biomarkers that can predict responses to radiotherapy to help develop personalized treatments in NSCLC.
Mutations in the EGFR gene and its downstream signaling pathways are major NSCLC driver mutations.5 Approximately 47% of patients with NSCLC in the Asia-Pacific region and 12% in Oceania have tumors associated with EGFR mutations.6 Targeted therapy based on EGFR mutations has been developed as standard first-line treatment for advanced NSCLC,7,8 and EGFR gene status has been identified as a prognostic biomarker for advanced NSCLC.
Clinical studies have recently shown that the EGFR gene status may correlate with radiosensitivity in patients with NSCLC. The response rate is higher, and progression-free survival and overall survival are longer in NSCLC patients with EGFR gene mutations than those in patients with wild-type (WT) EGFR, suggesting that EGFR gene status may also be a predictive biomarker for radiosensitivity in patients with NSCLC.9,10 However, many challenges remain with the use of EGFR mutations as diagnostic and prognostic biomarkers to measure the effectiveness of radiotherapy against advanced-stage NSCLC. For example, whether EGFR mutation increases the radiosensitivity of NSCLC cells remains controversial.11,12 Secondly, little is known regarding the correlation between radiosensitivity and EGFR mutation-triggered drug resistance to tyrosine kinase inhibitors (TKIs) in NSCLC cells. Thirdly, the molecular mechanisms underlying the radiosensitivity of EGFR-mutated NSCLC cells have not been extensively investigated.
To address the above issues, we examined the association between radiosensitivity and different EGFR mutation status in eight commonly used NSCLC cell lines. Moreover, the underlying mechanisms were investigated. Our study demonstrates that NSCLC cells with EGFR mutations are more sensitive to X-rays than those with wide-type EGFR genes. EGFR mutation status may be a potentially useful predictor of the effectiveness of radiotherapy for NSCLC.
Materials and methods
Cell lines and cell culture
NSCLC cell lines H226, A549, PC-9, HCC827, H3255, and H1975 were obtained from The Cell Bank of Chinese Academy of Sciences (Shanghai, China). PC-9/ZD and PC9/AB2 cells were generous gifts from Dr Fumiaki Koizumi (National Cancer Center, Japan) and Professor Li Zhang (Sun Yat-sen University Cancer Center, Guangzhou, China), respectively. Cells were grown in RPMI 1640 medium (Gibco, Gaithersburg, MD, USA) containing 10% fetal calf serum, 1% penicillin, and 1% streptomycin at 37°C in a humidified atmosphere of 5% CO2.
EGFR mutation analysis
Next-generation sequencing was conducted to validate EGFR mutations or other mutations in each NSCLC cell line. DNA profiling was undertaken using a commercially available capture-based sequencing panel, LungPlasma panel (Burning Rock Biotech, Guangzhou, Guangdong Province, China), targeting 168 genes and spanning 160K human genomic regions. DNA hybridization-based screening was carried out using magnetic beads and was amplified by PCR. A bioanalyzer high-sensitivity DNA assay was then used to assess the quality and size range. Thirty indexed samples were subjected to paired-end sequencing on a NextSeq 500 system (Illumina, USA). The EGFR mutation status of the eight NSCLC cell lines is shown in Table 1.
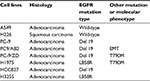  | Table 1 EGFR mutation status of NSCLC cell lines used in this study Abbreviations: NSCLC, non-small-cell lung cancer; EMT, epithelial–mesenchymal transition. |
Irradiation
6-MV X-ray irradiation was undertaken at an angle of 180° using a Varian Clinac 600C/D medical linear accelerator (Varian Medical Systems, Palo Alto, CA, USA) at 300 cGy/min with a source-to-axis distance of 100 cm and a field size of 40×40 cm2.
Clonogenic survival assay
Cells were seeded in 60-mm dishes at various densities and grown overnight. Cells were then exposed to single-dose X-ray irradiation ranging from 0 to 10 Gy, followed by an incubation at 37°C for 10–14 days. After fixation with paraformaldehyde (Beyotime, Shanghai, China), cells were stained with 0.5% crystal violet (Beyotime). Colonies containing more than 50 cells were counted and photographed with an Olympus microscope (Olympus, Tokyo, Japan). The surviving fraction (SF) was calculated as colony number/(plating cell number × plating efficiency). A survival curve was plotted as the logarithm of SF against the irradiation dose. The multi-target single-hit model was applied to calculate the average lethal dose (D0, Gy), quasi-threshold dose (Dq, Gy), and the extrapolation number (N). The linear-quadratic model was applied to calculate the cellular radiosensitivity parameters α (Gy-1), β (Gy-2), and α/β (Gy).13
Cell proliferation assay
Cells (1×103 cells/well) were plated in 96-well plates and grown overnight. Cells were then exposed to 4 Gy X-ray irradiation. Non-irradiated cells were used as a negative control. A Cell Counting Kit-8 (CCK-8; Dojindo, Kumamoto, Japan) assay was conducted to examine the effects of irradiation on cell proliferation at 24, 48, 72, 96, 120, and 144 hours, respectively, post-irradiation, according to the manufacturer’s instructions. Absorbance (OD value) was determined at an absorption wavelength of 450 nm using a microplate reader (Thermo Fisher Scientific, Waltham, MA, USA). The surviving fraction was calculated as (OD value of irradiated samples − OD value of blank well)/(OD value of negative control − OD value of blank well). The survival curves were plotted as the cell surviving fraction vs the incubation hours post-irradiation.
Cell apoptosis analyses by flow cytometry
Cells were seeded in 60-mm plates at a density of 1×105 cells in each plate and grown overnight. These cells were then exposed to 4 Gy X-ray and harvested at 0, 24, and 48 hours, respectively, post-irradiation. Cells were then rinsed with PBS, fixed with ice-cold ethanol, and resuspended in Annexin V-FITC solution (Beyotime) for cell apoptosis analyses prior to staining with propidium iodide working solution (PI; Beyotime). Results were analyzed using a FACS Aria II flow cytometer (BD Biosciences, San Jose, CA, USA).
Phosphorylated histone (γ-H2AX) foci assay
Cells were plated on coverslips in 35-mm dishes at a density of 1×106 cells per dish and grown overnight. At 0, 0.25, 0.5, 1, 2, 4, 6, 12, and 24 hours post-irradiation with 4 Gy, cells were fixed with ice-cold paraformaldehyde and washed with PBS. After treatment with 0.2% Triton-X100 in PBS, cells were blocked with BSA and then incubated with anti-γ-H2AX (Ser139; 1:500; Cell Signaling Technology, Danvers, MA, USA) at 37°C for 2 hours. Following PBS rinses, cells were incubated with Alexa Flour 488-conjugated secondary antibody (Ab; 1:600; Cell Signaling Technology) at 37°C in the dark for 1 hour. Hoechst 33342 (Beyotime) was used for nuclei staining. An IX70 inverted fluorescence microscope (Olympus Corporation, Tokyo, Japan) was used to acquire images at 10× fields. Three images containing more than 100 cells were randomly selected, and the cells exhibiting green fluorescence (γ-H2AX-positive cells) were counted by Image J software. γ-H2AX foci formation was evaluated as the number of γ-H2AX-positive cells (green)/the number of cells stained with Hoechst 33342 (blue).
Western blotting
Cells were seeded in 25-mm2 flasks at a density of 1×106 cells in each flask and allowed to adhere overnight. Cells were then exposed to 4 Gy X-ray irradiation and lysed for protein extraction at 0, 0.5, 1, 2, 4, 6, 12, and 24 hours post-irradiation. From this, 5–10 µg of each sample were separated by 10% SDS-PAGE and transferred to polyvinylidene difluoride membranes (Merck Millipore, Billerica, MA, USA), followed by 2 hours of blocking with 5% skimmed milk powder and overnight incubation with primary Ab against Bax, Bcl-2, Caspase-3 (Cell Signaling Technology, Danvers, MA, USA), or DNA-PKcs (Abcam, Cambridge, UK). The membranes were rinsed thrice with PBS containing 0.05% Tween-20 and then incubated with horseradish peroxidase-conjugated secondary Ab for 1 hour at 37°C. The chemiluminescent signals were detected using an enhanced chemiluminescence substrate (Millipore), visualized using a Minichemi imager system (Segacreation, Beijing, China), and quantified by Quantity One software (Bio-Rad, Laboratories Inc. Hercules, CA, USA).
Statistical analysis
Experiments were conducted in triplicates and repeated at least three times. Data are presented as the mean ± SD. Statistical significance was assessed using the Student’s t-test with SPSS V20.0 software (IBM Corporation, Armonk, NY, USA). P<0.05 was considered statistically significant.
Results
EGFR mutations promote the sensitivity of NSCLC cells to X-ray irradiation
To determine the sensitivity of each NSCLC cell line with different EGFR mutation status to X-ray irradiation, a colony-formation assay was conducted. As shown in Figure 1, compared to the WT EGFR cell lines A549 and H226, the EGFR-mutated cell lines exhibited high sensitivity to X-ray, as evidenced by significant decreases in the colony-forming activity (Figure 1) and the surviving fraction at different doses: The average surviving fraction was reduced by 50%, 75%, 90%, and 97% in response to 2, 4, 6, and 10 Gy of irradiation, respectively (all P<0.05; Figure 1 and Table 2).
  | Table 2 Surviving fraction of radiosensitivity between WT EGFR cell lines and EGFR-mutated cell lines was determined by colony-formation assay Abbreviation: WT, wild-type. |
To further confirm the radiosensitivity of EGFR-mutated cell lines, the cellular radiosensitivity parameters were measured using the multi-target single-hit and the linear-quadratic model. As shown in Table 3, the D0, Dq, and N values of EGFR-mutated cell lines were higher than those of WT EGFR cell lines (2- to 35-fold). Consistently, a generally opposite trend was observed for α/β ratios. In addition, the proliferation and surviving fraction of EGFR-mutated cell lines were markedly decreased in an irradiation time-dependent manner, compared to those of WT EGFR cell lines (P<0.001; Figure 2).
  | Table 3 Radiobiological parameters of radiosensitivity between WT EGFR cell lines and EGFR-mutated cell lines as determined by colony-formation assay Abbreviation: WT, wild-type. |
Taken together, these results suggest that EGFR-mutated NSCLC cell lines are more sensitive or less tolerant to X-ray irradiation than WT EGFR cell lines, which is likely due to the increased suppression of the cellular repair ability and proliferative ability of EGFR-mutated cell lines exposed to X-ray irradiation.
EGFR mutations increase the apoptosis of NSCLC cells in response to X-ray irradiation
Cell apoptosis contributes to the antitumor activity of radiotherapy. To investigate the mechanisms underlying the enhanced sensitivity of EGFR-mutated NSCLC cells to irradiation, we detected apoptosis in WT EGFR and EGFR-mutated NSCLC cells treated with X-ray irradiation using a flow cytometric assay. As shown in Figure 3A and B, the WT EGFR cells A549 and H226 did not exhibit apoptosis when irradiated with 4 Gy X-ray for 48 hours (P>0.05), whereas five cell lines with EGFR mutations (PC-9, PC9/AB2, PC-9/ZD, H1975, and HCC827) underwent significant apoptosis as early as 24 hours after the irradiation (P<0.01), and the apoptosis was further enhanced at 48 hours post-irradiation (P<0.001). Marked apoptosis of EGFR L858R-mutated H3255 cells occurs until 48 hours post-irradiation (P<0.001). These data indicate that EGFR mutation promotes the sensitivity of NSCLC cells to X-ray irradiation by inducing cell apoptosis.
EGFR mutations regulate apoptosis-related protein expression in the irradiation-treated NSCLC cells
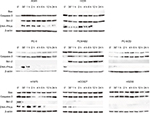
To further confirm that EGFR mutation may promote NSCLC cell apoptosis upon X-ray irradiation, Western blot analysis was undertaken to detect the expression of apoptosis-related proteins. As shown in Figure 4, proapoptotic protein Bax was nearly undetectable, whereas anti-apoptotic proteins Bcl-2 and DNA-dependent protein kinase catalytic subunit (DNA-PKcs) were substantially expressed in irradiation-treated WT EGFR NSCLC cells.
In contrast, there was appreciable production of Bax protein in the EGFR-mutated NSCLC cells after irradiation compared with WT EGFR NSCLC cells. The EGFR-mutated NSCLC cells had weak expression or no expression of Bcl-2 even under the irradiation condition, with the exception of PC9/AB2 cells in which Bcl-2 was significantly induced by the irradiation. DNA-PKcs was rapidly upregulated between 30 minutes and 1 hour after the irradiation in all NSCLC cells except for H3255. However, DNA-PKcs expression was then reduced to a basal level in PC-9, PC-9/ZD, H1975, and HCC827 cells 1 hour after the irradiation. In addition, Caspase-3 was constantly expressed in all eight NSCLC cells, regardless of the presence or absence of the irradiation. There appeared to be no significant difference in Caspase-3 expression between WT EGFR and EGFR-mutated NSCLC cells treated with X-ray irradiation. These results suggest that EGFR mutation promotes the apoptosis of irradiation-treated NSCLC by regulating apoptosis-related protein expression.
EGFR mutations sustain irradiation-induced DNA damage in NSCLC cells
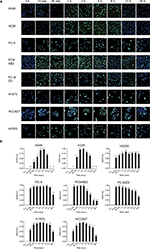
We next sought to determine whether EGFR mutation in NSCLC cells affects DNA damage – an important cellular event during radiotherapy. Because γ-H2AX foci formation is a key biomarker for DNA double-strand breaks,14 an γ-H2AX foci assay was conducted to detect DNA damage. As shown in Figure 5, γ-H2AX foci were formed as early as 15 minutes after 4 Gy X-ray irradiation in the EGFR-mutated NSCLC cells, peaked at 2 hours, and was sustained until 24 hours after irradiation.
In contrast, in the WT EGFR cells, γ-H2AX foci were detected at 30 minutes and disappeared at 12 hours post-irradiation. These data indicate that EGR mutation may contribute to DNA damage or inhibit the repair of DNA damage in response to X-ray irradiation, leading to decreased radiosensitivity in NSCLC cells.
Discussion
EGFR mutations are currently defined as the most prevalent genomically classified subgroup of NSCLC.15,16 This study investigated the association between EGFR mutations and radiosensitivity in NSCLC and found that EGFR-mutated NSCLC cells are more sensitive to X-ray irradiation than WT EGFR cells, which is consistent with a study by Das et al.11 However, Bokobza et al reported that EGFR-mutated NSCLC cells, such as PC-9 and HCC827, exhibit radioresistance when compared with the WT EGFR NSCLC cell line A549.17 This discrepancy is likely due to the difference in experimental conditions such as types and doses of radiation. For example, Das et al used X-ray, whereas Bokobza et al used gamma radiation from a cesium-137 source. Clinical evidence has shown that radiotherapy is more effective for patients with EGFR-mutated NSCLC than patients with WT EGFR NSCLC.18,19
EGFR T790M mutation in NSCLC accounts for more than 50% of acquired resistance to EGFR-TKIs.20 We found that the EGFR T790M mutation in NSCLC cells has no significant effect on radiosensitivity. NSCLC cell lines with Del19 or L858R mutation exhibit characteristic phenotypes of radiosensitivity, such as decreased colony-forming ability, suppressed cell proliferative ability, increased radiation-induced apoptosis, and sustained double-strand DNA breaks, which is independent of EGFR T790M mutation because there appears to be no appreciable changes in radiosensitivity between double mutations (Del 19 or L858R and T790M) and a single mutation (Del 19 or L858R) in the EGFR gene. These findings are consistent with the previous study.11 As the EGFR T790M mutation is uncorrelated with the radiosensitivity of NSCLC cells, we believe that radiotherapy may give rise to the same clinical outcomes for NSCLC patients with acquired resistance to EGFR-TKIs and those without the acquired resistance. Further clinical studies are needed to verify this hypothesis.
EGFR is a transmembrane glycoprotein consisting of an extracellular EGF-binding domain and an intracellular tyrosine kinase domain that plays an important role in cell proliferation through various signaling pathways.21 NSCLC cells with activating EGFR mutations (eg, Del 19, L858R, and T790M) render the cells more susceptible to irradiation damage. Indeed, our study demonstrates that EGFR mutations in NSCLC cells lead to sustained DNA damage (15 minutes–24 hours after irradiation), whereas WT EGFR NSCLC cells exhibit a weak response to irradiation, as evidenced by a relatively short time period of DNA damage (30 minutes–12 hours), allowing effective DNA repair and cellular recovery from irradiation damage. However, it remains to be elucidated whether this is the case when these cells are subjected to irreversible DNA damage in response to higher doses of irradiation. This requires further investigation.
However, the irradiation induces irreversible apoptosis in EGFR-mutated NSCLC, but not in WT EGFR NSCLC, further confirming the enhanced radiosensitivity of EGFR-mutated NSCLC. Mechanistically, EGFR mutations may promote cell apoptosis via the Bcl-2/Bax signaling pathway, as shown by irradiation-suppressed Bcl-2 expression and irradiation-induced Bax expressing in EGFR-mutated NSCLC cells – with the opposite trends being observed in WT EGFR cells. However, Bcl-2 protein expression appears to be highly induced by irradiation in PC9/AB2 cells, suggesting that other signaling pathways may participate in apoptotic processes in certain NSCLC cell subtypes with EGFR mutations. In addition, our findings revealed that DNA-PKcs was minimally expressed or not expressed in the majority of EGFR-mutated NSCLC cell lines.
DNA-PKcs has an anti-apoptotic function and is required for DNA repair.22,23 Previous studies have indicated that EGFR nuclear translocation is suppressed in EGFR-mutated cells exposed to irradiation, leading to the blockade of interaction between EGFR and DNA-PKcs and increased sensitivity to irradiation.24,25 There appears to be a significant correlation between DNA damage and cell apoptosis in irradiation-treated NSCLC cells. Emerging evidence consistently indicates that DNA damage and repair responses can trigger cell apoptosis26–28 and that apoptosis prevents further DNA repair and the propagation of DNA damage signaling.29
Conclusion
This study did not present data for the EGFR–TKI treatment of EGFR-mutated NSCLC cells, which limits our evaluation of the synergistic or combinative effects of irradiation and EGFR-TKI on the sensitivity of NSCLC cell subtypes. Despite this limitation, our study demonstrated that different EGFR mutation statuses may serve as potential diagnostic biomarkers for NSCLC radiosensitivity, which helps identify personalized treatment options for NSCLC patients with EGFR mutation and secondary resistance to EGFR–TKIs.
Acknowledgment
This work was financially supported by the National Natural Science Foundation of China (grant no. 81472172) and the Science and Technology Program of Guangzhou (grant no. 201804010222).
Disclosure
The authors report no conflicts of interest in this work.
References
Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83(5):584–594. | ||
Haslett K, Pöttgen C, Stuschke M, Faivre-Finn C. Hyperfractionated and accelerated radiotherapy in non-small cell lung cancer. J Thorac Dis. 2014;6(4):328–335. | ||
Curran WJ, Paulus R, Langer CJ, et al. Sequential vs. concurrent chemoradiation for stage III non-small cell lung cancer: randomized phase III trial RTOG 9410. J Natl Cancer Inst. 2011;103(19):1452–1460. | ||
Das AK, Bell MH, Nirodi CS, Story MD, Minna JD. Radiogenomics predicting tumor responses to radiotherapy in lung cancer. Semin Radiat Oncol. 2010;20(3):149–155. | ||
Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11(2):121–128. | ||
Midha A, Dearden S, Mccormack R. EGFR mutation incidence in non-small-cell lung cancer of adenocarcinoma histology: a systematic review and global map by ethnicity (mutMapII). Am J Cancer Res. 2015;5(9):2892–2911. | ||
Soria JC, Wu YL, Nakagawa K, et al. Gefitinib plus chemotherapy versus placebo plus chemotherapy in EGFR-mutation-positive non-small-cell lung cancer after progression on first-line gefitinib (IMPRESS): a phase 3 randomised trial. Lancet Oncol. 2015;16(8):990–998. | ||
Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31(27):3327–3334. | ||
Lee DW, Shin DY, Kim JW, et al. Additional prognostic role of EGFR activating mutations in lung adenocarcinoma patients with brain metastasis: integrating with lung specific GPA score. Lung Cancer. 2014;86(3):363–368. | ||
Wang TJ, Saad S, Qureshi YH, et al. Does lung cancer mutation status and targeted therapy predict for outcomes and local control in the setting of brain metastases treated with radiation? Neuro Oncol. 2015;17(7):1022–1028. | ||
Das AK, Sato M, Story MD, et al. Non-small-cell lung cancers with kinase domain mutations in the epidermal growth factor receptor are sensitive to ionizing radiation. Cancer Res. 2006;66(19):9601–9608. | ||
Oweida A, Sharifi Z, Halabi H, et al. Differential response to ablative ionizing radiation in genetically distinct non-small cell lung cancer cells. Cancer Biol Ther. 2016;17(4):390–399. | ||
Chapman JD, Gillespie CJ. The power of radiation biophysics-let’s use it. Int J Radiat Oncol Biol Phys. 2012;84(2):309–311. | ||
Ivashkevich A, Redon CE, Nakamura AJ, Martin RF, Martin OA. Use of the γ-H2AX assay to monitor DNA damage and repair in translational cancer research. Cancer Lett. 2012;327(1–2):123–133. | ||
Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350(21):2129–2139. | ||
Pao W, Miller V, Zakowski M, et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci U S A. 2004;101(36):13306–13311. | ||
Bokobza SM, Jiang Y, Weber AM, Devery AM, Ryan AJ. Short-course treatment with gefitinib enhances curative potential of radiation therapy in a mouse model of human non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2014;88(4):947–954. | ||
Lee HL, Chung TS, Ting LL, et al. EGFR mutations are associated with favorable intracranial response and progression-free survival following brain irradiation in non-small cell lung cancer patients with brain metastases. Radiat Oncol. 2012;7:181. | ||
Mak RH, Doran E, Muzikansky A, et al. Outcomes after combined modality therapy for EGFR-mutant and wild-type locally advanced NSCLC. Oncologist. 2011;16(6):886–895. | ||
Balak MN, Gong Y, Riely GJ, et al. Novel D761Y and common secondary T790M mutations in epidermal growth factor receptor-mutant lung adenocarcinomas with acquired resistance to kinase inhibitors. Clin Cancer Res. 2006;12(21):6494–6501. | ||
Bethune G, Bethune D, Ridgway N, Xu Z. Epidermal growth factor receptor (EGFR) in lung cancer: an overview and update. J Thorac Dis. 2010;2(1):48–51. | ||
Franco S, Murphy MM, Li G, et al. DNA-PKcs and Artemis function in the end-joining phase of immunoglobulin heavy chain class switch recombination. J Exp Med. 2008;205(3):557–564. | ||
Gurley KE, Moser R, Gu Y, Hasty P, Kemp CJ. DNA-PK suppresses a p53-independent apoptotic response to DNA damage. EMBO Rep. 2009;10(1):87–93. | ||
Chen DJ, Nirodi CS. The epidermal growth factor receptor: a role in repair of radiation-induced DNA damage. Clin Cancer Res. 2007;13(22 Pt 1):6555–6560. | ||
Liccardi G, Hartley JA, Hochhauser D. EGFR nuclear translocation modulates DNA repair following cisplatin and ionizing radiation treatment. Cancer Res. 2011;71(3):1103–1114. | ||
Strasser A, Harris AW, Jacks T, Cory S. DNA damage can induce apoptosis in proliferating lymphoid cells via p53-independent mechanisms inhibitable by Bcl-2. Cell. 1994;79(2):329–339. | ||
Nowsheen S, Yang ES. The intersection between DNA damage response and cell death pathways. Exp Oncol. 2012;34(3):243–254. | ||
Plesca D, Mazumder S, Almasan A. DNA damage response and apoptosis. Methods Enzymol. 2008;446:107–122. | ||
Wang J, Pabla N, Wang CY, et al. Caspase-mediated cleavage of ATM during cisplatin-induced tubular cell apoptosis: inactivation of its kinase activity toward p53. Am J Physiol Renal Physiol. 2006;291(6):F1300–F1307. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.